Abstract
Premature circuit clotting is a problem during continuous renal replacement therapy. We describe an algorithm for individualised anticoagulation with unfractionated heparin based on the patient's risk of bleeding and previous circuit life. The algorithm allows effective and safe nurse-led anticoagulation during continuous renal replacement therapy.
Introduction
Continuous renal replacement therapy (CRRT) has become an established treatment for patients with acute kidney injury in the intensive care unit (ICU). Premature circuit clotting is a common problem, leading to reduced circuit life, to reduced clearance and also to increased blood loss, work load and cost of therapy [1]. There are different ways of maintaining the circuit patent [2]. An international questionnaire showed that in the UK more than 98% of ICUs surveyed used unfractionated heparin [3]. The major advantages of unfractionated heparin are the low costs, familiarity, ease of administration and reversibility with protamine. CRRT is predominantly nurse-led [4]. After a decision is made to start CRRT, nurses usually prepare and manage the technique.
Unfractionated heparin is the first-line anticoagulant in our unit. In order to enable the nursing staff to manage CRRT effectively and safely, we aimed to have clear guidelines in place, including an algorithm for the use of heparin.
Methods
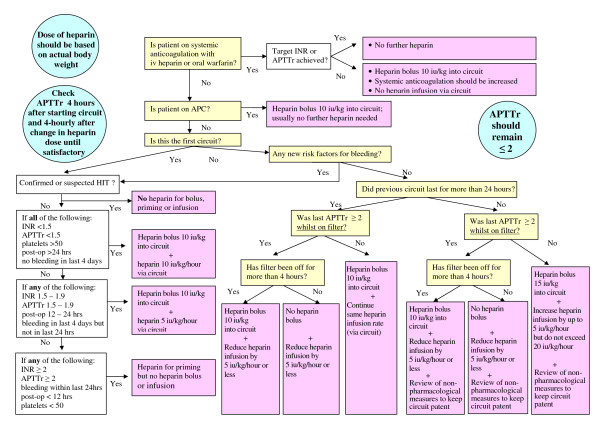
We contacted seven large ICUs in the UK and three units outside the UK. None of the ICUs contacted had a guideline for the use of unfractionated heparin during CRRT. We therefore designed an algorithm based on data from the literature and our own clinical experience (Figure 1).
Figure 1.
Algorithm for heparin anticoagulation during continuous renal replacement therapy. Algorithm is based on using 10,000 iu heparin in 40 ml of 0.9% NaCl. APC, activated protein C; APTTr, activated partial thromboplastin time ratio; CRRT, continuous renal replacement therapy; HIT, heparin-induced thrombocytopenia; INR, international normalised ratio; iv, intravenous; post-op, postoperative.
Results
The principles of the algorithm (Figure 1) are as follows. First, unfractionated heparin is administered via the circuit. Second, heparin is administered into the circuit priming solution before the blood is in contact with plastic surfaces (10,000 iu heparin/1,000 ml of 0.9% NaCl). Third, the dose of heparin is based on the patient's body weight. Fourth, the starting dose of heparin is individualised depending on the risk of bleeding and the previous circuit life - subsequent doses can be adjusted by the nursing staff according to the algorithm without the need for a medical review. Fifth, there is no target activated partial thromboplastin time ratio but this ratio is kept ≤ 2 to prevent over-anticoagulation. Sixth, regular attention is paid to nonpharmacological methods to maintain circuit patency (that is, change of vascular access, blood flow, predilution/postdilution ratio).
A recent audit covering the period May 2008 to May 2009 confirmed a mean circuit life of 19.8 hours using unfractionated heparin without any untoward incidents.
Copies of our algorithm have already been requested by several ICUs in the UK. The aim of the present paper is therefore to share our practice more widely.
Conclusion
Our heparin algorithm allows nurse-led effective and safe anticoagulation with unfractionated heparin during CRRT.
Abbreviations
CRRT: continuous renal replacement therapy; ICU: intensive care unit.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Marlies Ostermann, Email: Marlies.Ostermann@gstt.nhs.uk.
Helen Dickie, Email: Helen.Dickie@gstt.nhs.uk.
Linda Tovey, Email: Linda.Tovey@gstt.nhs.uk.
David Treacher, Email: David.Treacher@gstt.nhs.uk.
Acknowledgements
The authors would like to thank Ms Sam Lippett, former ICU pharmacist at Guy's & St Thomas' Hospital, for her contribution. The project was supported by internal departmental funds.
References
- Baldwin I. Factors affecting circuit patency and filter 'life'. Contrib Nephrol. 2007;156:178–184. doi: 10.1159/000102081. [DOI] [PubMed] [Google Scholar]
- Joannidis M, Oudemans-van Straaten HM. Clinical review: Patency of the circuit in continuous renal replacement therapy. Crit Care. 2007;11:218. doi: 10.1186/cc5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SE, Bodenham A, Short AIK, Turney JH. The provision and practice of renal replacement therapy on adult intensive care units in the United Kingdom. Anaesthesia. 2003;58:1063–1069. doi: 10.1046/j.1365-2044.2003.03449.x. [DOI] [PubMed] [Google Scholar]
- Baldwin I, Fealy N. Clinical nursing for the application of continuous renal replacement therapy in the intensive care unit. Semin Dial. 2009;22:189–193. doi: 10.1111/j.1525-139X.2008.00547.x. [DOI] [PubMed] [Google Scholar]