Abstract
Objectives
To identify and validate the biological significance of new genes/ proteins involved in the development of allergic airway disease in a murine asthma model.
Methods
Gene microarrays were used to identify genes with at least a 2-fold increase in gene expression in lungs of two separate mouse strains with high and low allergic susceptibility, respectively. Validation of mRNA data was obtained by western blotting and immunohistochemistry, followed by functional analysis of one of the identified genes in mice with targeted disruption of specific gene expression.
Results
Expression of two antioxidant enzymes, glutathione peroxidase-2 (Gpx-2) and glutathione-S-transferase Omega (GSTO) 1-1 was increased in both mouse strains after induction of allergic airway disease and localized in lung epithelial cells. Mice with targeted disruption of the Gpx-2 gene showed significantly enhanced airway inflammation compared to sensitized and challenged wild-type mice.
Conclusion
Our data indicate that genes encoding the antioxidants Gpx-2 and GSTO 1-1 are common inflammatory genes expressed upon induction of allergic airway inflammation, independently of allergic susceptibility. Furthermore, we provide evidence to illustrate the importance of a single antioxidant enzyme, Gpx-2, in protection from allergen-induced disease.
Keywords: Airway hyperreactivity, asthma, Glutathione peroxidase, glutathione S-transferase
Introduction
Allergic asthma is a polygenetic disease that unfolds through the interplay of various genes with environmental factors. Despite the identification of several proteins and pathways involved in this inflammatory process, clinical trials in which key mediators were inhibited revealed that other and yet unknown factors might be causally involved in the allergic cascade (1). In search for these putative candidates, it is intriguing to speculate that antioxidant defense systems might be involved in the regulation of airway inflammation, since airways are naturally exposed to higher oxygen concentrations than most other tissues. Recent studies have identified increased levels of oxidative stress and alterations of antioxidant enzymes in the lungs of allergic individuals and in allergic animal models resulting in the hypothesis that an increase in oxidative stress may contribute to the characteristic features of asthma (2). In search of new factors, different technologies may be applied. Several recent studies benefitted from microarray analysis of gene expression profiles in order to identify genes involved in the development of allergic airway inflammation, as reviewed in (3). This approach has significant advantages over conventional experimental approaches. Conventional approaches only permit the study of known mediators of inflammation, where previous studies usually already suggested a possible association with allergic airway disease. Deductive gene expression profiling via microarrays, on the other hand, might identify mediators without any known link to inflammation or airway disease, thereby introducing truly “novel” targets into the field of allergic airway research. To identify novel factors commonly involved in pulmonary inflammation, we therefore employed RNA microarray technology. Comparisons of naïve and treated mice, on the one hand side and two mouse strains with known different genetic suscepbility to the induction of allergic airway disease (4), on the other hand side, allowed us to identify common genes involved in pulmonary inflammation, indepently of genetic susceptibility to disease development. Among the identified genes were several genes involved in the regulation of oxidative stress, among these the antioxidative enzymes, glutathione peroxidase-2 (Gpx-2) and glutathione-S-transferase Omega 1-1 (GSTO 1-1). These two enzymes had not previously been recognized to be part of the allergen-mediated inflammation cascade. Our data indicate that GSTO 1-1 and Gpx-2 are upregulated in allergic airway inflammation. Furthermore, the absence of Gpx-2 leads to an increase in the allergic airway inflammation. Manipulating this pathway in future studies will test the hypotheses that oxidative stress is involved in the pathogenesis of asthma.
Methods
Animals
Specific-pathogen-free female BALB/c and C57BL/6 mice (Harlan-Winkelmann, Borchen, Germany), and C57BL/6 mice with a targeted disruption of Gpx-2 (Gpx-2-KO) (5), 6–8 weeks old at starting point of experiments, were used. Five animals/ group were analyzed and three independent experiments were conducted. All experimental procedures were approved by the animal care facility (Berlin Office for Occupational Safety, Protection of Health and Technical Safety-LAGeSo).
Sensitization and challenge protocol
Mice were sensitized by intraperitoneal (i.p.) injection of 20 μg ovalbumin (OVA) grade VI (Sigma-Aldrich, München, Germany) in 2 mg of aluminum hydroxide on days 1 and 14. Airway inflammation was induced by intranasal instillation of OVA grade V (Sigma-Aldrich,) (50 μg in 50 μl phosphate-buffered saline) on day 28 (for microarray analyses) and 29 (for quantitative real-time polymerase-chain reaction (RT-PCR)). For studies with Gpx-2-null animals, mice were systemically sensitized by i.p. injection of OVA and aluminum hydroxide. Non-sensitized mice received aluminum hydroxide without OVA. On days 28, 29 and 30, all mice were challenged with OVA, and killed at day 32. For microarray analyses, animals were sacrificed 16 h after the single intranasal challenge on day 28. For RT-PCR, Western blotting and for studies with Gpx-2-null animals, animals were sacrificed 48 h after last challenge, i.e. either 48h after two challenges on day 28 and 29 or 48h after challenges on day 28, 29 and 30.
Detection of the allergic phenotype
Immunoglobulins (Igs)
48 h after thelast challenge, blood was drawn from the tail vein, and serum levels of total IgE and OVA-specific IgE were measured by ELISA, as previously described (6).
Bronchoalveolar lavage
Sixteen hours after a single allergen challenge and forty-eight hours after multiple challenges (day 28 and 29 or days 28, 29 and 30, see above), lungs were lavaged and cytospin slides were prepared, stained with Diff-Quik (Dade Behring AG, Liederbach, Switzerland) and 200 cells were characterized according to morphologic criteria via light microscopy.
Airway reactivity
Airway reactivity was measured by whole body barometric plethysmography (WBP, corresponding to the Buxco-system provided by EMKA Technologies, Paris, France), as previously described (6).
Invasive lung function measurement in isolated perfused mouse lungs after three challenges (days 28, 29 and 30) of WT and KO mice
Mouse lungs were prepared, ventilated and analyzed as described (7). After a steady state period of 30 min, MCh was administered to the perfusate for 30 sec at 12-min intervals. Airway resistance values were determined at the end of the steady state period, as well as at the maximum level of resistance increase. The change in airway resistance was expressed as “relative fold airway resistance”, representing the increase in responsiveness due to OVA-sensitization by normalizing fold airway resistance values of OVA lungs to corresponding mean values of control groups.
Preparation of RNA
Total RNA was extracted from mouse lungs using Qiagen RNeasy Total RNA isolation kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
Microarray analysis
CRNAs were hybridized individually to mouse genome MG-U74Av2 chips (Affymetrix, High Wycombe, UK). In total, 8 lungs were analyzed, two treated mice vs. two controls in two different mouse strains (BALB/c and C57BL/6) respectively. The gene chips were scanned with an Affymetrix Gene Chip Instrument and scaled using Affymetrix’s Microarray Suite software 5.0 (MAS5). We made four-way comparison of the arrays; between two different mouse strains and between treated and controls (2x2 matrix). Only those genes which were found to be similarly regulated in all 4 comparisons were classified as differentially expressed genes. The signal log ratio (SLR) was converted to a standard on a logarithmic scale and the mean fold change of all 4 comparisons was calculated. We consecutively focused on those genes, which were similarly regulated in both strains of mice and with more than 2-fold changes between treated and untreated mice. A more detailed description of the microarray analysis is attached as supplementary material.
Real-time PCR
PCR amplification and analysis were performed using an ABI PRISM 7700 (Perkin Elmer, Rodgau, Germany) and SDS software version 1.7. (A more detailed description of the real time RT-PCR can be found in the supplementary materials)
Primer design and sequences
Complementary DNA PCR primers for amplification were designed using Primer3 Input software (primer3_www.cgi version 2.0) for DNA and RNA sequences obtained from GenBank.. The list of primers, and sequences were archived as supplementary material.
Protein preparation, SDS-PAGE and Western blotting
One gram of mouse lung tissue (snap-frozen, stored at −80°C) was homogenized in digestion buffer. Aliquots of the lung homogenates were analyzed via SDS-PAGE and western blotting using anti Gpx-2 or anti α-GSTO antibodies (8;9) (A detailed description is provided in the supplementary material).
Immunohistochemistry
Localization of the Gpx-2 and GSTO 1-1 proteins was detected via immunohistochemistry (IHC) using 4 μm paraffin sections of lung tissue. Antigen retrieval was performed by heating the tissue sections for 6 minutes in pre-heated Dako target retrieval solution (TRS, Dako, Hamburg, Germany), using a pressure cooker. For detection of GSTO 1-1, the rabbit antiserum was diluted 10,000 fold (9)and detection of Gpx-2 was performed with a rabbit polyclonal anti-Gpx-2 antibody (8). Biotinylated secondary anti-rabbit antibodies were used at a dilution of 1:10,000 (Amersham Pharmacia Biotech, Freiburg, Germany). For signal amplification and visualization of anti-GSTO 1-1 and anti-Gpx-2, a tyramine amplification system (CSA kit, Dako, Hamburg, Germany) was used. As chromogen for the peroxidase-reaction, 3,3’-diaminobenzidine tetrahydrochloride (DAB, Dako, Hamburg, Germany) was used.
Statistical analysis
Data pertaining to the allergic phenotype was analyzed statistically with the Mann-Whitney-U-Test. Micro-Array analysis was done with Affymetrix’s Microarray Suite software 5.0 (MAS5) using a non-parametric statistical test (Wilcoxon signed rank test).
Results
Analysis of the allergic phenotype
Systemic sensitization with the allergen ovalbumin (OVA) in BALB/c mice leads to a significant increase in both total and OVA-specific IgE as compared to animals that received only PBS (total IgE 1639 ng/ml versus 924 ng/ml; OVA-specific IgE 423 LU/ml versus < 6.2 LU/ml). The inflammatory reaction in the airways showed a specific and time-dependent pattern for the different cell types in the bronchoalveolar lavage (BAL) fluid (supplementary Table 1). 16 h after the single airway allergen challenge, mostly neutrophils, but virtually no eosinophils were detected. At this time point, airway hyperreactivity, measured via whole body plethysmography (6) had not yet developed (data not shown). At a later time point (48h), the BAL contained a robust eosinophilic and lymphocytic infiltration, corresponding to development of in vivo AHR.
Identification of upregulated inflammatory genes in lung tissues of sensitized and challenged animals
RNA isolated from whole lung tissue was used to generate Affymetrix based gene expression profiles. Lung tissue was obtained at 16h after a single allergen airway challenge to analyze genes at an early time point of AI in order to identify genes involved in the development of the characteristic Th2 phenotype of this model. Gene expression was compared between BALB/c mice and C57BL/6 mice because of their known differences in the development of AI and AHR (4). While both strains develop significant AI, AHR and systemic sensitization parameters such as allergen-specific IgE are much more pronounced in the BALB/c strain. We postulated that the comparison of these two strains would strengthen our aim (“two identify of signature genes of AI”) considerably compared to an approach utilizing only one mouse strain, increasing the probability of identifying genes truly relevant in the development of allergic AI. OVA-sensitized and -challenged C57BL/6 mice had an altered expression of 370 probe sets compared to the naive control mice, whereas OVA-challenged BALB/c mice had 2128 probe sets changed in their expression levels. Between these two sets, 95 probe sets were consistently up-regulated in both sensitized and challenged BALB/c and C57BL/6 mice, but only 31 probe sets coding for 27 different genes were up-regulated at least two-fold in both mouse strains (the list of up regulated genes after OVA challenge is provided in the supplementary material as Table II). Gene ontogeny analysis revealed that among these common inflammatory genes, several were involved in response to oxidative stress. Two of them, Gpx2 and GSTO1, had not yet been reported in the context of allergic airway reaction, and were thus analyzed further.
Upregulation of Gpx-2 and GSTO 1-1 in allergen-induced airway inflammation is confirmed by quantitative RT-PCR
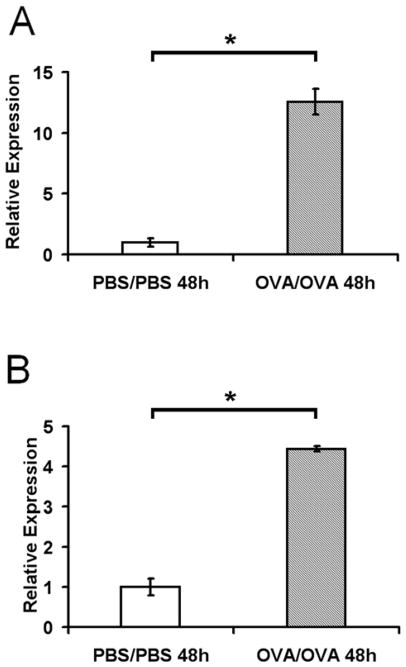
Quantitative RT-PCR was used to confirm the gene chip result. Sensitized and challenged mice (OVA/OVA) showed about two- and five-fold higher levels of Gpx2 and GSTO 1-1 mRNA in lung tissue as compared to animals in which airway inflammation was not induced (PBS/PBS) (Figure 1). Although the upregulation of Gpx-2 (Figure 1A) and GSTO 1-1 (Figure 1B)was found in both mouse strains after induction of allergic airway inflammation, in BALB/c mice the increase was even higher, as determined by the relative difference in fluorescence intensity between the target mRNAs and β-actin mRNA, a housekeeping gene.
Figure 1. Gpx-2 and GSTO 1-1 mRNA levels in the murine lungs.
mRNA levels of Gpx-2 gene (A) and GSTO-1 gene (B) were determined at 48 h after last challenge from mice subjected to sensitization and challenge (OVA/OVA) versus control mice (PBS/PBS) by real-time PCR. mRNA levels were initially normalized to β-actin mRNA levels. Comparisons were made by setting the value of control mice to one. Significance of mRNA-Expression (* p ≤ 0.05., Mann-Whitney-test) was calculated via ΔΔCT-method.
GPX-2 and GSTO 1-1 proteins are expressed at higher levels in mice with allergic airway inflammation
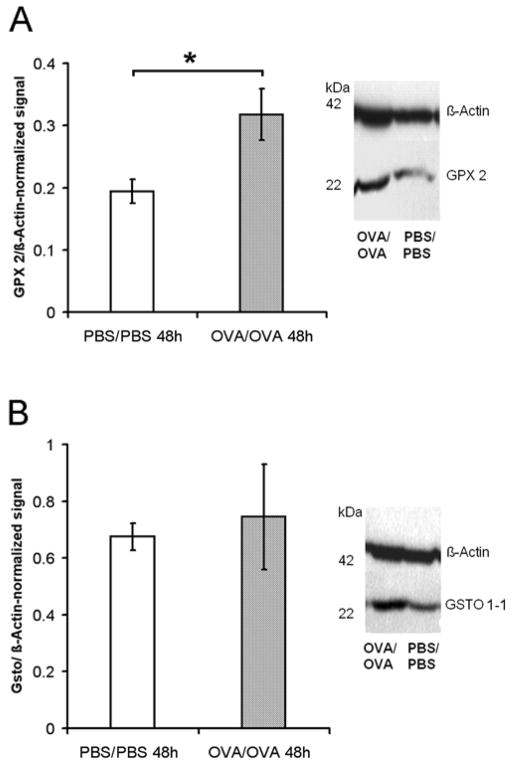
Western blotting with specific anti-Gpx-2 and anti-GSTO 1-1 antibodies was used to verify that an upregulation in mRNA levels leads to an increase in tissue protein levels of these enzymes in mice with allergic airway inflammation. Elevated expression levels of both proteins were detected in the lung tissue of mice challenged with OVA (OVA/OVA) as compared to PBS-treated control animals (PBS/PBS) (Figure 2). While these values attained statistical significance for Gpx-2 protein expression levels (Figure 2A), comparison of OVA-challenged mice to PBS-controls revealed only trends towards higher expression levels for GSTO 1-1.
Figure 2. Gpx-2 and GSTO 1-1 protein levels in murine lungs.
Relative quantity of Gpx-2 (A) or GSTO 1-1 (B) protein levels were compared to protein levels of ß-actin using integrated density values from western blot analysis (right hand of the graph) 48h after last challenge. * p ≤ 0.05, Mann-Whitney-test.
Expression pattern of Gpx-2 and GSTO1 in mouse lung
Immunohistochemistry for Gpx-2 and GSTO 1-1 revealed distinct expression patterns for these proteins in mouse bronchial epithelium (Figure 3). Expression patterns of both proteins were similar in the lungs of untreated animals as well as in sensitized and challenged animals with regards to localization. Gpx-2 expression, which so far had not been detected in the lung on a protein level, was found in basal cells (arrows in Figure 3A), revealing a pattern compatible with expression in the cells responsible for epithelial regeneration. GSTO 1-1 was found mainly in the apical parts of epithelial cells, sometimes appearing to be “budding” from the surface of the cells (arrows in Figure 3B), but secreted proteins were never detected by immunohistology inside the airway lumen.
Figure 3. Localization of Gpx-2 and GSTO 1-1 in murine lungs.
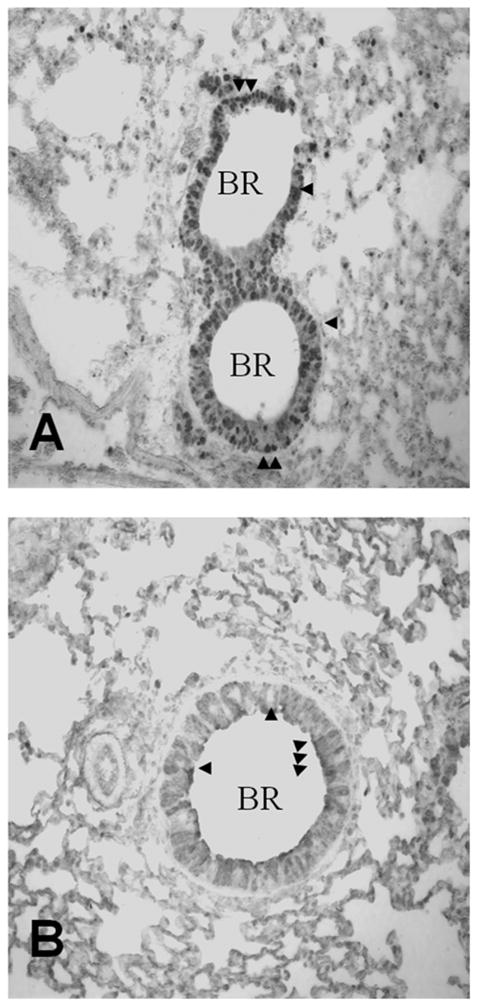
Immunohistochemical detection of Gpx-2 (A) and GSTO 1-1 (B) protein expression in murine lungs. The GSTO 1-1 protein was found mainly in apical parts of epithelial cells (arrows) while the Gpx-2 protein was localized in basal epithelial cells (arrows). (BR = bronchus). Protein expression was revealed via immunohistochemistry of paraffin cuts (400 x magnification) in lungs harvested 48h post-challenge
Gpx-2 protects against airway inflammation
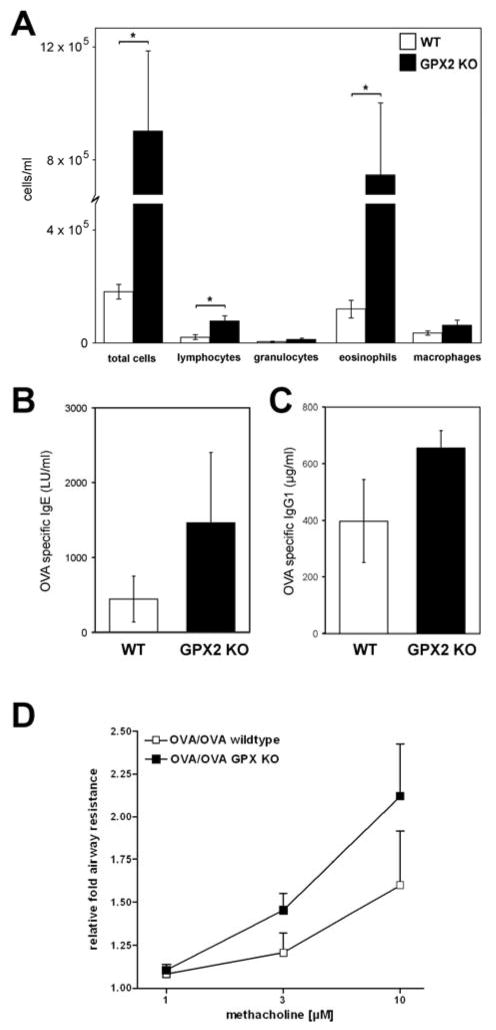
In order to evaluate the biological significance of our findings, we evaluated the consequences of Gpx-2 absence in the context of acute allergen-induced AI by utilizing mice genetically deficient for Gpx-2 expression. As shown in Figure 4, direct comparison of Gpx-2 knock-out (KO) mice with wild type (WT) littermates revealed significantly higher levels of AI in Gpx-2 knock-out mice, mainly due to significant increased number of lymphocytes and eosinophils (Figure 4A). OVA-specific total IgE and IgG1 levels were also increased in Gpx-2 knock-out mice but on a non significant level (Figure 4B and 4C). In order to evaluate functional consequences of Gpx-2 deficiency, we analyzed airway resistance after methacholine provocation in isolated and perfused lungs from WT and KO. Here, we observed a 32% increase in relative fold airway resistance in KO mice in comparison to WT mice (Figure 4D).
Figure 4. Gpx2 deficiency enhances allergic airway inflammation and airway reactivity.
Compared with WT littermates (wt, n=6), Gpx2 KO mice (n=6) showed increased airway inflammation, due to an increased influx of eosinophils and lymphocytes after sensitization and challenge with antigen. (A). OVA-spec. IgE and IgG1 were increased in Gpx-2 KOmice but to a significant level (B, C). Comparing airway resistance in isolated perfused lungs of Gpx2 KO and wild type mice (n= 6 each) after sensitization and challenge, we found a higher relative fold airway resistance in Gpx2 KO mice than in the corresponding WT controls (D).
* P < 0.05
Discussion
In the present study, we utilized gene expression profiling in lung tissues of two different mouse strains to identify novel and common inflammatory genes involved in allergic airway disease. We detected two antioxidants, GSTO 1-1 and Gpx-2, which had yet not been recognized in this context and which were significantly upregulated, both on the transcriptional and translational levels. Our data support recent evidence that chronic allergic airway inflammation is, in part, a result of and mediated by reactive oxygen species (ROS) (2). Furthermore, increased levels of inflammation and airway reactivity in Gpx-2-null mice support the notion that Gpx-2 plays a protective role in airway inflammation, similar to its anti-inflammatory role in the GI tract (5).
The family of Gpx consist of four selenoproteins, Gpx1-4, which are key enzymes in the redox cycle. Their differential expression patterns and additional enzymatic capacities indicate that they play an important role in exerting cell- and tissue-specific roles in metabolic regulation (10). Gpx-1-4 have all been reported to be expressed in human lungs (11), yet functional studies revealing their contributions to health and disease in this organ remain sparse. Hoffmann et al. have recently shown that Gpx-1, but not Gpx-4 protein was elevated (2.8-fold) in lung tissues of challenged C57BL/6J mice analyzed on day 29 (12). In our analysis, we were not able to reproduce this increase. However, induction of Gpx-1 gene expression might occur later in the time course of allergic inflammation than the time point analyzed in our study.
Most studies of GPX-2 so far were confined to the gastrointestinal tract ((5;13;14). Although mRNA Gpx-2 expression was found in mouse lungs, localization in this organ has not been elucidated (15). In humans, Gpx-2 protein expression was not detected in the lung (13) and Gpx activity in the lung was mainly attributed to Gpx-1 activity (16).
Gpx-2 is upregulated by nuclear factor erythroid 2-related factor 2, and increased levels in hyperoxia-induced lung injury in Nrf2-null mice points towards a role of Gpx-2 in protection against oxidative stress (15;17). The present study extends these findings to another adverse lung event that is known to generate ROS as well as inflammatory mediators: allergic airway inflammation. The finding of increased airway inflammation in the absence of Gpx-2 strongly supported a protective role in allergic airway inflammation for this protein.
Considering that Ho et al. have shown that 95% of the glutathione peroxidases activity in the lung is attributable to Gpx-1(16), it might be possible that the protective role of Gpx-2 in this organ is due to different enzymatic activities. In fact, glutathione peroxidases have been reported to inhibit prostaglandin synthesis (18), thus reducing the expression of pro-inflammatory mediators known to play an important role in the pathogenesis of allergic asthma,. Also, Gpx- 2 knock-out studies suggested an involvement in anti-inflammatory mechanisms (5). The exact mechanism by which Gpx-2 decreases airway inflammation will be the subject of further studies as our preliminary studies concerning changes in classical inflammatory mediators (IL-4, IL-5, IL-10, IFN-gamma) upon allergen restimulation did not show significant differences (data not shown).
The other gene identified in our present study belongs to the supergene family of glutathione S-transferases, of which we identified the mouse homologue of glutathione S-transferase Omega 1-1 (GSTO 1-1). In the human lung, the enzyme GSTO 1-1 is reported to be exclusively expressed in alveolar macrophages (19) while the mouse homologue has been shown to be expressed ubiquitously, with expression levels highest in the lung and liver (9).
Up to now, this enzyme has not been implicated in the pathology of bronchial asthma. The GST family was initially described to provide an important detoxification step for various ROS (20) but many of the six distinct subclasses perform additional reactions. (21). Human GSTO 1-1 acts as a glutathione-dependent-thioltransferase, which might serve to restore enzymatic function after exposure to oxidative stress (22). Human GSTO 1-1 has also been shown to inhibit IL-1β-dependent apoptosis via cytokine release inhibitory drugs (23), suggesting a new type of regulatory operation performed by this enzyme. The expression of the mouse homologue of GSTO 1-1, p28, was initially discovered in a radiation-resistant lymphoma line, pointing towards a possible role in conferring resistance to radiation-induced cell death (9). This role is supported by studies showing that GSTs inhibit certain stress kinases such as JNK (24), which in turn inhibits apoptosis and allows cell repair (25).
Integrating the functional results pertaining to GSTO 1-1 into our mouse model, inhibition of apoptosis by upregulation of GSTO 1-1 may lead to adverse effects in cells which under normal circumstances would be deleted. One hypothesis concerning the effects of ROS proposes three levels of response to oxidative stress. (i) Low amounts of oxidative stress induce protective responses via the induction of cytoprotective and anti-inflammatory mediators. (ii) An intermediate level of oxidative stress causes the induction of cytokines, chemokines, and adhesion molecules, leading to an inflammatory response. And finally, (iii) a high amount of oxidative stress causes apoptosis and necrosis, which leads to the induction of inflammation and remodeling in which induction of GSTO 1-1 might play a role, as reviewed in (25).
Other findings pointing towards a possible role of GSTO 1-1 and Gpx-2 in allergic airway diseases arise from studies on genetic heterogeneity. The individual’s ability to deal with an oxidant burden may depend in part on the genetic background. Polymorphisms of different subclasses of GSTs have been shown to be associated with asthma, lung function and susceptibility to xenobiotic enhancement of allergic symptoms (21;26;27). Recently, functional data have been added, suggesting that GSTs are able to modify the adjuvant effect of diesel exhaust particles and thereby attenuate local and systemic allergic inflammation (26). Such an association has not yet been reported for the GSTO 1-1 or the Gpx-2 iso-enzyme. Yet two independent studies were able to link the development of asthma to chromosome 14q24, which is the chromosomal location of Gpx-2 (28;29). Taken together, our data suggest that different activity levels of GSTO 1-1 and Gpx-2 due to genetic polymorphisms might contribute to the relative risk of disease development, a hypothesis which should be tested in association studies in disease cohorts.
In summary, we have identified two common inflammatory genes that were not previously recognized as being involved in the development of allergen-mediated airway disease. Knowledge of the mechanism underlying oxidative stress in the lungs may allow the development of novel antioxidant interventions. These strategies will then have to test the hypothesis that oxidative stress is involved in the pathogenesis of asthma, not only by direct injury to cells, but also as a fundamental factor in airway inflammation.
Supplementary Material
Acknowledgments
We thank Christine Seib for her excellent technical assistance.
This work was supported by grants from the German National Genome Research Network (Federal Ministry of Research (BMBF) 01GS0120) (EH), and the Crohn’s & Colitis Foundation of America (FFC), NIH R01 CA114569 (FFC)
Reference List
- 1.Leckie MJ, ten BA, Khan J, Diamant Z, O'Connor BJ, Walls CM, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000 Dec 23;356(9248):2144–8. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 2.Bowler RP, Crapo JD. Oxidative stress in allergic respiratory diseases. J Allergy Clin Immunol. 2002 Sep;110(3):349–56. doi: 10.1067/mai.2002.126780. [DOI] [PubMed] [Google Scholar]
- 3.Dittrich AM, Quarcoo D, Krokowski M, Ahrens B, Hamelmann E. Gene expression profiling as novel tool in experimental asthma research. Exp Toxicol Pathol. 2006 Jun;57(Suppl 2):31–3. doi: 10.1016/j.etp.2006.02.003. Epub;%2006 Mar 31.:31–3. [DOI] [PubMed] [Google Scholar]
- 4.Herz U, Braun A, Ruckert R, Renz H. Various immunological phenotypes are associated with increased airway responsiveness. Clin Exp Allergy. 1998 May;28(5):625–34. doi: 10.1046/j.1365-2222.1998.00280.x. [DOI] [PubMed] [Google Scholar]
- 5.Esworthy RS, Aranda R, Martin MG, Doroshow JH, Binder SW, Chu FF. Mice with combined disruption of Gpx1 and Gpx2 genes have colitis. Am J Physiol Gastrointest Liver Physiol. 2001 Sep;281(3):G848–G855. doi: 10.1152/ajpgi.2001.281.3.G848. [DOI] [PubMed] [Google Scholar]
- 6.Hamelmann E, Vella AT, Oshiba A, Kappler JW, Marrack P, Gelfand EW. Allergic airway sensitization induces T cell activation but not airway hyperresponsiveness in B cell-deficient mice. Proc Natl Acad Sci U S A. 1997 Feb 18;94(4):1350–5. doi: 10.1073/pnas.94.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witzenrath M, Ahrens B, Kube SM, Braun A, Hoymann HG, Hocke AC, et al. Detection of allergen-induced airway hyperresponsiveness in isolated mouse lungs. Am J Physiol Lung Cell Mol Physiol. 2006 Sep;291(3):L466–L472. doi: 10.1152/ajplung.00011.2005. [DOI] [PubMed] [Google Scholar]
- 8.Chu FF, Esworthy RS, Akman S, Doroshow JH. Modulation of glutathione peroxidase expression by selenium: effect on human MCF-7 breast cancer cell transfectants expressing a cellular glutathione peroxidase cDNA and doxorubicin-resistant MCF-7 cells. Nucleic Acids Res. 1990 Mar 25;18(6):1531–9. doi: 10.1093/nar/18.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kodym R, Calkins P, Story M. The cloning and characterization of a new stress response protein. A mammalian member of a family of theta class glutathione s-transferase-like proteins. J Biol Chem. 1999 Feb 19;274(8):5131–7. doi: 10.1074/jbc.274.8.5131. [DOI] [PubMed] [Google Scholar]
- 10.Brigelius-Flohe R. Tissue-specific functions of individual glutathione peroxidases. Free Radic Biol Med. 1999 Nov;27(9–10):951–65. doi: 10.1016/s0891-5849(99)00173-2. [DOI] [PubMed] [Google Scholar]
- 11.Chu FF, Esworthy RS, Doroshow JH, Doan K, Liu XF. Expression of plasma glutathione peroxidase in human liver in addition to kidney, heart, lung, and breast in humans and rodents. Blood. 1992 Jun 15;79(12):3233–8. [PubMed] [Google Scholar]
- 12.Hoffmann PR, Jourdan-Le SC, Hoffmann FW, Chang PS, Bollt O, He Q, et al. A role for dietary selenium and selenoproteins in allergic airway inflammation. J Immunol. 2007 Sep 1;179(5):3258–67. doi: 10.4049/jimmunol.179.5.3258. [DOI] [PubMed] [Google Scholar]
- 13.Chu FF, Doroshow JH, Esworthy RS. Expression, characterization, and tissue distribution of a new cellular selenium-dependent glutathione peroxidase, GSHPx-GI. J Biol Chem. 1993 Feb 5;268(4):2571–6. [PubMed] [Google Scholar]
- 14.Komatsu H, Okayasu I, Mitomi H, Imai H, Nakagawa Y, Obata F. Immunohistochemical detection of human gastrointestinal glutathione peroxidase in normal tissues and cultured cells with novel mouse monoclonal antibodies. J Histochem Cytochem. 2001 Jun;49(6):759–66. doi: 10.1177/002215540104900609. [DOI] [PubMed] [Google Scholar]
- 15.Singh A, Rangasamy T, Thimmulappa RK, Lee H, Osburn WO, Brigelius-Flohe R, et al. Glutathione peroxidase 2, the major cigarette smoke-inducible isoform of GPX in lungs, is regulated by Nrf2. Am J Respir Cell Mol Biol. 2006 Dec;35(6):639–50. doi: 10.1165/rcmb.2005-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho YS, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, et al. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Biol Chem. 1997 Jun 27;272(26):16644–51. doi: 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- 17.Cho HY, Jedlicka AE, Reddy SP, Kensler TW, Yamamoto M, Zhang LY, et al. Role of NRF2 in protection against hyperoxic lung injury in mice. Am J Respir Cell Mol Biol. 2002 Feb;26(2):175–82. doi: 10.1165/ajrcmb.26.2.4501. [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto H, Imai H, Nakagawa Y. Involvement of phospholipid hydroperoxide glutathione peroxidase in the modulation of prostaglandin D2 synthesis. J Biol Chem. 2000 Dec 22;275(51):40028–35. doi: 10.1074/jbc.M003191200. [DOI] [PubMed] [Google Scholar]
- 19.Yin ZL, Dahlstrom JE, Le Couteur DG, Board PG. Immunohistochemistry of omega class glutathione S-transferase in human tissues. J Histochem Cytochem. 2001 Aug;49(8):983–7. doi: 10.1177/002215540104900806. [DOI] [PubMed] [Google Scholar]
- 20.Hayes JD, Strange RC. Potential contribution of the glutathione S-transferase supergene family to resistance to oxidative stress. Free Radic Res. 1995 Mar;22(3):193–207. doi: 10.3109/10715769509147539. [DOI] [PubMed] [Google Scholar]
- 21.Strange RC, Spiteri MA, Ramachandran S, Fryer AA. Glutathione-S-transferase family of enzymes. Mutat Res. 2001 Oct 1;482(1–2):21–6. doi: 10.1016/s0027-5107(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 22.Board PG, Coggan M, Chelvanayagam G, Easteal S, Jermiin LS, Schulte GK, et al. Identification, characterization, and crystal structure of the Omega class glutathione transferases. J Biol Chem. 2000 Aug 11;275(32):24798–806. doi: 10.1074/jbc.M001706200. [DOI] [PubMed] [Google Scholar]
- 23.Laliberte RE, Perregaux DG, Hoth LR, Rosner PJ, Jordan CK, Peese KM, et al. Glutathione s-transferase omega 1-1 is a target of cytokine release inhibitory drugs and may be responsible for their effect on interleukin-1beta posttranslational processing. J Biol Chem. 2003 May 9;278(19):16567–78. doi: 10.1074/jbc.M211596200. [DOI] [PubMed] [Google Scholar]
- 24.Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, et al. Regulation of JNK signaling by GSTp. EMBO J. 1999 Mar 1;18(5):1321–34. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vignola AM, Chiappara G, Gagliardo R, Gjomarkaj M, Merendino A, Siena L, et al. Apoptosis and airway inflammation in asthma. Apoptosis. 2000 Nov;5(5):473–85. doi: 10.1023/a:1009661406440. [DOI] [PubMed] [Google Scholar]
- 26.Gilliland FD, Gauderman WJ, Vora H, Rappaport E, Dubeau L. Effects of glutathione-S-transferase M1, T1, and P1 on childhood lung function growth. Am J Respir Crit Care Med. 2002 Sep 1;166(5):710–6. doi: 10.1164/rccm.2112065. [DOI] [PubMed] [Google Scholar]
- 27.Gilliland FD, Li YF, Saxon A, az-Sanchez D. Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: randomised, placebo-controlled crossover study. Lancet. 2004 Jan 10;363(9403):119–25. doi: 10.1016/S0140-6736(03)15262-2. [DOI] [PubMed] [Google Scholar]
- 28.Mansur AH, Bishop DT, Markham AF, Morton NE, Holgate ST, Morrison JF. Suggestive evidence for genetic linkage between IgE phenotypes and chromosome 14q markers. Am J Respir Crit Care Med. 1999 Jun;159(6):1796–802. doi: 10.1164/ajrccm.159.6.9804036. [DOI] [PubMed] [Google Scholar]
- 29.Hakonarson H, Bjornsdottir US, Halapi E, Palsson S, Adalsteinsdottir E, Gislason D, et al. A major susceptibility gene for asthma maps to chromosome 14q24. Am J Hum Genet. 2002 Sep;71(3):483–91. doi: 10.1086/342205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.