In addition to its function as an Arp2/3 complex subunit, Arp1cb interacts with and stimulates Aurora A at centrosomes, functioning in cell cycle progression.
Abstract
Here we provide evidence in support of an inherent role for Arpc1b, a component of the Arp2/3 complex, in regulation of mitosis and demonstrate that its depletion inhibits Aurora A activation at the centrosome and impairs the ability of mammalian cells to enter mitosis. We discovered that Arpc1b colocalizes with γ-tubulin at centrosomes and stimulates Aurora A activity. Aurora A phosphorylates Arpc1b on threonine 21, and expression of Arpc1b but not a nonphosphorylatable Arpc1b mutant in mammalian cells leads to Aurora A kinase activation and abnormal centrosome amplification in a Pak1-independent manner. Together, these findings reveal a new function for Arpc1b in centrosomal homeostasis. Arpc1b is both a physiological activator and substrate of Aurora A kinase and these interactions help to maintain mitotic integrity in mammalian cells.
Introduction
Mitosis is a fundamental, regulated cellular process responsible for the generation of a mitotic spindle with two spindle poles, and daughter cells. Disrupted mitosis often leads to daughter cells with aberrant spindle poles and cellular pathogenesis (Sankaran and Parvin, 2006). Entry and progression through mitosis is a tightly regulated dynamic process involving activation of multiple kinases, including Aurora A (Marumoto et al., 2005). Association of Aurora A with centrosomes, spindle poles, aster microtubules, and the midbody supports its role in regulating centrosome maturation, duplication, and cell cycle progression, all of which are often compromised and dysregulated in the absence of Aurora A (Katayama et al., 2003). Loss of Aurora A in embryonic mice is lethal due to defects in mitotic spindle assembly and misaligned and lagging chromosomes (Sasai et al., 2008). In contrast, Aurora A up-regulation promotes centrosome amplification, aneuploidy, and cancer, and Aurora kinase expression is often elevated in many cancer types (Katayama et al., 2003). The paramount role of Aurora A in the biology of both normal and cancer cells has led to increasing interest in the molecular mechanisms responsible for Aurora A activation.
A number of Aurora A activators and substrates have been identified. For example, LATS2 and NDEL1 are Aurora A substrates that affect centrosome maturation, and Aurora A–mediated phosphorylation of TACC helps stabilize aster microtubules (Barros et al., 2005; Abe et al., 2006; Mori et al., 2007). Aurora A also phosphorylates tumor suppressors BRCA1 and p53 and influences their function in cell cycle progression (Katayama et al., 2004; Ouchi et al., 2004). Upstream activators of Aurora A, such as Ajuba in humans and Bora in Drosophila, participate in centrosome maturation (Hirota et al., 2003; Hutterer et al., 2006). The cofactor TPX2 targets Aurora A to mitotic spindle microtubules and plays an important role in spindle assembly in humans and in Xenopus (Bayliss et al., 2003). Aurora A activities and functions are also regulated by cytoskeleton remodeling components such as p21-activated kinase 1 (Pak1; Zhao et al., 2005), integrin-linked kinase (Fielding et al., 2008), the focal adhesion scaffolding factor Hef1 (Pugacheva and Golemis, 2005; Wu et al., 2006), and Rho GTPases (Ando et al., 2007), but the role of the actin cytoskeleton in Aurora A biology remains unknown.
The actin cytoskeleton undergoes dramatic cell cycle–dependent remodeling but its role in mitosis is not very well understood. G-actin is present both in the cytoplasm of interphase cells and in the mitotic phase of LLC-PK1 cells, COS, and CHO cells (Meijerman et al., 1999). Similarly, nuclear extracts from 293T cells contain all of the cofactors required for actin polymerization, including actin-related protein 3 (Arp3; Wu et al., 2006). Studies on Schizosaccharomyces pombe suggest a defective actin cytoskeleton results in a disoriented spindle and delayed cell division (Gachet et al., 2001). These observations predict a role for the actin cytoskeleton or actin-associated proteins in the regulation of mitosis and perhaps the cell cycle. The Arp2/3 complex is an actin regulator that initiates formation of new actin filaments (Zigmond, 1998; Goley and Welch, 2006). The complex consists of seven subunits known as Arp2, Arp3, Arpc1, Arpc2, Arpc3, Arpc4, and Arpc5. Arpc1 has two isoforms in humans, Arpc1a and Arpc1b. In earlier studies designed to isolate novel Pak1-interacting proteins during mitosis, we screened a complementary DNA expression library from mitotic HeLa cells with a GST-Pak1 solid-phase kinase assay, and identified Arpc1b as a Pak1-interacting substrate (Vadlamudi et al., 2004b). Pak1 phosphorylates Arpc1b on threonine 21 (T21) in the first repeat, a modification required for cell motility in growth factor–stimulated cells. Thus, we predict Arpc1b may have a role in mitosis.
Here we provide evidence that Arpc1b localizes on centrosomes and has a distinct role in cell cycle progression. Arpc1b interacts with and stimulates Aurora A activity and participates in the progression of the G2/M phase. Surprisingly, we discovered that Aurora A kinase phosphorylates Arpc1b on Thr21 and causes abnormal centrosome amplification in Pak1-deficient cells. These studies describe Arpc1b as a novel centrosome-associated protein that is a physiological activator and substrate of Aurora A kinase. Interactions of Arpc1b with Aurora A kinase are critical in the maintenance of mitotic integrity in mammalian cells.
Results
Arpc1b and tumorigenesis
A recent high-resolution expression profiling study suggested that Arpc1b is amplified in human pancreatic cancer cell lines (Mahlamäki et al., 2004). Thus, we initially explored whether Arpc1b is also up-regulated in human breast tumors using a limited number of paired samples. Arpc1b protein was up-regulated in 8 of 10 human breast tumors (Fig. S1 A). Because Arpc1b is dysregulated in human breast tumors, we examined the role of Arpc1b overexpression in the biology of ZR-75 breast cancer cells using pooled clones overexpressing Arpc1b (ZR-75/Arpc1b) or pcDNA empty vector (ZR-75/pcDNA) as model systems. Arpc1b up-regulation conferred a growth advantage to ZR-75 cells in anchorage-independent growth (Fig. S1 B) and tumorigenesis assays (Fig. S1, C and D). Interestingly, tumors in all seven mice with Arpc1b-overexpressing cells displayed a higher frequency of cells with multiple centrosomes whereas only one of seven mice with pcDNA developed a tumor, as assessed by anti-centrin antibodies (Fig. S1 E). Centrosomal amplification in tumors is intimately linked with stimulation of Aurora A kinase. We found elevated levels of activated Aurora A in mouse tumors isolated from ZR-75/Arpc1b compared with ZR-75/pcDNA cells, as revealed by immunohistochemical analysis with an anti–phospho-Aurora A (Thr288) antibody (unpublished data). These observations suggest that Arpc1b overexpression, which is a physiologically relevant event in human breast cancer, promotes tumorigenic properties of breast cancer cells. Subsequently, the ZR-75 cell line was used to specifically examine the role of Arpc1b in mitosis.
Arpc1b is an Aurora A–interacting protein that localizes to centrosomes
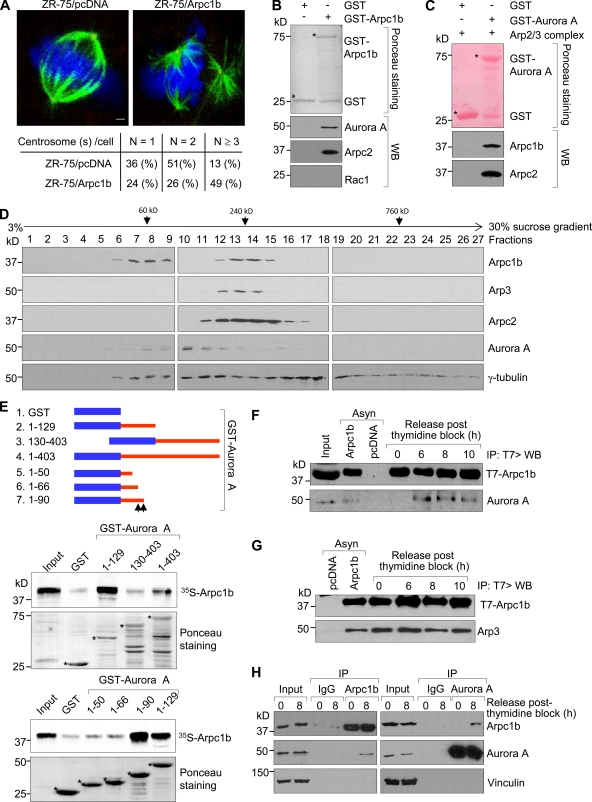
To explore the role of Arpc1b in mitosis, we initially used ZR-75 breast cancer cells overexpressing Arpc1b as a model system to study centrosome duplication, a hallmark of normal cell cycle regulation. Arpc1b overexpression was accompanied by centrosome amplification (Fig. 1 A), suggesting that Arpc1b helps determine the cellular integrity of the centrosome. Because Aurora kinases, particularly Aurora A, play an important role in maintaining spindle integrity and dysregulated activation of Aurora A causes centrosome amplification, we examined the possibility that Arpc1b interacts with Aurora A. GST-tagged Arpc1b interacted with Aurora A from ZR-75 cell lysates (Fig. 1 B). Because Arpc1b is a component of the Arp2/3 complex (Machesky and Gould, 1999; Goley and Welch, 2006), we wanted to evaluate whether purified Arp2/3 complex also interacts with Aurora A, as does GST-Arpc1b. Toward this end, GST-control or GST-Aurora A was incubated with purified Arp2/3 complex, and the immunoprecipitates were subjected to Western blot analysis using an anti-Arpc1b or anti-Arpc2 antibody. In addition to a direct interaction with Arpc1b (Fig. 1 B), Aurora A also interacted with the Arpc1b and Arpc2 as a part of the Arp2/3 complex (Fig. 1 C). To further distinguish the possibility that Arpc1b may also exist as a stand-alone protein in addition to acting as a component of the Arp2/3 complex, we performed a sucrose gradient sedimentation experiment using ZR-75 cell extracts following methods described previously (Humphries et al., 2002). Arpc1b had two distinct peaks (fractions 6–9 and 12–15; Fig. 1 D). In contrast, the two other components of the Arp2/3 protein complex, Arp3 and Arpc2 (Machesky and Gould, 1999; Goley and Welch, 2006), had only one peak (fractions 12–14 and 11–17, respectively; Fig. 1 D). In fact, Arp3 and Arpc2 comigrated with Arpc1b only in fractions 12–14 (Fig. 1 D). These results suggest that Arpc1b can indeed exist as a stand-alone protein and might play an independent role outside its established contribution to the Arp2/3 complex. Interestingly, Aurora A was present in fractions containing only Arpc1b (fractions 5–9) as well as those containing both Arpc1b and the Arp2/3 complex (fractions 12–15). These data are consistent with the notion that Aurora A interacts not only with Arpc1b alone, but also with the purified Arp2/3 complex.
Figure 1.
Arpc1b interacts with Aurora kinase A. (A) ZR-75 cells synchronized by double-thymidine block and released for 6 h were analyzed for centrosome numbers. α-Tubulin (green) is used to indicate spindle morphology and pericentrin (red) is used to indicate centrosomes. DNA is in blue. N, number of centrosomes per cell. Quantitation for the centrosome numbers in the different stable cell lines generated in ZR-75 cells is shown below panel A. Bar, 20 µm. (B) ZR-75 cell lysates were incubated with either GST or GST-Arpc1b and the protein complex precipitated was subjected to Western blot analysis with the antibodies. Ponceau-stained blot shows the quality of GST proteins used for the study. (C) GST control or GST-Aurora A was incubated with purified Arp2/3 complex and the immunoprecipitates were subjected to Western blot analysis using an anti-Arpc1b or anti-Arpc2 antibody. (D) ZR-75 cell extracts were fractionated onto 3–30% sucrose gradients by velocity sedimentation, and then subjected to Western blot analysis with the indicated antibodies. (E) GST-tagged deletion mutants of human Aurora kinase A. Aurora A, 1–403 is the full-length protein. In vitro–translated 35S-labeled Arpc1b was used to study binding of different GST-Aurora A deletion mutants. The extent of binding was estimated by measuring signal intensity. Ponceau S staining shows equal quantity of GST and GST-Aurora A deletions used in the reaction. Asterisk denotes the GST-fused protein of interest; black arrows indicate the interacting region of Aurora kinase A with Arpc1b. (F) Asynchronized cells or cells blocked with thymidine and released for various time points were used for immunoprecipitation (IP). Transfected T7-Arpc1b was immunoprecipitated using T7 antibody and the blot was probed for Aurora A. (G) Asynchronized cells or cells blocked with thymidine and released for various time points were subjected to the sequential IP/Western blot with the indicated antibodies. (H) Protein extract from ZR-75 cells released for 0 or 8 h after double-thymidine block were subjected to reciprocal immunoprecipitation assays with an anti-Aurora A or anti-Arpc1b antibody, followed by Western blot analysis with the indicated antibodies. kD, kilodaltons.
We performed in vitro GST pull-down assays to further determine whether a direct interaction between Arpc1b and Aurora A occurs. 35S-labeled, in vitro–translated Arpc1b protein bound strongly to the N-terminal domain, specifically the “A-box III” containing region encompassing amino acids 66–90 of Aurora A (Fig. 1 E). In cells, Aurora A levels peak during the late S phase and remain high during the G2-M phase of the cell cycle (Walter et al., 2000; Katayama et al., 2001). To determine at what point the interaction between endogenous Aurora A kinase and the transfected T7-tagged Arpc1b occurs, ZR-75 cells expressing T7-Arpc1b were arrested in the early S-phase using double-thymidine block and released into the cell cycle at various time points. The purity of cell synchronization was verified by flow cytometry and cyclin A expression (unpublished data). T7-tagged Arpc1b (T7-Arpc1b) interacted with endogenous Aurora A 6–10 h after release from the thymidine block (Fig. 1 F). In contrast, T7-Arpc1b was persistently associated with endogenous Arp3 at all of the time points (Fig. 1 G). To further examine whether endogenous levels of Arpc1b and Aurora A proteins may interact in a physiological context, ZR-75 cells were subjected to double-thymidine block and then released at the indicated time points. Protein extracts were subjected to the reciprocal immunoprecipitation analysis using an anti-Aurora A or anti-Arpc1b antibody, followed by Western blot analysis with the indicated antibodies. Consistent with the above findings, endogenous Aurora A and Arpc1b interacted at the 8-h time point (Fig. 1 H). Furthermore, when we probed the same membranes for vinculin, no bands were seen in the immunoprecipitates, even after long exposure (Fig. 1 H, bottom). Taken together, these results indicate that Arpc1b interacts with Aurora A in a cell cycle–dependent manner.
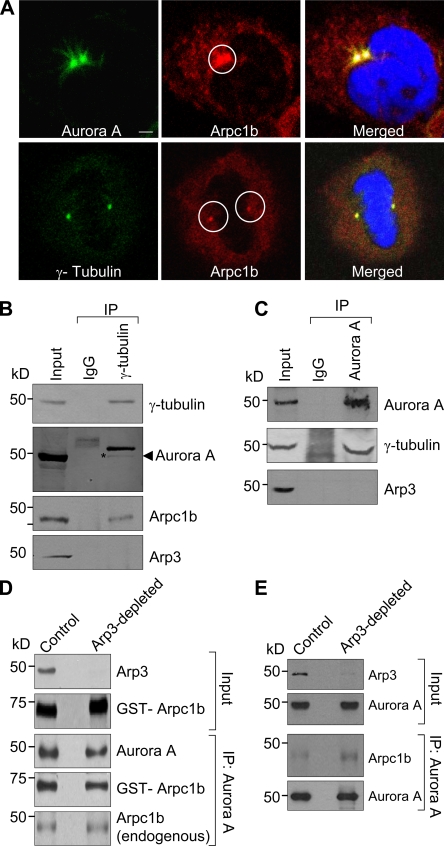
As a component of the Arp2/3 complex, Arpc1b primarily resides in the cytoplasm. However, a small amount of Arpc1b was reproducibly detected at the centrosome. Thus, we studied the subcellular localization of endogenous Arpc1b during the cell cycle after thymidine block. The majority of Arpc1b was cytoplasmic with a small fraction associated with the centrosome. A detailed confocal microscopic analysis indicated that endogenous Arpc1b colocalized with the centrosomal markers Aurora A and γ-tubulin (Fig. 2 A), and ectopic GFP-Arpc1b (Fig. S2) colocalized with γ-tubulin. Centrosomal staining of Arpc1b was confirmed using siRNA to Arpc1b, which led to the disappearance of Arpc1b staining at the centrosome with no effect on staining for Aurora A and γ-tubulin (Fig. S3 B). The specificity of Arpc1b knockdown was verified by probing for Arpc2, Arp3, and Aurora A as controls (Fig. S3 A). In support of these results, we found that γ-tubulin coimmunoprecipitated with endogenous Arpc1b and Aurora A but not Arp3 from ZR-75 cells arrested in the metaphase (Fig. 2 B). Furthermore, immunoprecipitation using an anti-Aurora A antibody failed to immunoprecipitate Arp3 (Fig. 2 C).
Figure 2.
Arpc1b localizes to the centrosome. (A) Synchronized ZR-75 cells were released for 6 h and analyzed for endogenous Arpc1b colocalization with centrosomal proteins Aurora A and γ-tubulin. Aurora A and γ-tubulin (green); endogenous Arpc1b (red); DNA (blue). Circle marks centrosome location. Bar, 5 µm. (B) Equal amount of cell extract was immunoprecipitated with control IgG or γ-tubulin antibody from the synchronized cells (top panel). The same blot was probed for Arpc1b (second last panel) and Arp3 (last panel). The blot was stripped and probed for Aurora A (second panel). (C) Equal amount of cell extract was immunoprecipitated with control IgG or Aurora A antibody (top panel). The same blot was probed for γ-tubulin (second panel) and Arp3 (last panel). Input, cell lysate used as positive control. (D) Xenopus egg extract was treated with either GST (control) or GST-WASP-CA (Arp3 depletion) and used for IP experiments. The Western blot shows Arp3 depletion in GST-WASP-CA–treated egg extract (top panel) and the GST-Aprc1b (second panel) used for the study. Aurora A was immunoprecipitated from the treated egg extract and incubated with GST-Arpc1b protein. The Western blot shows immunoprecipitated Aurora A and GST-Arpc1b or endogenous Arpc1b in complex with Aurora A in control and Arp3-depleted egg extract. (E) Aurora A was immunoprecipitated from the treated egg extract. Western blot shows immunoprecipitated Aurora A and endogenous Arpc1b in complex with Aurora A in control and Arp3-depleted egg extract. kD, kilodaltons.
The above results indicate two possibilities. Either the Arp2/3 complex fell apart during immunoprecipitation and, therefore, we did not observe Arp3 in the complex with either γ-tubulin or Aurora A, or the fraction of Arpc1b that exists independently of the Arp2/3 complex in cells was able to associate with Aurora A. To address these possibilities, Xenopus oocyte extracts were immunodepleted of Arp3 using GST-N WASP-CA (Yang et al., 2000) and used to study interactions of Arpc1b with Aurora A. There was no difference in the interaction of GST-Arpc1b or endogenous Arpc1b with Aurora A in control, GST-treated, or Arp3-depleted GST-N WASP-CA–treated extracts (Fig. 2 D). When a similar experiment was performed without adding exogenous GST-Arpc1b, the interaction between endogenous Arpc1b and Aurora A was improved (Fig. 2 E). These results, shown in Fig. 2, D and E, suggest that GST-Arpc1b added to the oocyte extract may compete with endogenous Arpc1b for binding to Aurora A. Alternatively, Arp3 depletion may destabilize the Arp2/3 complex. These findings collectively indicate that the interaction with Aurora A is specific to Arpc1b and independent of the Arp2/3 complex.
It was also of interest to investigate the previously observed interaction of Arpc1b with γ-tubulin. To accomplish this, we probed the same membranes from the sucrose density gradient experiments (Fig. 1 D) with a monoclonal anti–γ-tubulin antibody to determine the distribution of γ-tubulin on the sucrose density gradient based on the sedimentation profile. Similar studies from other laboratories have demonstrated that complexes of interacting proteins cofractionate on a sucrose density gradient after sedimentation (Ma et al., 1998). The results showed that γ-tubulin and Arpc1b coexisted in fractions 12–15 (Fig. 1 D, bottom). More interestingly, we also detected γ-tubulin in fractions 6–9, which represents the Arpc1b-only complex. Taken together, these experiments provide additional biochemical evidence to support the possibility that Arpc1b interacts with γ-tubulin, and the presence and physiological relevance of γ-tubulin in Arpc1b-only fractions warrant further investigation.
Arpc1b activates Aurora A
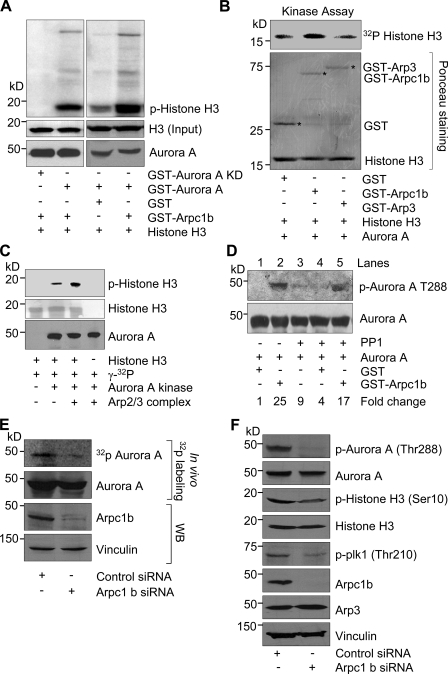
Because Arpc1b interacts with Aurora A and also localizes to centrosomes, we determined whether Arpc1b may activate Aurora A kinase. In an in vitro kinase assay using histone H3 as a substrate, Arpc1b enhanced Aurora A kinase activity (Fig. 3 A). Kinase-dead Aurora A was used as a negative control. Interestingly, neither Arp3 (Fig. 3 B) nor Arpc1a (Fig. S4 A), another two subunits of Arp2/3 complex (Machesky and Gould, 1999; Goley and Welch, 2006), enhanced the kinase activity of Aurora A, whereas the purified Arp2/3 complex did (Fig. 3 C). The observed effect of the Arp2/3 complex on Aurora A activity could be due, at least in part, to the presence of Arpc1b in the Arp2/3 complex.
Figure 3.
Arpc1b stimulates activity of Aurora A. (A) GST alone or GST-Arpc1b was used in an in vitro kinase assay with GST-Aurora A or GST-Aurora A KD protein. Phosphorylated histone H3 was visualized by autoradiography. Ponceau-stained blot shows equal amount of histone H3 used for both reactions. Western blot shows equal amount of Aurora A was used in all the reactions. (B) GST, GST-Arpc1b, or GST-Arp3 was used in an in vitro kinase assay with recombinant Aurora A using Histone H3 as a substrate. Phosphorylated histone H3 was visualized by autoradiography (top panel). Ponceau-stained blot shows equal amount of GST proteins and histone H3 used for all the reactions. Asterisk denotes the GST-fused protein of interest. (C) Aurora A kinase activity was studied using histone H3 as a substrate in the presence or absence purified Arp2/3 complex. Ponceau-stained blot shows equal amount of histone H3 and the Western blot shows equal amount of Aurora A used in the reactions. (D) GST or GST-Arpc1b was incubated with phosphatase (PP1)-treated Aurora A for 20 min in presence of [32P]γATP. Samples were analyzed for Aurora A phosphorylation (Thr-288, top panel) and total Aurora A (last panel). Fold change was expressed with respect to lane 1. (E) ZR-75 cells transfected with either control siRNA or Arpc1b siRNA were subjected to in vivo 32P-labeling. Protein extracts were subjected to IP with an anti-Aurora A antibody, and phosphorylated Aurora A was visualized by autoradiography. Knockdown of Arpc1b was verified by Western blot with an anti-Arpc1b antibody (bottom panel). (F) Immunoblots showing levels of phospho-Aurora A (Thr288), phospho-plk1 (Thr 210), and phospho-histone H3 (Ser 10) in control and Arpc1b siRNA-treated ZR-75 cell lysates. kD, kilodaltons.
Because Aurora A is phosphorylated in its activation loop at Thr288 (T288), presumably due to auto-phosphorylation (Littlepage et al., 2002), we investigated the significance of Arpc1b to Aurora A phosphorylation at this site. Treatment of Aurora A with purified type-1 protein phosphatase (PP1) reduced Aurora A kinase activity, and this decrease in activity corresponded to de-phosphorylation of T288, as detected by phospho-site–specific immunoblotting (Fig. 3 D). Adding GST-Arpc1b increased kinase activity of Aurora A with a corresponding increase in T288 phosphorylation (Fig. 3 D, lane 5) compared with a GST control (Fig. 3 D, lane 4). To further validate the role of Arpc1b in Aurora A activation, ZR-75 cells were transfected with specific siRNAs that target human Arpc1b or control siRNAs, and Aurora A phosphorylation was examined after 32P-labeling. Interestingly, knockdown of Arpc1b led to a decrease in Aurora A phosphorylation in situ (Fig. 3 E) as well as phosphorylation of its well-established downstream effectors, such as polo-like kinase 1 (plk-1; Macůrek et al., 2008) and histone H3 (Scrittori et al., 2001; Crosio et al., 2002; Fig. 3 F). Taken together, these findings suggest that Arpc1b is an interacting activator of Aurora A kinase.
Arpc1b is required for optimal cell cycle progression
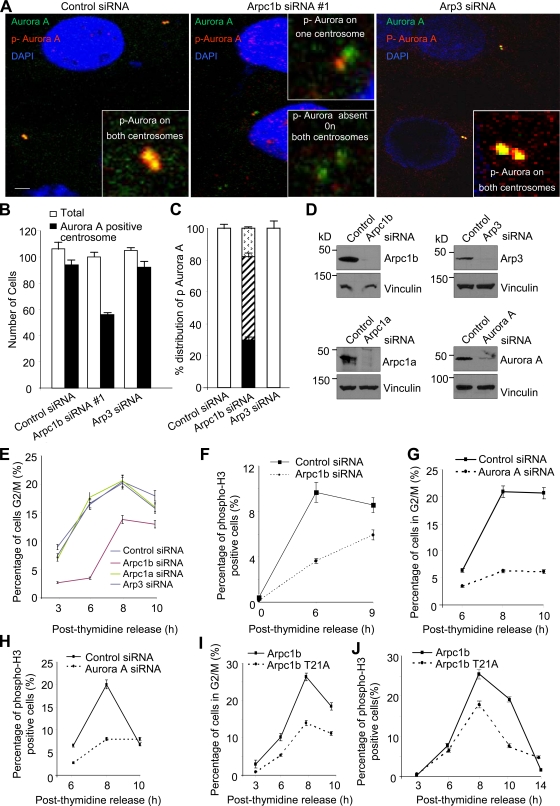
To determine the significance of Arpc1b during mitosis, we combined cell synchronization with siRNA silencing. ZR-75 cells were transfected with either control or Arpc1b siRNA or siRNA to two other components of the Arp2/3 complex, Arp3 and Arpc1a, in the interval between the two thymidine blocks. Cells transfected with Arpc1b-siRNA#1 (Fig. 4 A) or another Arpc1b-siRNA#2 (Fig. S4 B) were subjected to immunofluorescence analysis with antibodies against Aurora A and phospho-Aurora A (Thr288). Although Aurora A localized to the centrosome in cells transfected with Arpc1b siRNA, only ∼50% of cells displayed Aurora A activation, and Aurora A remained active either on only one centrosome or was inactive on both centrosomes (Fig. 4, A–C; and Fig. S4 B). In contrast to Arpc1b knockdown, siRNA of controls/Arp3 (Fig. 4, A–C) and Arpc1a (Fig. S4 C) had no effect on Aurora A activation on the centrosome.
Figure 4.
Arpc1b is essential for progression to G2-M phase of cell cycle. (A) Synchronized ZR-75 cells transfected with control, Arpc1b, or Arp3 siRNA and released for 7 h after G1-S arrest were immunostained with antibodies to Aurora A (green) or phospho-Aurora A T288 (red) as indicated. Insets represent higher magnification images of centrosomes. Bar plot shows percentage of cells with Aurora A–positive centrosomes (B), and distribution of phospho-Aurora A on centrosomes (C) on transfecting ZR-75 cells with control, Arpc1b, or Arp3 siRNA. Staining for phospho-Aurora A on both centrosomes: arrowheads in bar graph = absent from both centrosomes; diagonal lines = present on only one centrosome; black = reduced staining for both centrosomes. Bar, 5 µm. (D) ZR-75 cells were transfected with control siRNAs, or specific siRNAs targeting Arpc1b, Arp3, Arpc1a, or Aurora A, and protein extracts were subjected to Western blot analysis with the indicated antibodies. (E and G) Synchronized ZR-75 cells transfected with control or specific siRNAs targeting Arpc1b, Arpc1a, or Arp3 (E) or Aurora A (G) were scored for percentage of cells in G2-M phase at the indicated time points after release from G1-S arrest using FACS analysis; n = 3. (F and H) Synchronized ZR-75 cells transfected with either control or Arpc1b siRNA (F) or Aurora A siRNA (H) were scored for percentage of phospho-H3–positive cells at the indicated time points after release from G1-S arrest using FACS analysis; n = 3. (I and J) Synchronized ZR-75 cells stably expressing wild-type Arpc1b (ZR-75/T7-Arpc1b) or Arpc1bT21A (ZR-75/T7-Arpc1b T21A) were scored for percentage of cells in G2-M phase (I) or for percentage of phospho-H3–positive cells (J) at the indicated time points after release from G1-S arrest using FACS analysis; n = 3. kD, kilodaltons.
We also studied the effect of Arp2/3 complex knockdown on cell cycle progression. Depletion of Arpc1b but not Arpc1a or Arp3 drastically reduced the ability of cells to enter the G2/M phase of the cell cycle (Fig. 4 E). Knockdown effects of Arpc1b, Arpc1a, and Arp3 siRNAs were demonstrated by Western blot analysis with the indicated antibodies (Fig. 4 D). To confirm the role of Arpc1b in regulating progression to mitosis, we assessed the number of mitotic, phospho-H3–positive cells in control and Arpc1b-depleted samples. Knockdown of Arpc1b drastically reduced the phosho-H3–positive cells compared with control siRNA-treated samples (Fig. 4 F).
Given the evidence that Arpc1b depletion is responsible for decreased Aurora A activation (Fig. 3, E and F), we examined whether this negative effect was responsible for delayed mitotic entry or whether the low Aurora A activity is a consequence of blocked cell cycle progression. We used specific siRNAs targeting human Aurora A to knockdown endogenous Aurora A in ZR-75 cells, demonstrated by Western blot in Fig. 4 D (bottom right panel), and analyzed the cell cycle as described in Fig. 4, E and F. Aurora A knockdown significantly reduced cells in the G2/M phase (Fig. 4 G). This was further validated by phospho-histone H3 staining of control and Aurora A siRNA-transfected cells after thymidine release (Fig. 4 H). Thus, knockdown of Aurora A clearly compromised progression of cells in the G2/M phase, similar to the results shown in Fig. 4, E and F. These results indicate that the negative effect of Arpc1b depletion on Aurora A activity could be responsible for delayed mitotic entry in Arpc1b-depleted cells.
To further support these observations, we performed a molecular replacement experiment using an Arpc1b-T21A mutant that was able to function as a component of the Arp2/3 complex but unable to interact with or activate Aurora A. We performed the same experiments described in Fig. 4, E and F using stable clones of ZR-75 cells expressing T7-tagged wild-type Arpc1b (ZR-75/T7-Arpc1b) or the T7-Arpc1b-T21A mutation (ZR-75/T7-Arpc1b-T21A). As shown in Fig. 4, I and J, in contrast to wild-type controls, Arpc1b-T21A mutants exhibited compromised cell cycle progression and mitosis. These results suggest that the observed defects in cell cycle progression in Arpc1b-T21A–expressing cells may have resulted due to an inability to bind and activate Aurora A. Taken together, these findings suggest that the defects observed in Aurora A activation and cell cycle progression are specific to the knockdown of Arpc1b and that Arpc1b facilitates activation of Aurora A at centrosomes and regulates the progression of cells into mitosis.
Arpc1b-T21A mutant lacks the ability to bind and activate Aurora A
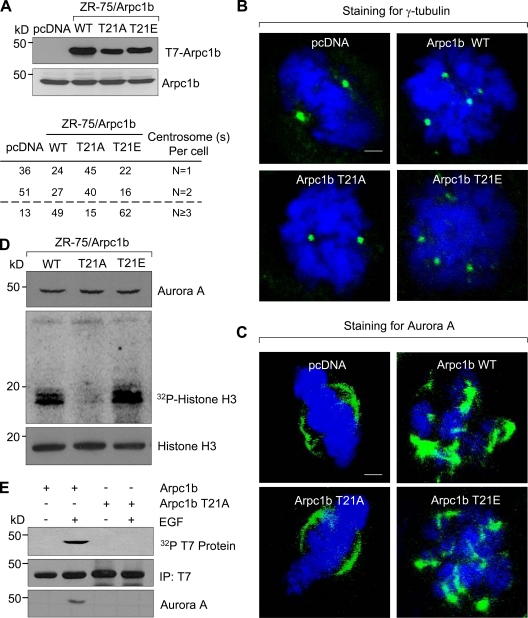
Because overexpression of Pak1 (Vadlamudi and Kumar, 2003; Kumar et al., 2006) or its substrate Arpc1b (Fig. 1 A) resulted in an accumulation of centrosomes, we hypothesized that Arpc1b may be a downstream effector of Pak1-mediated aberrant spindle formation. We addressed this by determining the effect of Arpc1b-T21 mutants on Aurora A activation and cell cycle progression. We generated pooled ZR-75 clones expressing a phosphorylation-defective T7-tagged Arpc1b-T21-Ala mutant (Arpc1b-T21A) or a phospho-mimetic mutant Arpc1b-T21-Glu (Arpc1bT21E; Fig. 5 A). Cells expressing T7-Aprc1b-WT or Arpc1b-T21E exhibited centrosome amplification, as indicated by an increased number of γ-tubulin (Fig. 5 B) and Aurora A foci (Fig. 5 C). Interestingly, we failed to observe such phenotypic changes in cells expressing T7-Arpc1b-T21A, which indicates a mechanistic role of the T21 site on Arpc1b function in the development of these phenotypes. To identify biochemical differences in the spindle phenotype observed in cells expressing T7-Arpc1b-WT or T7-Arpc1b-T21A, we determined the status of Aurora A activity in these stable cell lines. Cells expressing T7-Arpc1b-T21A had less Aurora A activity (∼40%) than did T7-Arpc1b or T7-Arpc1b-T21E cells (Fig. 5 D). Furthermore, Aurora A was pulled down in the complex with only Arpc1b in response to EGF, a known stimulant for Pak kinase (Menard and Mattingly, 2003), whereas Arpc1b-T21A failed to do so (Fig. 5 E).
Figure 5.
Phosphorylation of Arpc1b on Thr 21 regulates centrosome numbers in ZR-75 cells. (A) Expression of T7-Arpc1b (top) and endogenous Arpc1b (bottom) in stable cell lines generated in ZR-75 cells for indicated point mutations of Arpc1b. Quantitation for the centrosome numbers in the different stable cell lines generated in ZR-75 cells are shown below panel A. Synchronized cells released for 6 h after G1-S arrest were used for immunostaining. Representative images showing the distribution of γ-tubulin (green, B); Aurora A (green, C), and DNA (blue) in the stable cell lines. γ-Tubulin was used as a centrosome marker. Bars, 5 µm. (D) Synchronized cells released for 7 h after G1-S arrest were subjected to immunoprecipitation using agarose-tagged Aurora A antibody. The resulting precipitates were used for an in vitro kinase assay with histone H3 as substrate. Autoradiograph shows Aurora A, phosphorylated histone H3, and total histone H3. (E) 32P-labeled ZR-75 cells expressing either T7-Arpc1b or T7-Arpc1bT21A were treated with 100 ng/ml of EGF for 5 min. Arpc1b was immunoprecipitated using an anti-T7 antibody (second panel) and the in vivo phosphorylation of Arpc1b was analyzed using autoradiography (first panel). The same blot was probed for Aurora A (third panel). WT, wild type; kD, kilodaltons.
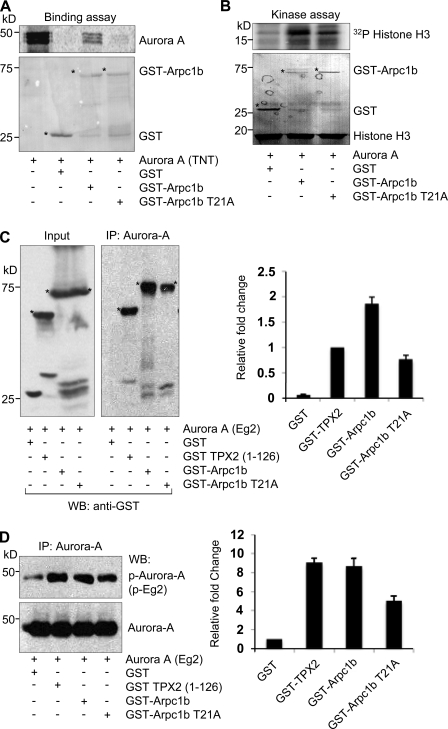
Consistent with the above findings, the direct interaction of Aurora A with GST-Arpc1b-T21A was substantially lower than that of wild-type Arpc1b (Fig. 6 A). GST-Arpc1b-T21A was less able to activate Aurora A (1.01-fold) than was GST-Arpc1b (1.9-fold; Fig. 6 B). The Arp complex has an important function in the interphase cytoskeleton. The spindle defects observed in ZR-75 cells overexpressing Arpc1b could be an indirect effect of perturbing interphase cytoskeletal functions. To determine whether Arpc1b has a direct role in Aurora A activation and centrosome function, we turned to mitotic phase Xenopus oocyte extracts and compared the relative activation of Aurora A by wild-type Arpc1b versus the positive control, the N-terminal domain of Xenopus TPX2 (amino acids 1–126), which binds and activates Aurora A (Bayliss et al., 2003). In these assays, we measured the activation of Xenopus Aurora A (Eg2) using phospho-peptide antibodies (P-Eg2) specific for the region of Eg2 that includes T295, which is equivalent to T288 in human Aurora A kinase (Tsai et al., 2003). Although GST-Arpc1b showed stronger binding to Xenopus Aurora A (Fig. 6 C) compared with TPX2, both TPX2 and Arpc1b comparably activated Aurora A (Fig. 6 D). Similar to that reported for binding inefficiency, GST-Arpc1b-T21A also exhibited reduced activation of Aurora A compared with that of wild-type Arpc1b or TPX2 (Fig. 6, C and D). Together, these findings suggest an inherent role of Arpc1b-T21 phosphorylation in binding and activating Aurora A and that the Arpc1b–Aurora A pathway is involved in maintaining spindle organization.
Figure 6.
Phosphorylation of Arpc1b on Thr 21 regulates its interaction with Aurora A. (A) In vitro–translated 35S-labeled Aurora A was used to study its binding with GST, GST-Arpc1b, or GST-Arpc1b T21A. The extent of binding was estimated by measuring signal intensity. Ponceau-stained blot shows equal quantity of GST-tagged proteins used for each reaction. (B) GST, GST-Arpc1b, and GST-Arpc1b T21A were used in an in vitro kinase assay with recombinant Aurora A protein. Phosphorylated histone H3 was visualized by autoradiography. Ponceau-stained blot shows equal amount of histone H3 and GST-tagged proteins used for the study. Circle marks GST-fused protein of interest. (C) The 1C1 monoclonal antibody to Xenopus Aurora A (Eg2) was used to immunoprecipitate Eg2 from CSF egg extract supplemented with GST control, GST-TPX2 (1–126), GST-Arpc1b, or GST-Arpc1b T21A and the bound proteins were analyzed by Western blotting using anti-GST antibodies. Asterisk denotes the GST-fused protein of interest. Bar plot shows fold change quantitated from three experiments. (D) A phospho-specific antibody that recognizes phospho-Thr 295 of Eg2 was used to study the extent of activation of Aurora A (top panel). Bottom panel shows equal amount of Aurora A used in all the reactions. Bar plot shows fold change quantitated from three experiments. kD, kilodaltons.
Arpc1b supports centrosome amplification in the absence of Pak1
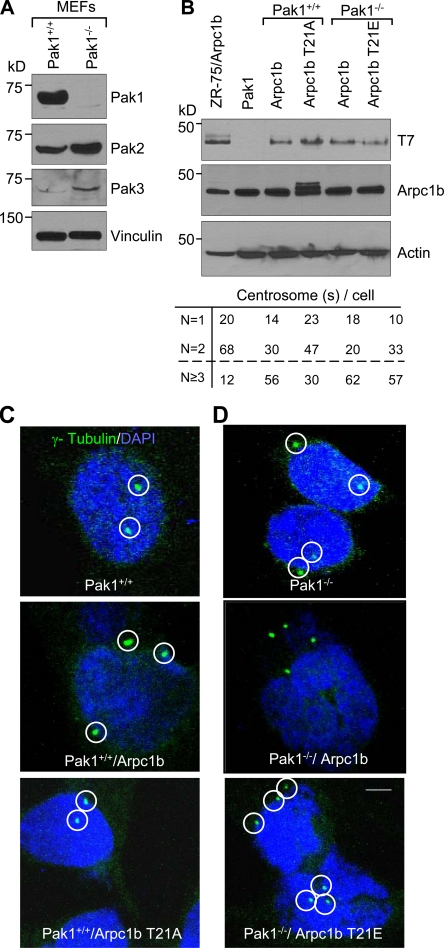
Because Aurora A can be activated by Pak1 (Zhao et al., 2005) and Arpc1b, as demonstrated in this study, we determined the significance of Pak1 in Arpc1b-mediated centrosome amplification by using Pak1-knockout (Pak1−/−) or wild-type (Pak1+/+) mouse embryonic fibroblasts (MEFs). Depletion of Pak1 was accompanied by some compensatory effects on Pak2 and Pak3 expression (Fig. 7 A). We generated stable pooled MEF clones expressing T7-Arpc1b, T7-Arpc1b-T21A, or T7-Arpc1b-T21E (Fig. 7 B). Pak1+/+/Arpc1b and Pak1−/−/Arpc1b-T21E displayed multiple centrosomes, whereas Pak1+/+/Arpc1b-T21A exhibited normal centrosome numbers (Fig. 7 C). Surprisingly, Pak1−/− MEFs overexpressing wild-type Arpc1b also displayed an increased number of centrosomes (Fig. 7 D). These findings suggest that Arpc1b expression alone, but not Arpc1b-T21A, may lead to abnormal centrosome amplification and is independent of Pak1 status. We hypothesize that the T21 site in Arpc1b is a critical regulator of its functions in the maintenance of centrosomal amplification. Our studies also indicate that this site on Arpc1b can be regulated by cellular kinases other than Pak1. We studied Arpc1b phosphorylation by Pak2 and Pak3 (Fig. S5). Both Pak2 and Pak3 phosphorylated Arpc1b on sites other than Thr 21. This suggests that the T21 site on Arpc1b is specific to Pak1 protein and not other Pak kinases.
Figure 7.
Role of Pak1 in Arpc1b-mediated centrosome amplification. (A) Western blot analysis showing expression of different Pak isoforms in the Pak1+/+ or Pak1−/− MEFs. Vinculin was used as an internal control. (B) Western blot analysis showing expression of T7-Arpc1b and endogenous Arpc1b levels in stable cell lines generated in the Pak1+/+ or Pak1−/− MEFs for indicated point mutations of Arpc1b. Actin was used as an internal control. Quantitation of the centrosome numbers in the different stable cell lines generated in the Pak1+/+ or Pak1−/− MEFs cells are shown below panel B. (C and D) Representative images showing distribution of γ-tubulin (green) and DNA (blue) in the stable cell lines. γ-Tubulin was used as a centrosome marker. Circle marks centrosome location. Bar, 5 µm. kD, kilodaltons.
Aurora A is an Arpc1b kinase
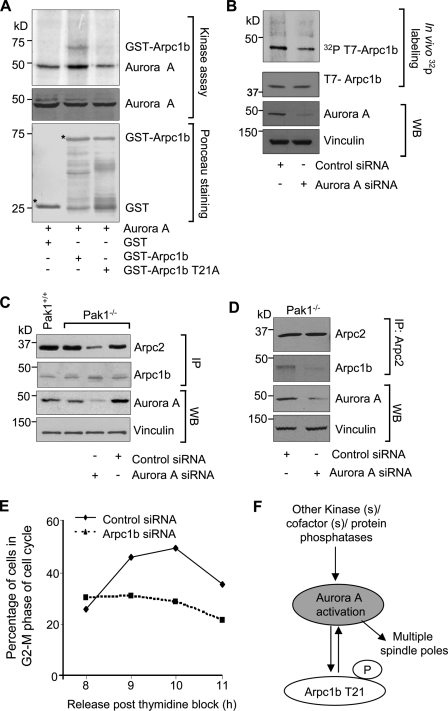
Upon analyzing the amino acid sequence of Arpc1b, we identified T21 as a potential Aurora A kinase phosphorylation site with the sequence R/K/N-R-X-S/T-B, where B is any hydrophobic amino acid but proline. To test this possibility, we examined the ability of purified Aurora A to phosphorylate GST-Arpc1b or the GST-Arpc1b-T21A mutant. Aurora A phosphorylated Arpc1b but not Arpc1b-T21A (Fig. 8 A). We used specific siRNAs targeting Aurora A to study the ability of Aurora A to phosphorylate T7-Arpc1b in vivo. Selective knockdown of Aurora A in Pak1−/− MEFs resulted in inhibition of Arpc1b phosphorylation (Fig. 8 B). This suggests Aurora A, in addition to Pak1, is responsible for the phosphorylation of Arpc1b. We explored the effect of Aurora A knockdown on the interactions of Arpc1b with other Arp2/3 complex components in Pak1−/− MEFs. Arpc1b continued to interact with Arpc2 in the absence of Pak1, but these interactions were drastically reduced upon knockdown of Aurora kinase A (Fig. 8 C). To further confirm these findings, we knocked down Aurora A in the Pak1−/− MEFs and then performed a reverse immunoprecipitation assay with an anti-Arpc2 antibody, followed by Western blot analysis with an anti-Arpc1b antibody. Consistent with our previous results, knockdown of Aurora A resulted in the dissociation of Arp2/3 complex (Fig. 8 D). Together, these findings reveal that Arpc1b-T21 phosphorylation by Aurora A or by other kinases such as Pak1 modulates its ability to interact with other components of the Arp2/3 complex.
Figure 8.
Arpc1b: a new Aurora A substrate. (A) Kinase assay for GST-Arpc1b and GST-Arpc1b T21A proteins in presence of Aurora A. Aurora A in the middle panel indicates comparable amounts of protein loading. Ponceau-stained blot shows equal quantity of GST-tagged proteins used for the study. Asterisk denotes the GST-fused protein of interest. (B) In vivo phosphorylation of T7-tagged Arpc1b in the Pak1−/− MEFs treated with control or Arpc1b siRNA. (Top to bottom): [32P]T7-Arpc1b, T7-Arpc1b, Aurora A, and vinculin. (C) Western blot showing extent of Arpc2 immunoprecipitated with Arpc1b from Pak1+/+ or Pak1−/− cells after Aurora A knockdown (top panel), effective Arpc1b pull-down (second panel), and effective knockdown of Aurora A (third panel). Vinculin was used as an internal control. (D) Western blot showing extent of Arpc1b immunoprecipitated with Arpc2 from the Pak1−/− cells after Aurora A knockdown (second panel), effective Arpc2 pull-down (top panel), and effective knockdown of Aurora A (third panel). Vinculin was used as an internal control. (E) Pak1−/− cells (synchronized in G1-S phase) transfected with either control or Arpc1b siRNAs were scored for the percentage of cells in G2-M phase at the indicated time points after release from G1-S arrest using FACS analysis; n = 3. (F) Proposed model explaining the role for threonine 21 phosphorylation on Arpc1b in Aurora A activation leading to centrosome amplification. WB, Western blot; IP, immunoprecipitation; kD, kilodaltons.
To investigate the significance of Arpc1b in Aurora A activation and cell cycle progression, we again combined cell synchronization with siRNA silencing. Initially, we determined the effect of Pak1 depletion on cell cycle progression. Pak1−/− cells exhibited delayed entry into the G2/M phase (9 h after double-thymidine block; unpublished data) compared with the Pak1+/+ MEFs (6 h after double-thymidine block; unpublished data). Next, we depleted Arpc1b in the Pak1−/− cells and studied the effects on cell cycle progression. Knockdown of Arpc1b in the Pak1−/− MEFs drastically reduced the ability of cells to enter the G2/M phase of the cell cycle (Fig. 8 E). Taken together, these results reveal a crucial role of Arpc1b in Aurora A activation and the regulation of cell cycle progression (Fig. 8 F).
Discussion
We identified Arpc1b, a component of the Arp2/3 complex, as a novel substrate and activator of Aurora A kinase. Aurora A plays an important role in the regulation of spindle formation and mitosis. Ectopic overexpression of Aurora A in cultured cells leads to polyploidy and centrosome amplification (Zhou et al., 1998; Katayama et al., 2003; Stenoien et al., 2003). In addition to its activation by genomic amplification, Aurora A is also activated by the binding of its protein activators. In this context, our present study adds Arpc1b to the list of physiological upstream activators of Aurora A. Overexpression of Arpc1b, but not its phosphorylation-inactive mutant, bound and activated Aurora A and caused centrosomal amplification. This suggests an inherent critical role of T21 phosphorylation in executing the newly discovered centrosomal function of Arpc1b in epithelial (Fig. 5 B) or fibroblast cells (Fig. 7 C). We also found that selective knockdown of Arpc1b in ZR-75 and Pak1−/− cells delayed the entry of most cells into the mitotic phase. Thus, there appears to be a natural role of Arpc1b in cell cycle progression from the S to the G2/M phase, presumably due to its inherent role in the activation of Aurora A. This study also implies the existence of a feedback regulatory mechanism between Arpc1b and Aurora A in maintaining spindle organization through mitosis.
The existence of Arpc1b in a complex with γ-tubulin provides supportive evidence to its role in centrosome duplication involving expansion of pericentriolar material. Arpc1b thus serves as a cofactor to Aurora A, serving as a substrate as well as an activator of the kinase involved in the regulation of centrosome duplication. However, it is important to remember that in addition to Arpc1b, other proteins lacking enzymatic activity, such as cofactors Tpx2 (Bayliss et al., 2003), Hef1 (Pugacheva and Golemis, 2005), Ajuba (Hirota et al., 2003), and Bora (Hutterer et al., 2006) or even protein phosphatases, may also regulate the initial activation of Aurora A. Interestingly, LATS2, the downstream substrate of Aurora A, has also been reported to interact with and phosphorylate the Aurora A cofactor Ajuba, although the functional relevance of this phosphorylation has not yet been demonstrated. Collectively, the findings reported here and those of previous studies suggest that a complex network of Aurora A substrates and activators coordinately regulate centrosome maturation and, consequently, its function during mitosis. Emerging lines of evidence also point toward a role for actin and actin-related proteins in reliable chromosome segregation during mitosis (Gerisch et al., 2004; Hurst et al., 2004; Igarashi et al., 2005). In conjunction with our studies of Arpc1b, these findings open up a new avenue of investigation in determining the role of actin-related proteins in cellular processes other than actin polymerization, such as organization of centrosomal and spindle machineries.
Materials and methods
Cell culture and tissue samples
ZR-75 cells were purchased from American Tissue Culture Collection (Manassas, VA) and were cultured in Dulbecco’s modified Eagle’s medium/F12 (DMEM/F-12) (Mediatech) supplemented with 10% fetal calf serum (Hyclone). Human breast cancer samples were from the tumor bank at M.D. Anderson Cancer Center (Houston, TX).
Antibodies and reagents
GST-Aurora A, GST-Aurora A kinase-dead (KD), and anti-centrin antibody were obtained from Dr. Subrata Sen’s laboratory (M.D. Anderson Cancer Center, Houston, TX). (His)6-tagged or GST-fused recombinant full-length human Aurora A was purchased from Millipore and Cell Signaling Technology, respectively. Antibody against T7-epitope (Novagen); actin, vinculin, γ-tubulin (Sigma-Aldrich); Aurora A, Rac1 (BD; Cell Signaling Technology); Pak1, Pak2, phopho-Histone H3 (Ser10), Aurora A, GST, and Arp3 (Cell Signaling Technology); Arpc1b (Imgenex; Santa Cruz Biotechnology, Inc.); Arpc2, Arp3 (Millipore); Arpc2 and Pak3 (Santa Cruz Biotechnology, Inc.); Phospho-Aurora A (Thr288) (Novus Biologicals); and Plk-1, phospho-plk1 (Thr210) (BioLegend) were used. Purified Arp2/3 complex was purchased from Cytoskeleton, Inc. Horseradish peroxidase (HRP)–conjugated anti–mouse, anti–goat, or anti–rabbit secondary antibodies were purchased from GE Healthcare or eBioscience.
Stable clones
Pak1+/+ or Pak1−/− MEFs (generated in Dr. J. Chernoff’s laboratory) were transfected with T7-Arpc1b or T7-Arpc1bT21A or T7-Arpc1b T21A constructs using FuGENE 6 transfection reagent (Roche) according to the standard protocols. Stable clones were selected using G418 selection (1 mg/ml), as previously reported (Vadlamudi et al., 2004b).
siRNA transfection
RNA interference against human control siRNA, Arpc1b, Arpc1a, Arp3, and Aurora A were purchased from Thermo Fisher Scientific. Cells were transfected with 100 nmol/L siRNA (control, Arpc1b, Arpc1a, Aurora A, or Arp3 siRNA), 4 µl Oligofectamine (Invitrogen) in 6-well plates according to the manufacturer’s protocol. Specific protein knockdown was checked 48 h after transfection by Western blot analysis.
GST pull-down assay
In vitro transcription and translation of Arpc1b or Aurora kinases A was performed using a T7-TNT kit (Promega) in which 1 µg of cDNA in pcDNA 3.1 vector was translated in the presence of [35S]methionine and diluted in GST-binding buffer (25 mM Tris-HCl, pH 8.0, 50 mM NaCl, 10% glycerol, and 0.1% NP-40). An equal aliquot was used for each GST pull-down. The GST pull-down assays were performed by incubating equal amounts of GST, GST-tagged full-length proteins, and GST-tagged mutation constructs immobilized on glutathione Sepharose beads (GE Healthcare) with in vitro–translated 35S-labeled protein. Bound proteins were isolated by incubating the mixture for 3 h at 4°C in GST binding buffer. After washing, the proteins were eluted in 2× SDS buffer, separated by SDS-PAGE and visualized by autoradiography.
Dephosphorylation of Aurora A by phosphatase
Recombinant Aurora A expressed in bacteria was treated with PP1 (New England Biolabs, Inc.) using standard protocol (Katayama et al., 2001) and incubated with GST or GST-Arpc1b at 30°C for 30 min. The reaction was resolved on SDS-PAGE, transferred to nitrocellulose membrane, and analyzed using a PhosphorImager.
Aurora kinase assay
The kinase reactions were performed using standard protocol (Hirota et al., 2003). In brief, 140 ng of Aurora kinase was incubated with either 1 µg of GST or GST-Arpc1b in kinase buffer (20 mM Hepes, pH 7.5, 10 mM MgCl2, 1 mM DTT, and 10 mM KCl) containing 10 µg histone H3 (Active Motif), 5 µCi of γ-[32P]ATP, and 5 µM ATP. The reactions were incubated at 30°C for 30 min and resolved on SDS-PAGE, transferred to polyvinylidene difluoride membranes, and analyzed using a PhosphorImager.
Immunoblotting and immunoprecipitation
Cells were grown in complete medium containing 10% fetal bovine serum (Hyclone) and antibiotic-antimycotic solution (Invitrogen). Where indicated, cells were incubated with either 2 mM thymidine (Sigma-Aldrich) or with 0.4 µg/ml nocodazole (Sigma-Aldrich) overnight. In case of thymidine, cells were subjected to double-thymidine block. After treatments, cells were washed thrice with PBS and lysed in buffer C (20 mM Hepes, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 10 mM NaF, 1 mM NaVO4, 0.5% Nonidet P-40, protease inhibitor mixture [Roche], and phosphatase inhibitor mixture [Sigma-Aldrich]) for 30 min on ice. Cell lysates containing equal amounts of protein were resolved on a 10% SDS-PAGE gel, transferred to nitrocellulose, probed with the appropriate antibodies, and detected using an enhanced chemiluminescence method. Immunoprecipitation was then performed overnight at 4°C using 1 µg of antibody/mg of protein. Complexes were collected with protein G beads for 4 h at 4°C. After extensive washing in 20 mM Tris-HCl, pH 8.0, 50 mM NaCl, and 1 mM EDTA, proteins were detected as described above.
Immunofluorescence and confocal microscopy
Cells were grown on glass coverslips. After 24 h, nocodazole was added to the medium to a final concentration of 10 ng/ml. After a 16-h treatment with nocodazole, cells were washed three times with PBS followed by fixation and permeabilization using Methanol (−20°C) for 10 min. Cells were washed three times with PBS and stained for γ-tubulin, Phospho and total Aurora kinase A, Arpc1b, or centrin using the appropriate antibodies followed by incubation with secondary antibody conjugated with Alexa 488 (green; Invitrogen) or Alexa 546 (red; Invitrogen). The coverslips were counterstained with DAPI (blue; Invitrogen) for DNA. Coverslips were mounted in SlowFade mounting medium (Invitrogen) and sealed onto glass slides. Samples were imaged using a laser-scanning confocal microscope (Fluoview FV300; Olympus) equipped with a 60×/1.4 NA objective in accordance with established methods. Quantitative analysis was conducted by using MetaMorph 6.1 (MDS Analytical Technologies). The images were taken at room temperature and each representative image is at the same cellular level and magnification. Images were prepared using Photoshop (version 7.0; Adobe).
Xenopus egg extract preparation and immunoprecipitation
The cytostatic factor (CSF)–arrested Xenopus egg extracts were prepared as described previously (Tsai et al., 2003). For immunoprecipitation experiments, protein G–conjugated Dynabeads 280 (Invitrogen) were coated with 1C1 monoclonal anti-Eg2 antibody (5 µg/20 µl of beads). Beads were washed twice with PBS and twice with XB (10 mM K-Hepes, pH 7.7, 50 mM sucrose, 100 mM KCl, 2 mM MgCl2, 0.1 mM CaCl2, and 5 mM EGTA). For immunoprecipitations, Aurora A beads were added to 100 µl of fresh egg extract and incubated at room temperature for 1 h with rotation. Immunoprecipitates were analyzed by Western blotting to determine either the quantity or the phosphorylation state of Aurora A (also called Eg2) using rabbit anti-Xenopus Aurora A or rabbit antibodies specific to phosphorylated Aurora A. The bound proteins were analyzed by Western blotting using anti-GST antibodies.
Depletion of Arp3 from Xenopus egg extracts
Glutathione agarose beads (70 mg; Sigma-Aldrich) were resuspended and allowed to swell in 1 ml of XB Buffer (1 mM MgCl2, 100 mM KCl, and 0.1 mM CaCl2) for 30 min at room temperature. 100 µl of the beads were divided into two separate tubes and then washed 3× with cold XB + 10% glycerol + 2 mM DTT. They were then incubated with 500 µg of either purified GST-CA or GST (as control) proteins for 1 h at 4°C on a rotator. After 1 h, the beads were washed 3× with cold XB + 10% glycerol + 2 mM DTT. For depletion of the actin-nucleating protein Arp3, 100 µl of CSF-arrested mitotic Xenopus egg extracts were subjected to two rounds of incubation with either 50 µl of GST-conjugated (control) or GST-WASP-CA–conjugated beads (GST-WASP-CA binds Arp3) at 4°C for 30 min. After each round of depletion, the egg extracts were centrifuged on a table-top centrifuge for ∼15–30 s to pellet the glutathione beads. The egg extracts were then separated from the beads (to get rid of the Arp3 protein) and incubated with 75 µl of unconjugated glutathione beads to bind the residual unbound GST or GST-WASP-CA proteins in the respective egg extracts at 4°C for 30 min. The egg extracts (control and Arp3 depleted) were separated from the glutathione beads by centrifugation as described earlier. These egg extracts were then used to perform Aurora A immunoprecipitation as described above.
Mouse xenograft studies
For the tumorigenesis studies, total 5 × 106 cells of ZR-75/pcDNA or ZR-75/Arpc1b clones were bilaterally injected into the mammary fat pads, as described previously (Vadlamudi et al., 2004a). Each group had seven animals. Tumor growth was monitored for 52 wk, and tumor volume was measured. All animal procedures were done in compliance with the Institute Animal Care and Use Committee and the National Institutes of Health Policy on Humane Care and Use of Laboratory Animals.
Soft-agar experiments and sucrose gradient sedimentation
Soft-agar colony growth assays (Mazumdar et al., 2001) and sucrose gradient sedimentation (Humphries et al., 2002) were performed as described previously (Mazumdar et al., 2001). In brief, 1 ml of 0.6% DIFCO agar in DME supplemented with 10% FBS and insulin was layered onto 60 × 15-mm tissue-culture plates. Cells (10,000 cells) were mixed with 1 ml of 0.36% Bactoagar solution in DME prepared in a similar manner and layered on top of the 0.6% Bactoagar layer. Plates were incubated at 37°C in 5% CO2 for 21 d. For sucrose gradient sedimentation experiments, 10-ml sucrose gradients (3–30%) were poured in ultra-clear tubes for an SW41 rotor (Beckman Coulter). Cell lysates were precleared by centrifugation for 15 min, 70,000 rpm, 4°C in a TLA100.3 rotor (Beckman Coulter). 400 µl supernatant or high molecular weight gel filtration size standards (GE Healthcare) were layered over each gradient. Samples were centrifuged for 15 h at 34,000 rpm, 4°C in an SW41 rotor, and 0.2-ml fractions were collected. Samples of each fraction were run on SDS-PAGE gels, blotted, and probed with antibodies to determine the positions of proteins in the gradients.
Online supplemental materials
Fig. S1 shows the results from initial exploratory experiments leading to this study. Fig. S2 shows the localization of GFP-Arpc1b. Fig. S3 shows the verification of the specificity of Arpc1b siRNA. Fig. S4 demonstrates that Arpc1a fails to stimulate Aurora A activity. Fig. S5 shows Arpc1b phosphorylation by Pak2 and Pak3. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200908050/DC1.
Acknowledgments
We thank Andrew M. Hudson and Lynn Cooley (Department of Genetics, Yale University School of Medicine, New Haven, CT) for experiments in Drosophila (not included here) and Rui-An Wang (M.D. Anderson Cancer Center, Houston, TX) for generating Aurora kinase A deletion constructs.
Work in Jonathan Chernoff’s laboratory leading to creation of MEFs was supported by NIH grant CA117884 (to J. Chernoff). This study was supported by NIH grant CA90970 (to R. Kumar).
Footnotes
Abbreviations used in this paper:
- Arp
- actin-related protein
- MEF
- mouse embryonic fibroblast
- Pak
- p21-activated kinase
References
- Abe Y., Ohsugi M., Haraguchi K., Fujimoto J., Yamamoto T. 2006. LATS2-Ajuba complex regulates gamma-tubulin recruitment to centrosomes and spindle organization during mitosis. FEBS Lett. 580:782–788 10.1016/j.febslet.2005.12.096 [DOI] [PubMed] [Google Scholar]
- Ando Y., Yasuda S., Oceguera-Yanez F., Narumiya S. 2007. Inactivation of Rho GTPases with Clostridium difficile toxin B impairs centrosomal activation of Aurora-A in G2/M transition of HeLa cells. Mol. Biol. Cell. 18:3752–3763 10.1091/mbc.E07-03-0281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros T.P., Kinoshita K., Hyman A.A., Raff J.W. 2005. Aurora A activates D-TACC-Msps complexes exclusively at centrosomes to stabilize centrosomal microtubules. J. Cell Biol. 170:1039–1046 10.1083/jcb.200504097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss R., Sardon T., Vernos I., Conti E. 2003. Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol. Cell. 12:851–862 10.1016/S1097-2765(03)00392-7 [DOI] [PubMed] [Google Scholar]
- Crosio C., Fimia G.M., Loury R., Kimura M., Okano Y., Zhou H., Sen S., Allis C.D., Sassone-Corsi P. 2002. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol. Cell. Biol. 22:874–885 10.1128/MCB.22.3.874-885.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding A.B., Dobreva I., McDonald P.C., Foster L.J., Dedhar S. 2008. Integrin-linked kinase localizes to the centrosome and regulates mitotic spindle organization. J. Cell Biol. 180:681–689 10.1083/jcb.200710074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachet Y., Tournier S., Millar J.B., Hyams J.S. 2001. A MAP kinase-dependent actin checkpoint ensures proper spindle orientation in fission yeast. Nature. 412:352–355 10.1038/35085604 [DOI] [PubMed] [Google Scholar]
- Gerisch G., Faix J., Köhler J., Müller-Taubenberger A. 2004. Actin-binding proteins required for reliable chromosome segregation in mitosis. Cell Motil. Cytoskeleton. 57:18–25 10.1002/cm.10150 [DOI] [PubMed] [Google Scholar]
- Goley E.D., Welch M.D. 2006. The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 7:713–726 10.1038/nrm2026 [DOI] [PubMed] [Google Scholar]
- Hirota T., Kunitoku N., Sasayama T., Marumoto T., Zhang D., Nitta M., Hatakeyama K., Saya H. 2003. Aurora-A and an interacting activator, the LIM protein Ajuba, are required for mitotic commitment in human cells. Cell. 114:585–598 10.1016/S0092-8674(03)00642-1 [DOI] [PubMed] [Google Scholar]
- Humphries C.L., Balcer H.I., D’Agostino J.L., Winsor B., Drubin D.G., Barnes G., Andrews B.J., Goode B.L. 2002. Direct regulation of Arp2/3 complex activity and function by the actin binding protein coronin. J. Cell Biol. 159:993–1004 10.1083/jcb.200206113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst I.R., Zuo J., Jiang J., Holliday L.S. 2004. Actin-related protein 2/3 complex is required for actin ring formation. J. Bone Miner. Res. 19:499–506 10.1359/JBMR.0301238 [DOI] [PubMed] [Google Scholar]
- Hutterer A., Berdnik D., Wirtz-Peitz F., Zigman M., Schleiffer A., Knoblich J.A. 2006. Mitotic activation of the kinase Aurora-A requires its binding partner Bora. Dev. Cell. 11:147–157 10.1016/j.devcel.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Igarashi R., Suzuki M., Nogami S., Ohya Y. 2005. Molecular dissection of ARP1 regions required for nuclear migration and cell wall integrity checkpoint functions in Saccharomyces cerevisiae. Cell Struct. Funct. 30:57–67 10.1247/csf.30.57 [DOI] [PubMed] [Google Scholar]
- Katayama H., Zhou H., Li Q., Tatsuka M., Sen S. 2001. Interaction and feedback regulation between STK15/BTAK/Aurora-A kinase and protein phosphatase 1 through mitotic cell division cycle. J. Biol. Chem. 276:46219–46224 10.1074/jbc.M107540200 [DOI] [PubMed] [Google Scholar]
- Katayama H., Brinkley W.R., Sen S. 2003. The Aurora kinases: role in cell transformation and tumorigenesis. Cancer Metastasis Rev. 22:451–464 10.1023/A:1023789416385 [DOI] [PubMed] [Google Scholar]
- Katayama H., Sasai K., Kawai H., Yuan Z.M., Bondaruk J., Suzuki F., Fujii S., Arlinghaus R.B., Czerniak B.A., Sen S. 2004. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat. Genet. 36:55–62 10.1038/ng1279 [DOI] [PubMed] [Google Scholar]
- Kumar R., Gururaj A.E., Barnes C.J. 2006. p21-activated kinases in cancer. Nat. Rev. Cancer. 6:459–471 10.1038/nrc1892 [DOI] [PubMed] [Google Scholar]
- Littlepage L.E., Wu H., Andresson T., Deanehan J.K., Amundadottir L.T., Ruderman J.V. 2002. Identification of phosphorylated residues that affect the activity of the mitotic kinase Aurora-A. Proc. Natl. Acad. Sci. USA. 99:15440–15445 10.1073/pnas.202606599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Rohatgi R., Kirschner M.W. 1998. The Arp2/3 complex mediates actin polymerization induced by the small GTP-binding protein Cdc42. Proc. Natl. Acad. Sci. USA. 95:15362–15367 10.1073/pnas.95.26.15362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky L.M., Gould K.L. 1999. The Arp2/3 complex: a multifunctional actin organizer. Curr. Opin. Cell Biol. 11:117–121 10.1016/S0955-0674(99)80014-3 [DOI] [PubMed] [Google Scholar]
- Macůrek L., Lindqvist A., Lim D., Lampson M.A., Klompmaker R., Freire R., Clouin C., Taylor S.S., Yaffe M.B., Medema R.H. 2008. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 455:119–123 10.1038/nature07185 [DOI] [PubMed] [Google Scholar]
- Mahlamäki E.H., Kauraniemi P., Monni O., Wolf M., Hautaniemi S., Kallioniemi A. 2004. High-resolution genomic and expression profiling reveals 105 putative amplification target genes in pancreatic cancer. Neoplasia. 6:432–439 10.1593/neo.04130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumoto T., Zhang D., Saya H. 2005. Aurora-A - a guardian of poles. Nat. Rev. Cancer. 5:42–50 10.1038/nrc1526 [DOI] [PubMed] [Google Scholar]
- Mazumdar A., Wang R.A., Mishra S.K., Adam L., Bagheri-Yarmand R., Mandal M., Vadlamudi R.K., Kumar R. 2001. Transcriptional repression of oestrogen receptor by metastasis-associated protein 1 corepressor. Nat. Cell Biol. 3:30–37 10.1038/35050532 [DOI] [PubMed] [Google Scholar]
- Meijerman I., Blom W.M., de Bont H.J., Mulder G.J., Nagelkerke J.F. 1999. Changes of G-actin localisation in the mitotic spindle region or nucleus during mitosis and after heat shock: a histochemical study of G-actin in various cell lines with fluorescent labelled vitamin D-binding protein. Biochim. Biophys. Acta. 1452:12–24 10.1016/S0167-4889(99)00119-6 [DOI] [PubMed] [Google Scholar]
- Menard R.E., Mattingly R.R. 2003. Cell surface receptors activate p21-activated kinase 1 via multiple Ras and PI3-kinase-dependent pathways. Cell. Signal. 15:1099–1109 10.1016/S0898-6568(03)00087-1 [DOI] [PubMed] [Google Scholar]
- Mori D., Yano Y., Toyo-oka K., Yoshida N., Yamada M., Muramatsu M., Zhang D., Saya H., Toyoshima Y.Y., Kinoshita K., et al. 2007. NDEL1 phosphorylation by Aurora-A kinase is essential for centrosomal maturation, separation, and TACC3 recruitment. Mol. Cell. Biol. 27:352–367 10.1128/MCB.00878-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi M., Fujiuchi N., Sasai K., Katayama H., Minamishima Y.A., Ongusaha P.P., Deng C., Sen S., Lee S.W., Ouchi T. 2004. BRCA1 phosphorylation by Aurora-A in the regulation of G2 to M transition. J. Biol. Chem. 279:19643–19648 10.1074/jbc.M311780200 [DOI] [PubMed] [Google Scholar]
- Pugacheva E.N., Golemis E.A. 2005. The focal adhesion scaffolding protein HEF1 regulates activation of the Aurora-A and Nek2 kinases at the centrosome. Nat. Cell Biol. 7:937–946 10.1038/ncb1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran S., Parvin J.D. 2006. Centrosome function in normal and tumor cells. J. Cell. Biochem. 99:1240–1250 10.1002/jcb.21003 [DOI] [PubMed] [Google Scholar]
- Sasai K., Parant J.M., Brandt M.E., Carter J., Adams H.P., Stass S.A., Killary A.M., Katayama H., Sen S. 2008. Targeted disruption of Aurora A causes abnormal mitotic spindle assembly, chromosome misalignment and embryonic lethality. Oncogene. 27:4122–4127 10.1038/onc.2008.47 [DOI] [PubMed] [Google Scholar]
- Scrittori L., Hans F., Angelov D., Charra M., Prigent C., Dimitrov S. 2001. pEg2 aurora-A kinase, histone H3 phosphorylation, and chromosome assembly in Xenopus egg extract. J. Biol. Chem. 276:30002–30010 10.1074/jbc.M102701200 [DOI] [PubMed] [Google Scholar]
- Stenoien D.L., Sen S., Mancini M.A., Brinkley B.R. 2003. Dynamic association of a tumor amplified kinase, Aurora-A, with the centrosome and mitotic spindle. Cell Motil. Cytoskeleton. 55:134–146 10.1002/cm.10120 [DOI] [PubMed] [Google Scholar]
- Tsai M.Y., Wiese C., Cao K., Martin O., Donovan P., Ruderman J., Prigent C., Zheng Y. 2003. A Ran signalling pathway mediated by the mitotic kinase Aurora A in spindle assembly. Nat. Cell Biol. 5:242–248 10.1038/ncb936 [DOI] [PubMed] [Google Scholar]
- Vadlamudi R.K., Kumar R. 2003. P21-activated kinases in human cancer. Cancer Metastasis Rev. 22:385–393 10.1023/A:1023729130497 [DOI] [PubMed] [Google Scholar]
- Vadlamudi R.K., Bagheri-Yarmand R., Yang Z., Balasenthil S., Nguyen D., Sahin A.A., den Hollander P., Kumar R. 2004a. Dynein light chain 1, a p21-activated kinase 1-interacting substrate, promotes cancerous phenotypes. Cancer Cell. 5:575–585 10.1016/j.ccr.2004.05.022 [DOI] [PubMed] [Google Scholar]
- Vadlamudi R.K., Li F., Barnes C.J., Bagheri-Yarmand R., Kumar R. 2004b. p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. EMBO Rep. 5:154–160 10.1038/sj.embor.7400079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A.O., Seghezzi W., Korver W., Sheung J., Lees E. 2000. The mitotic serine/threonine kinase Aurora2/AIK is regulated by phosphorylation and degradation. Oncogene. 19:4906–4916 10.1038/sj.onc.1203847 [DOI] [PubMed] [Google Scholar]
- Wu X., Yoo Y., Okuhama N.N., Tucker P.W., Liu G., Guan J.L. 2006. Regulation of RNA-polymerase-II-dependent transcription by N-WASP and its nuclear-binding partners. Nat. Cell Biol. 8:756–763 10.1038/ncb1433 [DOI] [PubMed] [Google Scholar]
- Yang C., Huang M., DeBiasio J., Pring M., Joyce M., Miki H., Takenawa T., Zigmond S.H. 2000. Profilin enhances Cdc42-induced nucleation of actin polymerization. J. Cell Biol. 150:1001–1012 10.1083/jcb.150.5.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z.S., Lim J.P., Ng Y.W., Lim L., Manser E. 2005. The GIT-associated kinase PAK targets to the centrosome and regulates Aurora-A. Mol. Cell. 20:237–249 10.1016/j.molcel.2005.08.035 [DOI] [PubMed] [Google Scholar]
- Zhou H., Kuang J., Zhong L., Kuo W.L., Gray J.W., Sahin A., Brinkley B.R., Sen S. 1998. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat. Genet. 20:189–193 10.1038/2496 [DOI] [PubMed] [Google Scholar]
- Zigmond S.H. 1998. Actin cytoskeleton: the Arp2/3 complex gets to the point. Curr. Biol. 8:R654–R657 10.1016/S0960-9822(07)00415-0 [DOI] [PubMed] [Google Scholar]