Abstract
In this issue, three groups (Hewitt et al. 2010. J. Cell Biol. doi:10.1083/jcb.201002133; Maciejowski et al. 2010. J. Cell Biol. doi:10.1083/jcb.201001050; Santaguida et al. 2010. J. Cell Biol. doi:10.1083/jcb.201001036) use chemical inhibitors to analyze the function of the mitotic checkpoint kinase Mps1. These studies demonstrate that Mps1 kinase activity ensures accurate chromosome segregation through its recruitment to kinetochores of mitotic checkpoint proteins, formation of interphase and mitotic inhibitors of Cdc20, and correction of faulty microtubule attachments.
Mps1 is an essential mitotic kinase in organisms from yeast to man. Originally discovered in a yeast genetic screen for mutants producing monopolar spindles (Winey et al., 1991), Mps1’s primary role is in the mitotic checkpoint (Weiss and Winey, 1996; Abrieu et al., 2001; Stucke et al., 2002; Fisk and Winey, 2004). This checkpoint, also known as the spindle assembly checkpoint, prevents cell cycle advance from metaphase to anaphase before attachment of every chromosome to spindle microtubules. The signaling device for this safeguard mechanism is the unattached kinetochore, which generates one or more inhibitors of Cdc20, an essential activator of anaphase-promoting complex/cyclosome (APC/C), the E3 ubiquitin ligase which targets cyclin B and securin for destruction (Figs. 1 and 2; Kops et al., 2005; Musacchio and Salmon, 2007). In the absence of a functional mitotic checkpoint, as occurs when Mps1 function is lost, cells become rapidly aneuploid and subsequently die (Kops et al., 2005; Janssen et al., 2009), observations which have lead to the proposal that Mps1 is an attractive anticancer drug target.
Figure 1.
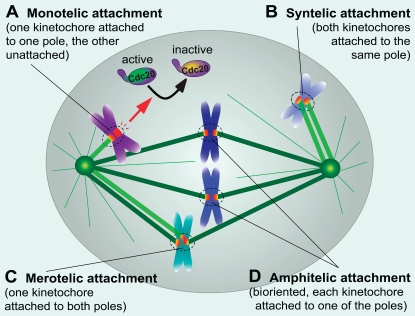
Microtubule–kinetochore attachments. Four types of kinetochore–microtubule attachments are highlighted. (A) Monotelic attachment with only one kinetochore attached. Unattached kinetochores produce the mitotic checkpoint inhibitor that delays advance to anaphase by inactivating Cdc20, an activator of the ubiquitin ligase APC/C. (B) Syntelic attachment with both kinetochores attached to microtubules from the same pole. (C) Merotelic attachment with one kinetochore attached to microtubules from both poles. (D) Bioriented attachment (also known as amphitelic) with the two kinetochores of each chromatid pair attached to opposite spindle poles.
Figure 2.
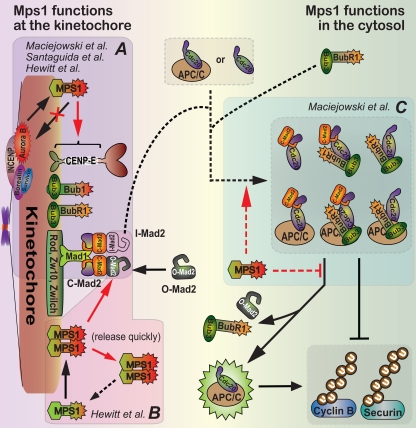
Mps1 functions at multiple steps to inhibit Cdc20–APC/C. (A) All three groups (Hewitt et al., 2010; Maciejowski et al., 2010; Santaguida et al., 2010) demonstrate that at unattached kinetochores, Mps1 kinase activity is required to recruit other mitotic checkpoint components, including Mad1, Mad2, Bub1, BubR1, Bub3, and the Rod–Zw10–Zwilch complex. Discrepancies exist on exactly which components depend on Mps1 activity (see Table I for details). (B) Hewitt et al. (2010) show that Mps1 kinase activity maintains the recruitment at unattached kinetochores of O-Mad2 to the stably bound Mad1–C-Mad2 template. The molecular mechanism is yet to be elucidated. Mps1 may dimerize and be activated by self phosphorylation at kinetochores followed by quick release into the cytosol. (C) Maciejowski et al. (2010) demonstrate that Mps1 kinase activity in the cytosol promotes the assembly and/or prevents the disassembly of Cdc20–APC/C inhibitory complexes. Although the relative abundance and contribution of specific Cdc20–APC/C inhibitory complexes are unclear, all inhibit Cdc20 to prevent polyubiquitination of the key mitotic regulators cyclin B and securin by APC/C, an event which targets them for degradation as an irreversible trigger for anaphase entry.
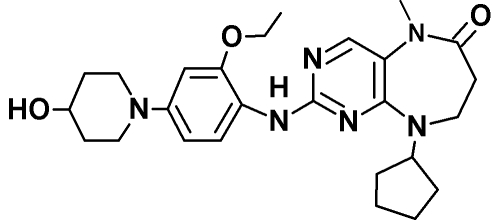
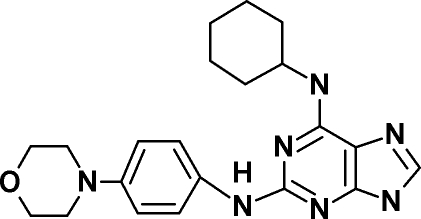
Three novel Mps1 inhibitors have been described this year. A first pair, Mps1-IN-1 and Mps1-IN-2, have half maximal inhibitory concentrations (IC50) of between 100 and 300 nM (Kwiatkowski et al., 2010). In this issue, two more Mps1 inhibitors are reported (Table I). Hewitt et al. describe AZ3146, which has an IC50 of ∼35 nM toward recombinant Mps1 and does not inhibit Cdk1 and Aurora B at that concentration. Concurrently, Santaguida et al. discover that reversine, a purine derivative named after its ability to promote dedifferentiation of C2C12 myoblasts into multipotent cells (Chen et al., 2004), is actually a highly potent Mps1 inhibitor. Although previously proposed to be an Aurora B inhibitor (D’Alise et al., 2008), reversine is the most potent of the Mps1 inhibitors with an IC50 of 3 nM toward full-length Mps1 (Santaguida et al., 2010). It is also the most exhaustively characterized for specificity, and it is 35 times more potent an inhibitor of Mps1 than of Aurora B. In addition, Mps1 inhibition by reversine is rapidly reversed after removal of the drug from culture media, a valuable experimental property which attributes another meaning to the name.
Table I.
Summary of studies using chemical inhibitors of human Mps1 kinase activity
| Property | Studies | ||||||
| Hewitt et al., 2010 | Kwiatkowski et al., 2010 | Santaguida et al., 2010 | Maciejowski et al., 2010 | Sliedrecht et al., 2010 | Tighe et al., 2008 | ||
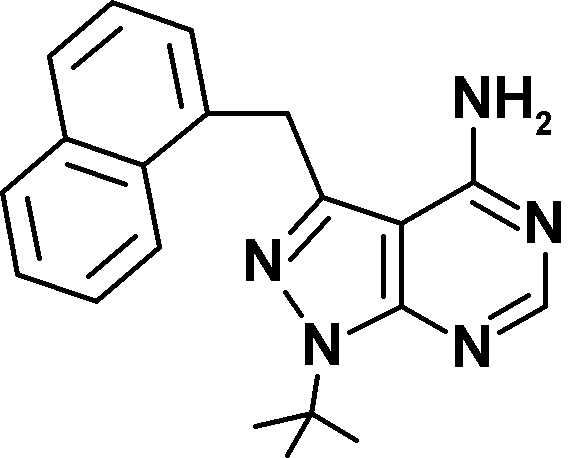
| Inhibitor | AZ3146 | Mps1-IN-1 | Mps1-IN-2 | Reversine | 3MB-PP1 | 23-dMB-PP1 | 1-NM-PP1 |
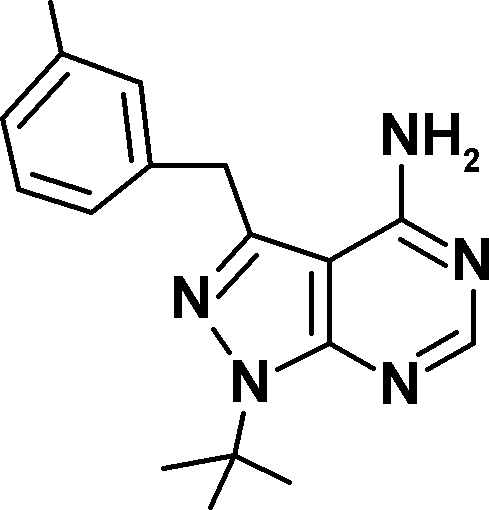
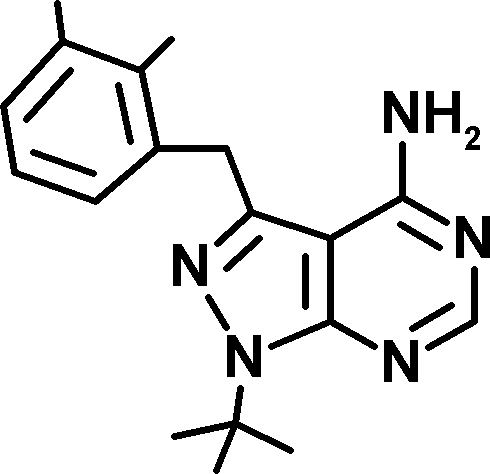
| Structure | 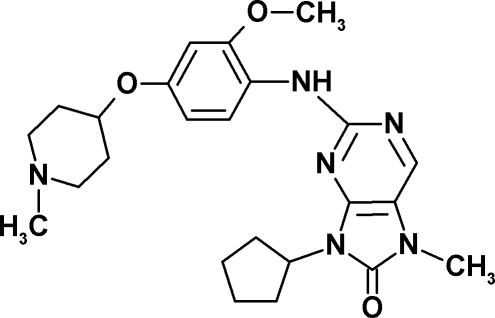 |
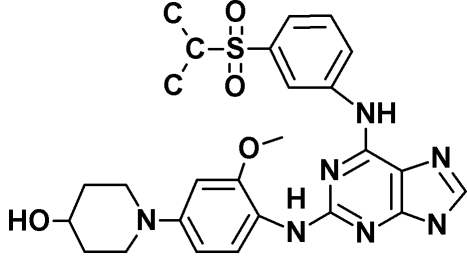 |
 |
 |
 |
 |
 |
| IC50 (nM)a | 35 | 370 | 145 | 3/6b | ND | ND | ND |
| Conc. used in cell (µM) | 2 | 10 | 10 | 0.5 | 10 | 1 | 10 |
| Drug target | Endogenous Mps1 | Endogenous Mps1 | Endogenous Mps1c | Endogenous Mps1d | Mps1-ase | Mps1-ase | Mps1-ase |
| Approach | Inhibitor; siRNA | Inhibitor; stable shRNA | Inhibitor; stable shRNA | Inhibitor; siRNA | Gene knockout + stable transgene | stable shRNA + stable transgene | shRNA + transgene |
| Cell line used | HeLa | U2OS/HCT116/HeLa/RPE1 | U2OS | HeLa | hTERT-RPE1 | U2OS/HCT116 | HeLa |
| TMitosis (min)f | ∼90 | ND | ND | ∼45 | ∼42 | ∼22/18 | ND |
| TMitosis+Inhibitor (min) | ∼32 | ∼45 | ND | ∼30 | ∼12 | ∼12/10 | ∼36 |
| Kinetochore localization inhibitedg | O-Mad2/CENP-E | Mad2/Mad1 | ND | Mad1/Spindly/Rod/Zw10/Zwilch | Mad2/Mad1/Bub1/BubR1/Zw10/Plk1/CENP-E/pH2A/Sgo1 | Mad2/Mad1 Bub1/Cdc20 | Mad2 |
| Kinetochore localization not inhibited | Mad1h/Zwilchh pCENP-A/Aurora B/pAurora B/ACA | CREST | ND | Bub1/BubR1/KNL1/Mis12/Ndc80/Zwint/Aurora B/pCENP-A/CENP-C/CREST | Ska3/Ndc80/KNL1/Zwint/CENP-A/Aurora B/INCENP/CREST | CENP-E/BubR1/ACA | Mad1/ACA |
| Chromosome misalignment | ND | Yes | ND | Yes | Yes | Yes | ND |
| Defect in error correction | Yes | NA | ND | Yes | ND | Yes | ND |
| Affect Aurora B kinase activity | No | Yes | ND | No (at 0.5 µM) | No | Yes | ND |
Conc., concentration.
The half maximal inhibitory concentration.
For full-length and kinase domain Mps1, respectively.
Binds Plk1 with higher affinity.
Inhibits Aurora B with an IC50 of 98 nM.
Analogue-sensitive Mps1.
Time cells spend in mitosis.
Scoring >50% reduction of signal intensity.
Depends on when drugs are applied.
Using a complementary approach, in this issue, Maciejowski et al. produced the highest selectivity in Mps1 inhibition by building human diploid cells in which the sole Mps1 gene contained a mutation that resulted in an enlarged ATP-binding pocket (frequently called a Shokat allele) that can accept (and be inhibited by) a bulky purine analogue (3MB-PP1). The modified Mps1 (termed Mps1-as), already reduced in activity by ∼90% relative to unmodified Mps1, could be highly selectively inhibited by the addition of 3MB-PP1. Collectively, these new chemical tools have enabled dissection of the multiple signaling pathways regulated by Mps1 in human cells and may mark a starting point for the development of therapeutic drugs targeting Mps1.
Each of the new Mps1 inhibitors overrides mitotic checkpoint–mediated mitotic arrest in cells in which spindle assembly is blocked with microtubule inhibitors (Hewitt et al., 2010; Maciejowski et al., 2010; Santaguida et al., 2010). These data confirm an indispensible role for Mps1 kinase activity in the mitotic checkpoint (Abrieu et al., 2001; Dorer et al., 2005; Jones et al., 2005). It should be noted that among the current and previous studies, there are marked discrepancies on exactly which of the checkpoint proteins require Mps1 activity for their kinetochore localization (Fig. 2 A and Table I). Our view is that these differences are likely due to the extent and timing of Mps1 inhibition, as well as the use of different cell types. Mps1’s role in the checkpoint had previously been attributed to its requirement for kinetochore recruitment of the checkpoint protein Mad2 (Abrieu et al., 2001; Jelluma et al., 2008; Tighe et al., 2008; Kwiatkowski et al., 2010; Sliedrecht et al., 2010). In engineered human retinal pigment epithelial (RPE) cells (Maciejowski et al., 2010), all conserved mitotic checkpoint components, including Bub1, BubR1, Mad1, and Mad2, were evicted from kinetochores when Mps1 was inhibited or deleted. Also, all were recruited in the cells containing only the Mps1-as allele but to which no inhibitor had been added, demonstrating that the 10% of normal kinase activity provided by this allele is sufficient for Mps1’s essential role.
In contrast, by using the AZ3146 inhibitor, Hewitt et al. (2010) found that Mps1 activity is required to recruit Mad1 to kinetochores but not maintain it there. Stably bound Mad1 forms a tight complex with closed Mad2 (C-Mad2) at unattached kinetochores, which serves as a template to dock an additional, initially open Mad2 conformer (O-Mad2; the latent inactive form that spontaneously binds Cdc20 very slowly). This produces a putative, transient kinetochore-bound intermediate (I-Mad2) that can convert into C-Mad2 that is bound to Cdc20, thereby inactivating it (Fig. 2; Musacchio and Salmon, 2007). Interestingly, Hewitt et al. (2010) report that recruitment of O-Mad2 to the C-Mad2–Mad1 template is blocked if Mps1 is inhibited with AZ3146 after cells enter mitosis. Thus, both C-Mad2 and O-Mad2 molecules require Mps1 activity for loading at kinetochores, but the stably bound C-Mad2 does not require continuing Mps1 activity. How Mps1 kinase activity controls the docking of O-Mad2 to the C-Mad2–Mad1 template is now a central, unresolved question (Fig. 2 B).
In addition to the generation of a Cdc20 inhibitor by unattached kinetochores in prometaphase, a Cdc20 inhibitor is generated in interphase so as to prevent immediate anaphase onset after mitotic entry, thereby providing time for newly assembled kinetochores to generate their own mitotic checkpoint inhibitor. This premade inhibitor has been referred to as a timer (Meraldi et al., 2004), as the minimum length of mitosis is set by the time needed to inactivate it. Use of each of the Msp1 inhibitors has revealed that Mps1 inhibition shortens mitosis (Table I). Most striking among these, inhibition of Mps1-as with 3MB-PP1 shortens mitosis in human RPE cells from ∼42 to ∼12 min (Maciejowski et al., 2010). This dramatic acceleration of mitosis supports Mps1 as a component needed for production of the interphase-produced M phase timer, in addition to Mad2 and BubR1 that directly bind Cdc20.
Although the exact molecular constituents of either the mitotic or interphase Cdc20 inhibitors are yet to be defined (Fig. 2 C), Maciejowski et al. (2010) found that the amounts of Mad2 and BubR1 associated with Cdc20 are significantly reduced after Mps1 inhibition in both interphase and mitotic cells. Furthermore, they found that a truncated Mps1 mutant that fails to localize to kinetochores is sufficient to support the assembly of a Mad2–Cdc20 complex and delay anaphase onset. Because Mad2 and BubR1 can associate with and inhibit Cdc20 in vitro without Mps1 (Kulukian et al., 2009), Mps1 may either catalyze these associations in vivo and/or inhibit a yet to be identified pathway that actively disassociates Mad2 and BubR1 from Cdc20 (Fig. 2 C). An unresolved question is how cytosolic Mps1 is inhibited after checkpoint silencing at the kinetochore.
Using the chemical inhibitors, all three groups found that the kinase activity of human Mps1 is required for proper chromosome alignment and accurate chromosome segregation (Hewitt et al., 2010; Maciejowski et al., 2010; Santaguida et al., 2010), in agreement with previous studies using short hairpin RNA (shRNA) to reduce Mps1 (Jelluma et al., 2008; Tighe et al., 2008). In particular, correction of syntelic attachments, in which both kinetochores of a mitotic chromatid pair are attached to the same pole (Fig. 1 B), are inhibited in the absence of Mps1 kinase activity (Hewitt et al., 2010; Maciejowski et al., 2010; Santaguida et al., 2010), as previously shown in yeast (Maure et al., 2007). It has been proposed that Mps1 acts upstream of Aurora B to correct syntelic attachments (Jelluma et al., 2008; Sliedrecht et al., 2010). However, all three current studies failed to detect changes of Aurora B activity upon Mps1 inhibition; instead, Santaguida et al. (2010) and Hewitt et al. (2010) found that the kinetochore localization of Mps1 depends on Aurora B activity and propose that Aurora B acts upstream of Mps1. Indeed, consideration of the divergent outcomes for which kinase comes first (Table I) makes it unlikely, at least in our view, that there is a strictly linear pathway with one kinase upstream of the other; rather, there is likely to be a network nature of mitotic kinase signaling. Additionally, Mps1 and Aurora B kinase activities may converge on a common substrate or substrates. The kinetochore motor CENP-E is a good candidate for such a target, as it is required for chromosome congression and is phosphorylated by both Mps1 (Espeut et al., 2008) and Aurora B (Kim et al., 2010). Aurora B is also required for correcting merotelic attachment errors (Fig. 1 C; Cimini et al., 2006; Knowlton et al., 2006), a condition which does not occur in budding yeast. It remains to be tested whether human Mps1 also corrects this type of attachment error and, if so, whether it partners with Aurora B. The chemical tools recently developed should greatly facilitate the field to dissect out these underlying mechanisms.
Beyond an understanding of how Mps1 functions at a molecular level, an ultimate goal is the design of better anticancer drugs. The current Mps1 inhibitors serve as a starting point to directly evaluate whether Mps1 can be used as an effective anticancer drug target. A caveat of this approach is the requirement for >90% inhibition before any observable mitotic phenotype. Nevertheless, initial studies have demonstrated that inhibiting Mps1 with chemical inhibitors kills cultured tumor cells (Kwiatkowski et al., 2010; Sliedrecht et al., 2010). We wait to see if this promise holds true in clinical settings.
References
- Abrieu A., Magnaghi-Jaulin L., Kahana J.A., Peter M., Castro A., Vigneron S., Lorca T., Cleveland D.W., Labbé J.C. 2001. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell. 106:83–93 10.1016/S0092-8674(01)00410-X [DOI] [PubMed] [Google Scholar]
- Chen S., Zhang Q., Wu X., Schultz P.G., Ding S. 2004. Dedifferentiation of lineage-committed cells by a small molecule. J. Am. Chem. Soc. 126:410–411 10.1021/ja037390k [DOI] [PubMed] [Google Scholar]
- Cimini D., Wan X., Hirel C.B., Salmon E.D. 2006. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr. Biol. 16:1711–1718 10.1016/j.cub.2006.07.022 [DOI] [PubMed] [Google Scholar]
- D’Alise A.M., Amabile G., Iovino M., Di Giorgio F.P., Bartiromo M., Sessa F., Villa F., Musacchio A., Cortese R. 2008. Reversine, a novel Aurora kinases inhibitor, inhibits colony formation of human acute myeloid leukemia cells. Mol. Cancer Ther. 7:1140–1149 10.1158/1535-7163.MCT-07-2051 [DOI] [PubMed] [Google Scholar]
- Dorer R.K., Zhong S., Tallarico J.A., Wong W.H., Mitchison T.J., Murray A.W. 2005. A small-molecule inhibitor of Mps1 blocks the spindle-checkpoint response to a lack of tension on mitotic chromosomes. Curr. Biol. 15:1070–1076 10.1016/j.cub.2005.05.020 [DOI] [PubMed] [Google Scholar]
- Espeut J., Gaussen A., Bieling P., Morin V., Prieto S., Fesquet D., Surrey T., Abrieu A. 2008. Phosphorylation relieves autoinhibition of the kinetochore motor Cenp-E. Mol. Cell. 29:637–643 10.1016/j.molcel.2008.01.004 [DOI] [PubMed] [Google Scholar]
- Fisk H.A., Winey M. 2004. Spindle regulation: Mps1 flies into new areas. Curr. Biol. 14:R1058–R1060 10.1016/j.cub.2004.11.047 [DOI] [PubMed] [Google Scholar]
- Hewitt L., Tighe A., Santaguida S., White A.M., Jones C.D., Musacchio A., Green S., Taylor S.S. 2010. Sustained Mps1 activity is required in mitosis to recruit O-Mad2 to the Mad1–C-Mad2 core complex. J. Cell Biol. 190:25–34 10.1083/jcb.201002133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen A., Kops G.J., Medema R.H. 2009. Elevating the frequency of chromosome mis-segregation as a strategy to kill tumor cells. Proc. Natl. Acad. Sci. USA. 106:19108–19113 10.1073/pnas.0904343106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelluma N., Brenkman A.B., van den Broek N.J., Cruijsen C.W., van Osch M.H., Lens S.M., Medema R.H., Kops G.J. 2008. Mps1 phosphorylates Borealin to control Aurora B activity and chromosome alignment. Cell. 132:233–246 10.1016/j.cell.2007.11.046 [DOI] [PubMed] [Google Scholar]
- Jones M.H., Huneycutt B.J., Pearson C.G., Zhang C., Morgan G., Shokat K., Bloom K., Winey M. 2005. Chemical genetics reveals a role for Mps1 kinase in kinetochore attachment during mitosis. Curr. Biol. 15:160–165 10.1016/j.cub.2005.01.010 [DOI] [PubMed] [Google Scholar]
- Kim Y., Holland A.J., Lan W., Cleveland D.W. 2010. Aurora kinases and protein phosphatase 1 mediate chromosome congression through regulation of CENP-E. Cell. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton A.L., Lan W., Stukenberg P.T. 2006. Aurora B is enriched at merotelic attachment sites, where it regulates MCAK. Curr. Biol. 16:1705–1710 10.1016/j.cub.2006.07.057 [DOI] [PubMed] [Google Scholar]
- Kops G.J., Weaver B.A., Cleveland D.W. 2005. On the road to cancer: aneuploidy and the mitotic checkpoint. Nat. Rev. Cancer. 5:773–785 10.1038/nrc1714 [DOI] [PubMed] [Google Scholar]
- Kulukian A., Han J.S., Cleveland D.W. 2009. Unattached kinetochores catalyze production of an anaphase inhibitor that requires a Mad2 template to prime Cdc20 for BubR1 binding. Dev. Cell. 16:105–117 10.1016/j.devcel.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski N., Jelluma N., Filippakopoulos P., Soundararajan M., Manak M.S., Kwon M., Choi H.G., Sim T., Deveraux Q.L., Rottmann S., et al. 2010. Small-molecule kinase inhibitors provide insight into Mps1 cell cycle function. Nat. Chem. Biol. 6:359–368 10.1038/nchembio.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejowski J., George K.A., Terret M.-E., Zhang C., Shokat K.M., Jallepalli P.V. 2010. Mps1 directs the assembly of Cdc20 inhibitory complexes during interphase and mitosis to control M phase timing and spindle checkpoint signaling. J. Cell Biol. 190:89–100 10.1083/jcb.201001050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maure J.F., Kitamura E., Tanaka T.U. 2007. Mps1 kinase promotes sister-kinetochore bi-orientation by a tension-dependent mechanism. Curr. Biol. 17:2175–2182 10.1016/j.cub.2007.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P., Draviam V.M., Sorger P.K. 2004. Timing and checkpoints in the regulation of mitotic progression. Dev. Cell. 7:45–60 10.1016/j.devcel.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Musacchio A., Salmon E.D. 2007. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8:379–393 10.1038/nrm2163 [DOI] [PubMed] [Google Scholar]
- Santaguida S., Tighe A., D’Alise A.M., Taylor S.S., Musacchio A. 2010. Dissecting the role of MPS1 in chromosome biorientation and the spindle checkpoint through the small molecule inhibitor reversine. J. Cell Biol. 190:73–87 10.1083/jcb.201001036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliedrecht T., Zhang C., Shokat K.M., Kops G.J. 2010. Chemical genetic inhibition of Mps1 in stable human cell lines reveals novel aspects of Mps1 function in mitosis. PLoS One. 5:e10251 10.1371/journal.pone.0010251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucke V.M., Silljé H.H., Arnaud L., Nigg E.A. 2002. Human Mps1 kinase is required for the spindle assembly checkpoint but not for centrosome duplication. EMBO J. 21:1723–1732 10.1093/emboj/21.7.1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tighe A., Staples O., Taylor S. 2008. Mps1 kinase activity restrains anaphase during an unperturbed mitosis and targets Mad2 to kinetochores. J. Cell Biol. 181:893–901 10.1083/jcb.200712028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Winey M. 1996. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J. Cell Biol. 132:111–123 10.1083/jcb.132.1.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M., Goetsch L., Baum P., Byers B. 1991. MPS1 and MPS2: novel yeast genes defining distinct steps of spindle pole body duplication. J. Cell Biol. 114:745–754 10.1083/jcb.114.4.745 [DOI] [PMC free article] [PubMed] [Google Scholar]