Summary
Supernumerary centrioles lead to abnormal mitosis [1,2] which in turn promotes tumorigenesis [3,4]. Thus, centriole duplication must be coordinated with the cell cycle to ensure that the number of centrioles in the cell doubles precisely during each cell cycle [5]. However, in some transformed cells centrioles undergo multiple rounds of duplication (reduplication) during prolonged interphase [6-8]. Mechanisms responsible for centriole reduplication are poorly understood. Here, we report that centrioles reduplicate consistently in cancerous and non-transformed human cells during G2 arrests and this reduplication requires the activity of Polo-like kinase 1 (Plk1). We also find that cell’s ability to reduplicate centrioles during S-arrests depends on the presence of activated (T210-phosphorylated) Plk1 at the centrosome. In the absence of activated Plk1, nascent procentrioles remain associated with mother centrioles, which prevent centriole reduplication. In contrast, if Plk1(pT210) appears at the centrosome, procentrioles mature, disengage from mother centrioles, and ultimately duplicate. Plk1 activity is not required for the assembly of procentrioles, however. Thus, the role of Plk1 is to coordinate centriole duplication cycle with the cell cycle. Activation of Plk1 during late-S-G2 induces procentriole maturation and after this point the centriole cycle can be completed autonomously, even in the absence of cell cycle progression.
Results and Discussion
Centrioles Reduplicate in G2-arrested Cells
Normally, new centrioles (procentrioles) assemble at G1/S transition and they remain associated with mother centrioles until the ensuing mitosis [9]. If the cell cycle is arrested with hydroxyurea, in many cell types (e.g., HeLa) procentrioles form once and they remain associated with mother centrioles indefinitely. Interestingly, these procentrioles also remain significantly shorter than their mothers [7] and they lack hPOC5 (Figure S1, A-E), a protein normally recruited to the distal parts of procentrioles during G2 [10]. In contrast, some transformed cells (e.g., U2-OS) continuously reduplicate centrioles upon HU arrest [6,11]. In these cell types procentrioles develop to full length and recruit hPOC5 prior to disengaging from their mothers, and ultimately reduplicating (Figure S1, F-J). These differences prompted us to speculate that HU-arrested U2-OS cells progress farther toward G2 phase, achieving conditions under which procentrioles can naturally mature into daughter centrioles. If this assumption is correct, then the ability of U2-OS cells to reduplicate centrioles is not due to abnormalities intrinsic to the centriole cycle. Instead, the difference between ‘reduplicating’ and ‘non-reduplicating’ cell types is based on the stringency of the cell-cycle arrest upon HU-treatment. A more stringent S phase arrest should prevent centriole reduplication in U2-OS cells. Conversely, centrioles should reduplicate in HeLa cells if the cell cycle is arrested during later S or G2.
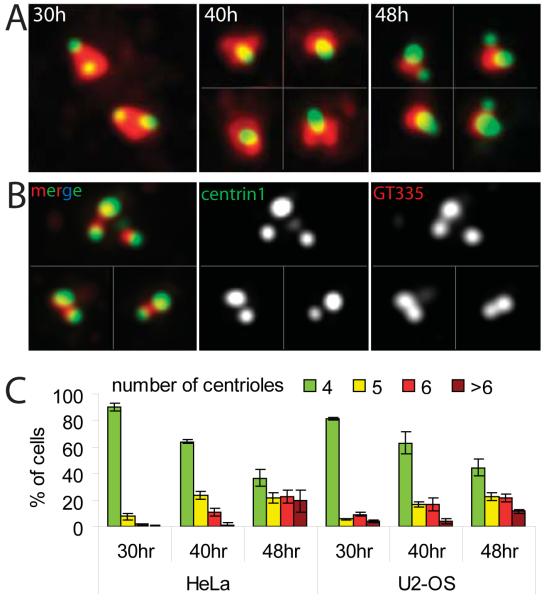
To test whether centrioles can reduplicate in G2-arrested cells, we used a small molecule inhibitor RO-3306 (hereafter, RO) that reversibly arrests cells in late G2, by inhibiting the activity of Cdk1 [12]. Immunofluorescence analyses in synchronous cell populations revealed that the number of cells with more than four centrin-GFP spots gradually increased to >60% after ~18 hr of G2 arrest (48 hr after shake-off; see Supplementary Experimental Procedures and Figure S2A-B). These additional spots also contained polyglutamylated tubulin and were consistently surrounded by clouds of γ-tubulin (Figure 1), suggesting that they were bona-fide centrioles. Importantly, at later time points most cells contained a mixture of diplosomes and individual centrioles (Figure 1), suggesting that centriole amplification occurred via repetitive rounds of centriole disengagement and duplication [7]. Therefore, both U2-OS and HeLa cells readily reduplicate centrioles during RO-induced G2 arrests.
Figure 1.
Centrioles reduplicate in G2-arrested cells. (A) Examples of typical centrosome configurations in RO-treated HeLa cells at indicated time points (30 hr corresponds to the beginning of G2 arrest, see Supplementary Experimental Procedures). Similar configurations were also observed in U2-OS cells (not shown). (30 hr) Two diplosomes in one cell; (40 hr) four individual centrioles in one cell; (48 hr) four diplosomes in one cell. Green, centrin-GFP; red, γ-tubulin (B) Centrin-GFP-positive structures also contain polyglutamylated tubulin, as evident from staining with GT335 antibody. (C) Percentages of cells with different number of centrioles as determined via centrin-GFP signal. (Also see Figure S2).
It has previously been demonstrated that centrioles can assemble de novo if all preexisting centrioles are removed from the cell. The de novo assembly occurs during S but not during G1 phase [13,14]. To test whether centrioles can assemble de novo during RO-induced G2 arrest, we used a laser microbeam to ablate all resident centrioles in HeLa cells 28-48 hr after mitotic shake-off (0-18 hr of G2 arrest). In all 26 cases, laser ablation induced formation of multiple centrin-GFP foci that also contained other centriolar and centrosomal proteins (Figure S2 C-D). Therefore, centriole de novo assembly can be initiated during G2.
We also observed centriole reduplication during RO-induced G2 arrests in non-transformed diploid human RPE-1 cells (Figure S2 E-H). This result suggested that centriole reduplication upon G2 arrest is a universal phenomenon that is not limited to transformed cells.
Centrioles Do Not Reduplicate in Emi1-depleted DNA-reduplicating Cells
Our findings suggest that centriole reduplication requires certain activities that normally emerge during G2. In this regard, HU-arrested U2-OS and CHO cells that reduplicate centrioles are known to accumulate higher levels of cyclins A and B. In contrast, in HeLa cells levels of these proteins remain relatively low [15,16]. This prompted us to investigate whether centriole reduplication occurs in U2-OS cells under conditions that prevent progression into G2 and accumulation of mitotic cyclins.
We used siRNA to deplete Early Mitotic Inhibitor 1 (Emi1) in U2-OS and HeLa cells. Emi1 is an interphase suppressor of the anaphase promoting complex/cyclosome (APC/C) required for proper coordination between DNA duplication and mitosis [17,18]. In Emi1-depleted cells, APC/C remains active, leading to degradation of cyclins A and B (Figure S3 A), as well as geminin [19]. In contrast, cyclin E remains stable, and its activity is sufficient to drive DNA synthesis [18]. As a result of Emi1 depletion, the cells enter a perpetual DNA reduplication, a state manifested by the increase in the size of the nucleus [18].
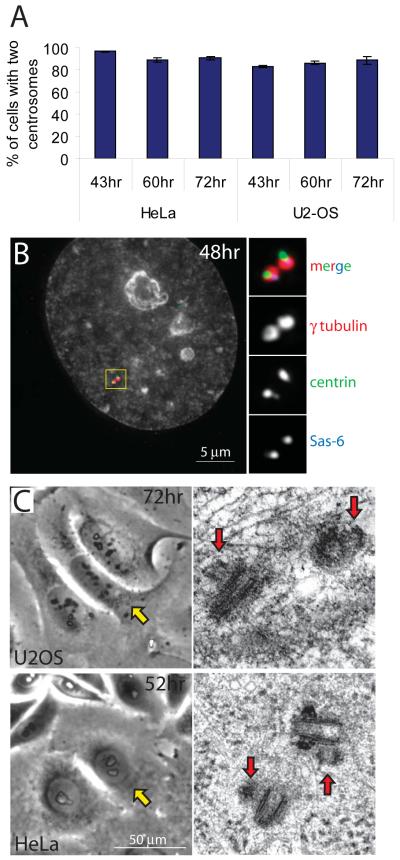
Despite the perpetuate S-phase, ~90% of either U2-OS or HeLa cells depleted of Emi1 (see Supplemental Experimental procedures) contained just two centrosomes, as revealed by γ-tubulin staining (Figure 2A). Only one prominent centrin spot was consistently found within each γ-tubulin cloud (Figure 2B). However, the centrin spot was in most cases distorted, manifesting the initial stages of centriole duplication [7]. Further, Sas6, the protein shown to be recruited to the nascent procentrioles [20] was consistently associated with the centrin spot (Figure 2B). Finally, serial-section EM analyses (one HeLa and five U2-OS cells) confirmed that each cell contained only two diplosomes, each comprising one fully grown centriole and a short procentriole (Figure 2C).
Figure 2.
Procentrioles form, but do not mature, in Emi1-depleted cells. (A) Percentage of Emi1-depleted HeLa and U2-OS cells with two centrosomes at various time points after transfection. (B) Centrosome configuration in Emi1-depleted U2-OS cells 46 h after transfection. See text for details. (C) Serial-section EM analyses confirm that Emi1-depleted cells with grossly enlarged nuclei (yellow arrowheads) contain just two diplosomes with 150-200-nm short procentrioles (red arrows). (See also Figure S2 and Video S1).
We also laser-ablated all resident centrioles in Emi1-depleted cells 24-27 hr after mitotic shake-off. This treatment resulted in the activation of de novo centriole assembly in all 13 cells studied (Figure S3B). Further, selective ablation of procentrioles within diplosomes [7], in Emi1-depleted HeLa cells, resulted in the formation of new procentrioles on the same mother centriole (Figure S3C). Thus, cytoplasmic conditions in Emi1-depleted cells remain permissive for the assembly of procentrioles, but not for their development into full-sized daughter centrioles.
Activated Plk1 Is Required for Procentriole Maturation and Centriole Reduplication
Thus far, our results confirmed that procentrioles assemble but do not mature during S phase. In contrast, procentrioles assemble, develop into full-sized daughter centrioles, and disengage from their mothers in G2-arrested cells. This finding prompted us to seek the molecular pathways responsible for procentriole maturation during late-S/G2.
Several cell cycle regulators have been linked to the ability of cells to reduplicate centrioles during interphase arrest [16,21,22]. For example, overexpression of cyclin A induces centriole reduplication in HU-arrested HeLa cells [16]. Depletion of Plk1 by siRNA prevents centriole reduplication in HU-arrested arrested U2-OS cells [22]. Both cyclin A and Plk1 accumulate during late-S and G2 phases, a fact consistent with their potential role in procentriole maturation. Furthermore, a recent study suggested that Plk1 activity during early mitosis is required for subsequent disengagement of centrioles, at the onset of anaphase [23].
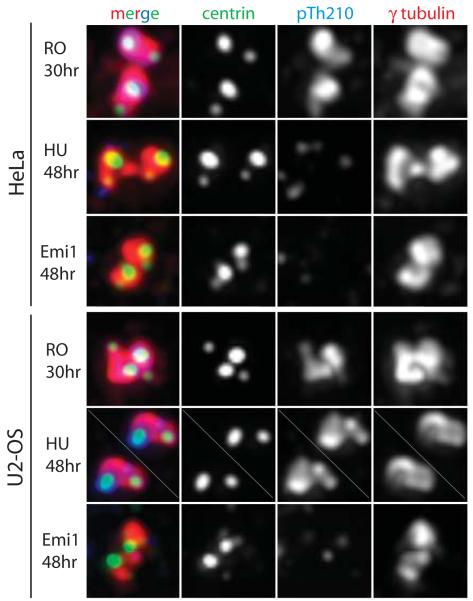
Phosphorylation of Thr210 by Aurora A has been shown to activate Plk1 during late-S and G2 phases [24]. Interestingly, T210-phosphorylated Plk1 was detected on the centrosome, although the significance of this observation was not clear [24]. We found pT210-Plk1 to be present at the centrosome in RO-treated, but not in HU-treated or Emi1-depleted HeLa cells (Figure 3). In contrast, phosphorylated Plk1 was found at the centrosome in RO-treated and HU-treated, but not in Emi-depleted U2OS cells, signifying that the presence of p210-Plk1 on the centrosome strictly correlates with the conditions that allow procentrioles to mature and disengage.
Figure 3.
pThr210 Plk1 is present at centrosomes under conditions that allow procentriole maturation and centriole reduplication. Cells either treated with HU, RO-3306, or depleted of Emi1 were fixed at indicated time points and analyzed by fluorescence microscopy. The pThr210 signal was prominent at the centrosome in RO-treated HeLa as well as in RO- and HU-treated U2-OS cells. In contrast, pThr210 was not detectable at the centrosome in HU-treated HeLa or in Emi1-depleted HeLa or U2-OS cells.
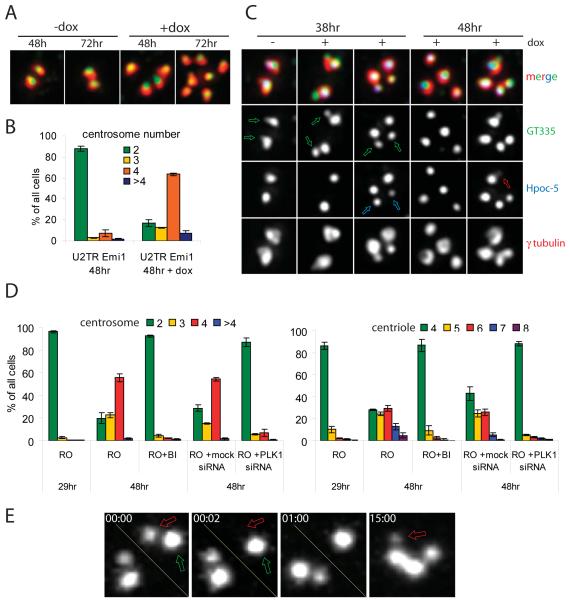
To determine whether active Plk1 is required for procentriole maturation, we employed U2TR, a U2-OS-derived cell line engineered to express, upon induction with doxycycline, a constitutively active phospho-mimicking (T210D) mutant of human Plk1 [24]. Similar to the wild-type U2-OS cells, non-induced U2TR cells do not reduplicate centrioles upon Emi1 depletion (Figure 4A). However, induction of Plk1(T210D) expression in Emi1-depleted U2TR cells by 1-mM doxycycline resulted in the rapid accumulation of supernumerary centrosomes (Figure 4B). Importantly, doxycycline-induced cells contained a mixture of diplosomes and individual centrioles, indicating that centriole amplification occurred via repetitive rounds of centriole duplication and disengagement. Also, doxycycline-induced expression of constitutively active Plk1 dramatically accelerated centriole reduplication in HU-arrested U2TR cells, a process known to occur via repetitive rounds of duplication and disengagement (Figure S4A).
Figure 4.
Activated Plk1 is necessary for centriole reduplication. (A-C) Expression of phosphomimicking Plk1 mutant (T210D) induces centriole reduplication in Emi1-depleted U2TR cells. Mitotic cells (collected by shake-off) were plated on coverslips and 1.5 hr later transected with Emi1 siRNA. Doxycycline (1 μM) was added 24-26h after shake off. (A) Typical centrosome configurations in non-induced (−dox) vs. induced (+dox) Emi1-depleted U2TR cells. Red, γ-tubulin (PCM); green, polyglutamylated tubulin (centrioles). (B) Percentage of cells with various numbers of centrosomes under two treatment conditions. (C) Procentrioles mature and disengage from mother centrioles upon expression of Plk1(T210D). Notice that in non-induced cells all centrioles are duplicated (green arrows in 38 hr −dox). However, procentrioles are small and do not contain hPOC-5 which is normally recruited to the distal end of growing procentrioles during G2. In induced cells, procentrioles become more prominent (green arrows in 38 hr +dox) and eventually do recruit hPOC-5 (blue arrows in 38 hr +dox). At a later time, induced cells contain both individual centrioles and diplosomes. hPOC5 is found in all individual centrioles. However, some procentrioles lack hPOC-5 or contain minimal amounts of this protein (red arrow, 48 hr +dox). (See also Figure S4). (D-E) Inhibition of Plk1 prevents centriole reduplication but not initiation of procentriole assembly. Mitotic HeLa cells (collected by shake-off) were plated on coverslips and 1.5 hr later treated with RO. 100 nM Plk1 inhibitor BI2536 was added 29 hr after shake-off. Alternatively, 1.5 hr after shakeof cell were transfected with siRNA against Plk1. (D) Percentage of cells with various centriole and centrosome numbers under different conditions. (E) Procentriole assembly in G2-arrested HeLa cells after selective ablation of the original procentriole. A procentriole (red arrow in 00:00) was ablated within the diplosome (compare 00:00 and 00:02) at 31h after shake-off. This operation resulted in the formation of a new procentriole (arrow in 15:00) on the mother centriole, as evidenced by centrin-GFP signal. (See also Figure S5).
We used immunofluorescence LM and EM to directly determine whether procentrioles mature upon expression of Plk1(T210D) in Emi1-depleted U2TR cells. In non-induced cells, hPOC5 protein was consistently present on the mother centrioles but was absent from the immature procentrioles. Already ~12 hr after induction of Plk1(T210D) expression by doxycycline, hPOC5 was found on the procentrioles of Emi1-depleted U2TR cells (Figures 4C and S4B). Accumulation of hPOC5 on the procentrioles occurred prior to their disengagement from the mothers, and precedes centriole reduplication. We also found that another centriole maturation marker, CEP170 [25] accumulated at all centrosomes in doxycycline-induced Emi1-depleted U2TR cells (Figure S4 C). Serial-section EM analysis confirmed that 72 hr after doxycycline induction, Emi1-depleted U2TR cells contained multiple diplosomes and individual daughter centrioles (2 cell, data not shown).
Expression of Plk1(T210D) in Emi1-depleted HeLa cells also increased the number of cells with supernumerary centrioles from ~5.4% to ~19.4% (data not shown). The lower percentage of centriole-reduplicating cells in this experimental system is likely due to cumulative inefficiency of two consecutive rounds of transfection (Emi1 siRNA followed by PLK1-expression plasmid). However, genetic differences between U2-OS and HeLa cells cannot be ruled out at this point.
As expected, cyclin A was not detectable in doxycycline-induced Emi1-depleted U2TR cells that had reduplicated centrioles (Figure S5), meaning that centriole maturation and reduplication, under the conditions used, occur without the accumulation of this mitotic cyclin. Although cyclin A was previously implicated in governing centriole duplication [8], our findings are not completely unexpected as cyclins A and E likely play redundant roles in centriole duplication [reviewed in 26]. In fact, a recent study demonstrates that complete ablation of cyclin A does not affect cell proliferation in fibroblasts [27].
Up to this point our experiments suggested that expression of active Plk1 triggers procentriole maturation and reduplication during S phase. To determine whether Plk1 activity is required for centriole reduplication during G2 arrests, we used the specific small-molecule inhibitor BI2536 [28] as well as siRNA against Plk1. We found that centriole reduplication in RO-treated HeLa cells did not occur if Plk1 activity had been inhibited by 100-nM BI2536 as well as if Plk1 was siRNA-depleted (Figure 4D). Under both conditions, cells remained with two diplosomes for at least 48 hr. Furthermore, BI2536 prevented centriole reduplication in HU-arrested CHO cells (Figure S6A). However, laser-ablation of procentrioles within the diplosomes resulted in the formation of new procentrioles (Figure 4E) in 7 of 8 cells tested. Also, inhibition of Plk1 in G2-arrested HeLa cells did not affect the ability of centrioles to assemble de novo (Figure S6B; N=9). Thus, Plk1 activity is not required for the initiation of procentriole assembly in G2-arrested cells.
Daniel Mazia’s classic experiments on the continuation of the centriole cycle in cells arrested during mitosis suggested, however, that the two cycles are not continuously synchronized. Instead, synchronization is achieved by linking the two cycles only at certain key time points [5]. One such time point is the transition from G1 into S phase, a time when both DNA and centrioles begin to duplicate, due to activation of cyclin/Cdk2 [9,29]. A second synchronization point has recently been suggested to exist during M phase, when activation of separase results in separation of chromatids and disengagement of daughter centrioles from their mothers [30]. However, it is clear that centrioles can also disengage via separase-independent mechanisms. In fact, although centriole disengagement is clearly delayed in separase-null cells, most diplosomes still manage to break down eventually [23]. Further, centriole disengagement occurs in some, but not in all, cell types, when the cell cycle is arrested via HU or aphidicolin treatment [6,7]. Under these conditions centrioles disengage and reduplicate asynchronously within a single cell, suggesting that the process is regulated by the factors intrinsic to the centrosome [7].
Although several molecular pathways have been implicated in the ability of certain cells to reduplicate centrioles during a prolonged S period [9,29,31], the mechanism of centriole reduplication remains poorly understood. Previous work suggested that centriole reduplication occurs when cells manage to disengage their centrioles during S arrests, and raised the question of what molecular mechanism is responsible for the disengagement [7,32]. Our results presented here identify Plk1-mediated maturation of procentrioles into full-size daughter centrioles as the event both necessary and sufficient for centriole disengagement. Importantly, Plk1 activity is not required for the assembly of new centrioles. We propose that Plk1 activity provides additional synchronization mechanism between the centriole cycle and the cell cycle, ensuring that centrioles do not disengage or reduplicate if progression towards mitosis is delayed. Given that both normal and transformed cells readily reduplicate their centrioles if the cell cycle is arrested after Plk1 is already activated, late-G2 arrests are likely to have severe negative effects on cell proliferation.
Experimental procedures
Centrin1-GFP-expressing HeLa cells have been previously described [7,33]. U2-OS, CHO (both from American Type Culture Collection, Manassas, VA), and RPE-1 (Clontech, Palo Alto, CA) cells were stably transfected with the previously described CenGFP lentivirus [7]. U2TRT210D, an inducible clone of U2-OS expressing the T210D phospho-mimicking Plk1 mutant [24] was a kind gift from Dr. R. Medema (University of Utrecht, The Netherlands). Plk1(T210D) expression was induced with 1 μg/ml doxocycline (Sigma, St Louis, MO). Cultures were maintained in DMEM medium (Invitrogen) supplemented with 10% fetal calf serum (Cellgro), 100 U penicillin, and 1μg/ml streptomycin (Gibco). Cells were grown in humidified 5% CO2 atmosphere at 37°C.
Details on cell synchronization, drug treatment, siRNA, and immunostaining are provided in Supplementary Experimental Procedures.
Laser microsurgery and microscopy
Laser ablations and live-cell imaging were conducted on a custom-build microsurgery workstation detailed elsewhere [7]. 3-D datasets of fixed cells were collected on a DeltaVision workstation and deconvolved with SoftWorX 2.1 software (Applied Precision). Same-cell LM/serial EM analyses were conducted as previously described [7].
Quantification and statistics
Centrosome or centriole numbers: histograms present average values of at least three independent experiments, with at least 200 cells counted per each experimental condition or time point. Error bars indicate standard error of mean. Quantification of centriole-associated hPOC5 signal was measured in average-intensity projections of deconvolved 3-D datasets. Fluorescence signal was integrated within a 9×9 pixel (~0.6×0.6 μm) region of interest centered on the centriole and background corrected. Minimum of 7 cells were quantified for each experimental condition.
Highlights
Centrioles reduplicate in both, normal and transformed G2-arrested cells
Inhibition of Plk1 prevents centriole reduplication in G2-arrested cells
Centrioles do not reduplicate in Emi1-depleted DNA-reduplicating cells
Expression of active Plk1 allows centrioles to reduplicate in Emi1-depleted cells
Plk1 activity is not required for procentriole formation
Activated Plk1(pT210) at the centrosome is required for procentriole maturation
Supplementary Material
Acknowledgements
We thank Dr. Yimin Dong for her expert help with EM. Special thanks to Drs. René H. Medema and Libor Macůrek for sharing the U2TRT210D cell line. We are grateful to the members of our lab for helpful discussions and to Drs. C.B. O’Connell and G. Sluder for critical comments on the manuscript. This work was supported by a grant from the National Institutes of Health (GM GM59363) to A.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silkworth WT, Nardi IK, Scholl LM, Cimini D. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS ONE. 2009;4:e6564. doi: 10.1371/journal.pone.0006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weaver BA, Silk AD, Montagna C, Verdier-Pinard P, Cleveland DW. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell. 2007;11:25–36. doi: 10.1016/j.ccr.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Basto R, Brunk K, Vinogradova T, Peel N, Franz A, Khodjakov A, Raff JW. Centrosome amplification can initiate tumorigenesis in flies. Cell. 2008;133:1032–1042. doi: 10.1016/j.cell.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazia D. The chromosome cycle and the centrosome cycle in the mitotic cycle. Internat. Review Cytology. 1987;100:49–92. doi: 10.1016/s0074-7696(08)61698-8. [DOI] [PubMed] [Google Scholar]
- 6.Balczon R, Bao L, Zimmer WE, Brown K, Zinkowski RP, Brinkley BR. Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J. Cell Biol. 1995;130:105–115. doi: 10.1083/jcb.130.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loncarek J, Hergert P, Magidson V, Khodjakov A. Control of daughter centriole formation by the pericentriolar material. Nature Cell Biology. 2008;10:322–328. doi: 10.1038/ncb1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meraldi P, Lukas J, Fry AM, Bartek J, Nigg EA. Centrosome duplication in mammalian somatic cells requires E2F and cdk2-cyclin A. Nature Cell Biology. 1999;1:88–93. doi: 10.1038/10054. [DOI] [PubMed] [Google Scholar]
- 9.Nigg EA. Centrole duplication: of rules and licenses. Trends in Cell Biology. 2007;17:215–221. doi: 10.1016/j.tcb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Azimsadeh J, Hergert P, Delouvee A, Euteneuer U, Formstecher E, Khodjakov A, Bornens M. hPOC5 is a centrin-binding protein required for assembly of full-length centrioles. J. Cell Biol. 2009;185:101–114. doi: 10.1083/jcb.200808082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stucke VM, Sillje HH, Arnaud L, Nigg EA. Human Mps1 kinase is required for the spindle assembly checkpoint but not for centrosome duplication. EMBO Journal. 2002;21:1723–1732. doi: 10.1093/emboj/21.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vassilev LT, Tovar C, Chen S, Knezevic D, Zhao X, Sun H, Heimbrook DC, Chen L. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc. Natl. Acad. Sci. U. S. A. 2006;103:10660–10665. doi: 10.1073/pnas.0600447103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Terra S, English CN, Hergert P, McEwen BF, Sluder G, Khodjakov A. The de novo centriole assembly pathway in HeLa cells: cell cycle progression and centriole assembly/maturation. J. Cell Biol. 2005;168:713–720. doi: 10.1083/jcb.200411126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uetake Y, Loncarek J, Nordberg JJ, English CN, La Terra S, Khodjakov A, Sluder G. Cell cycle progression and de novo centriole assembly after centrosomal removal in untransformed human cells. J. Cell Biol. 2007;176:173–182. doi: 10.1083/jcb.200607073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kung AL, Sherwood SW, Schimke RT. Differences in the regulation of protein synthesis, cyclin B accumulation, and cellular growth in response to the inhibition of DNA synthesis in Chinese hamster ovary and HeLa S3 cells. J. Biol. Chem. 1993;268:23072–23080. [PubMed] [Google Scholar]
- 16.Balczon RC. Overexpression of cyclin A in human HeLa cells induces detachment of kinetochores and spindle pole/centrosome overproduction. Chromosoma. 2001;110:381–392. doi: 10.1007/s004120100157. [DOI] [PubMed] [Google Scholar]
- 17.Di Fiore B, Pines J. Defining the role of Emi1 in the DNA replication-segregation cycle. Chromosoma. 2008;117:333–338. doi: 10.1007/s00412-008-0152-x. [DOI] [PubMed] [Google Scholar]
- 18.Di Fiore B, Pines J. Emi1 is needed to couple DNA replication with mitosis but does not regulate activation of the mitotic APC/C. J. Cell Biol. 2007;177:425–437. doi: 10.1083/jcb.200611166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machida YJ, Dutta A. The APC/C inhibitor, Emi1, is essential for prevention of rereplication. Genes & Development. 2007;21:184–194. doi: 10.1101/gad.1495007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strnad P, Leidel S, Vinogradova T, Euteneuer U, Khodjakov A, Gonczy P. Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Developmental Cell. 2007;13:203–213. doi: 10.1016/j.devcel.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasbek C, Yang CH, Yusof AM, Chapman HM, Winey M, Fisk HA. Preventing the degradation of mps1 at centrosomes is sufficient to cause centrosome reduplication in human cells. Molec. Biol. of the Cell. 2007;18:4457–4469. doi: 10.1091/mbc.E07-03-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Erikson RL. Activation of Cdc2/cyclin B and inhibition of centrosome amplification in cells depleted of Plk1 by siRNA. Proc. Natl. Acad. Sci. U. S. A. 2002;99:8672–8676. doi: 10.1073/pnas.132269599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsou MF, Wang WJ, George KA, Uryu K, Stearns T, Jallepalli PV. Polo kinase and separase regulate the mitotic licensing of centriole duplication in human cells. Developmental Cell. 2009;17:344–354. doi: 10.1016/j.devcel.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macurek L, Lindquist A, Lim D, Lampson MA, Klompmaker R, Freire R, Clouin C, Taylor SS, Yaffe MB, Medema RH. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 2008;445:119–123. doi: 10.1038/nature07185. [DOI] [PubMed] [Google Scholar]
- 25.Guarguaglini G, Duncan PI, Stierhof YD, Holmstrom T, Duensing S, Nigg EA. The forkhead-associated domain protein Cep170 interacts with Polo-like kinase 1 and serves as a marker for mature centrioles. Molec. Biol. of the Cell. 2005;16:1095–1107. doi: 10.1091/mbc.E04-10-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinchcliffe EH, Sluder G. Two for two: Cdk2 and its role in centrosome doubling. Oncogene. 2002;21:6154–6160. doi: 10.1038/sj.onc.1205826. [DOI] [PubMed] [Google Scholar]
- 27.Kalaszczynska I, Geng Y, Iino T, Mizuno S, Choi Y, Kondratiuk I, Silver DP, Wolgemuth DJ, Akashi K, Sicinski P. Cyclin A is redundant in fibroblasts but essential in hematopoietic and embryonic stem cells. Cell. 2009;138:352–365. doi: 10.1016/j.cell.2009.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenart P, Petronczki M, Steegmaier M, Di Fiore B, Lipp JJ, Hoffmann M, Rettig WJ, Kraut N, Peters JM. The Small-Molecule Inhibitor BI 2536 Reveals Novel Insights into Mitotic Roles of Polo-like Kinase 1. Curr. Biol. 2007;17:304–315. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 29.Hinchcliffe EH, Sluder G. “It takes two to tango”: understanding how centrosome duplication is regulated throughout the cell cycle. Genes & Development. 2001;15:1167–1181. doi: 10.1101/gad.894001. [DOI] [PubMed] [Google Scholar]
- 30.Tsou MF, Stearns T. Mechanism limiting centrosome duplication to once per cell cycle. Nature. 2006;442:947–951. doi: 10.1038/nature04985. [DOI] [PubMed] [Google Scholar]
- 31.Loncarek J, Khodjakov A. Ab ovo or de novo? Mechanisms of centriole duplication. Molecules and Cells. 2009;27:135–142. doi: 10.1007/s10059-009-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong C, Stearns T. Centrosome number is controlled by a centrosome-intrinsic block to reduplication. Nature Cell Biology. 2003;5:539–544. doi: 10.1038/ncb993. [DOI] [PubMed] [Google Scholar]
- 33.Piel M, Meyer P, Khodjakov A, Rieder CL, Bornens M. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J. Cell Biol. 2000;149:317–330. doi: 10.1083/jcb.149.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.