Abstract
Question
Does water loss during drought stress represent an important physiological constraint on the evolution of flower size?
Organism
A genetically diverse population of Mimulus guttatus (yellow monkeyflower) originally sampled from an alpine meadow in Oregon, USA.
Methods
We grew plants of three different genotypic classes (small, medium, and large flowered) under both well-watered and drought-stress conditions and measured water use efficiency using stable carbon isotopes.
Results
There was no difference in water use efficiency among flower size genotypes under well-watered conditions, but the water use efficiency of small-flowered plants was substantially lower than that of medium or large genotypes under drought stress. Whether this paradoxical result is a direct effect of flower size or an indirect (i.e. pleiotropic) effect, the presence of a genetic correlation between floral and physiological traits indicates that selection of one does impact the other.
Keywords: carbon isotopes, drought, genetic correlations, Mimulus guttatus, water use efficiency
INTRODUCTION
The diversity of floral morphologies in angiosperms, and the association between particular floral features and animal pollinators, provide textbook examples of adaptation and co-evolution (Faegri and Van der Pijl, 1979; Johnson, 2006). However, pollinators are only one of several selection pressures that act on floral traits. For example, the majority of angiosperms reproduce via self-fertilization at least occasionally. Selfing implies different optima for the size, number, and coordination of floral parts than outcrossing, and as a consequence, highly selfing species typically exhibit a distinct ‘syndrome’ of features (Ornduff, 1969). Floral traits may also be developmentally linked to other physiological, phenological, and life-history characters of the plant. These developmental linkages create genetic constraints in that floral characters become subject to the evolutionary forces that act on correlated traits (Arnold, 1992).
Galen describes a specific floral/physiological constraint wherein the amount of water lost through corolla tissue increases with flower size (Galen et al., 1999; Galen, 2000; Carroll et al. 2001). With sufficient water, large flowers are favoured owing to their attractiveness to pollinators or because they produce more ovules and/or pollen grains. However, under drought conditions, large flowers are disfavoured because they cause more rapid desiccation of the plant. Field and laboratory studies from Epilobium angustifolium (Carroll et al., 2001) and Polemonium viscosum (Galen et al., 1999; Galen, 2000) are consistent with this model. However, it is important to recognize that water loss and flower size may be related by other proximate mechanisms. For example, if flower size and total leaf area are positively correlated, then transpiration through leaves might generate a correlation between drought stress and flower size.
In this study, we test for a genetic correlation between flower size (corolla width) and water use efficiency in Mimulus guttatus, the yellow monkeyflower. Water use efficiency is the ratio of biomass accumulated through photosynthesis to water lost through transpiration. We estimate the genetic correlation between water use efficiency and corolla width for an annual population located on Iron Mountain in the Cascade Mountains of Oregon (Willis, 1993, 1996). Corolla width is genetically variable within the Iron Mountain population (Kelly and Arathi, 2003) and has been shown to affect pollinator recruitment in this species (Martin, 2004). Individuals bolt from the seedling stage following snow melt, usually in early June. They flower and then die, due primarily to desiccation, about a month and a half later. Drought is progressive – that is, water availability deteriorates continuously over the lifespan of the plant as snowmelt diminishes.
We used artificial selection to disrupt the continuous genetic variability in flower size of the natural population into divergent ‘types’. If corolla width and water use efficiency are genetically correlated, then selection on corolla width should produce a correlated response in water use efficiency (Falconer and Mackay, 1996). Over six generations, we imposed selection (in different experimental populations) for both larger and smaller corollas (Kelly, 2008). Our experiment, which included an unselected control population, produced the seeds for the three flower size categories of the present study: small, medium (control), and large. Under well-watered conditions, the average corolla widths were 22.3 mm (large, n = 48), 17.5 mm (medium, n = 58), and 12.7 mm (small, n = 6), respectively.
METHODS
Seeds from each of the three experimental populations were germinated in the greenhouse. Ten days later, approximately 130 seedlings from each group were transplanted into 98-well flats and moved to a growth room. There was some transplant death, but mortality was generally low and unrelated to treatment or flower size category. Flats were equally divided into two treatment categories, wet and dry. Flats in the wet treatment were submerged in water continuously. Dry flats were submerged in water for 5 min each day from day 15 to day 20, for 5 min every other day from day 20 to days 20–27, and for 5 min every third day from day 27 to day 44 of the experiment. The plants began to flower on day 20 and corolla width was measured on the day that each plant produced its first flower. On day 44, plants were harvested, the total number of flowers counted, and the above-ground vegetative tissue placed in an envelope and dried.
We estimated the water use efficiency of plants grown under both well-watered and drought-stressed conditions using carbon isotope analysis (Farquhar et al., 1982, 1989). Carbon isotope ratios (δ13C) of leaf tissue provide a time-integrated estimate of stomatal conductance (ci/ca), where ci and ca are the leaf-internal and ambient CO2 concentrations, respectively. A higher δ13C value usually indicates a higher water use efficiency, assuming that ca is constant and that the gradient in water-vapour pressure between the leaf and atmosphere is similar across treatments (Ehleringer, 1993). In our experiment, all plants were grown in close proximity under common CO2, temperature, and humidity conditions. Samples (1–2 mg) of dried tissue from the youngest leaves of each plant were sent (in tin capsules) to the Stable Isotope Mass Spectrometry Laboratory (Kansas State University, Manhattan, KS) for analysis. δ13C was measured using an elemental analyser (Carla Erba, Model 1110, Milano, Italy) coupled to a ThermoFinnigan Delta Plus gas isotope mass spectrometer (Bremen, Germany) using the standard formula: δ13C = (Rsample/Rstandard − 1) × 1000, where R is the ratio of the heavy isotope (13C) to the lighter isotope (12C). The standard was PDB (belemnite carbonate standard from the PeeDee Formation, SC). The precision of δ13C measurements was ± 0.2‰.
RESULTS
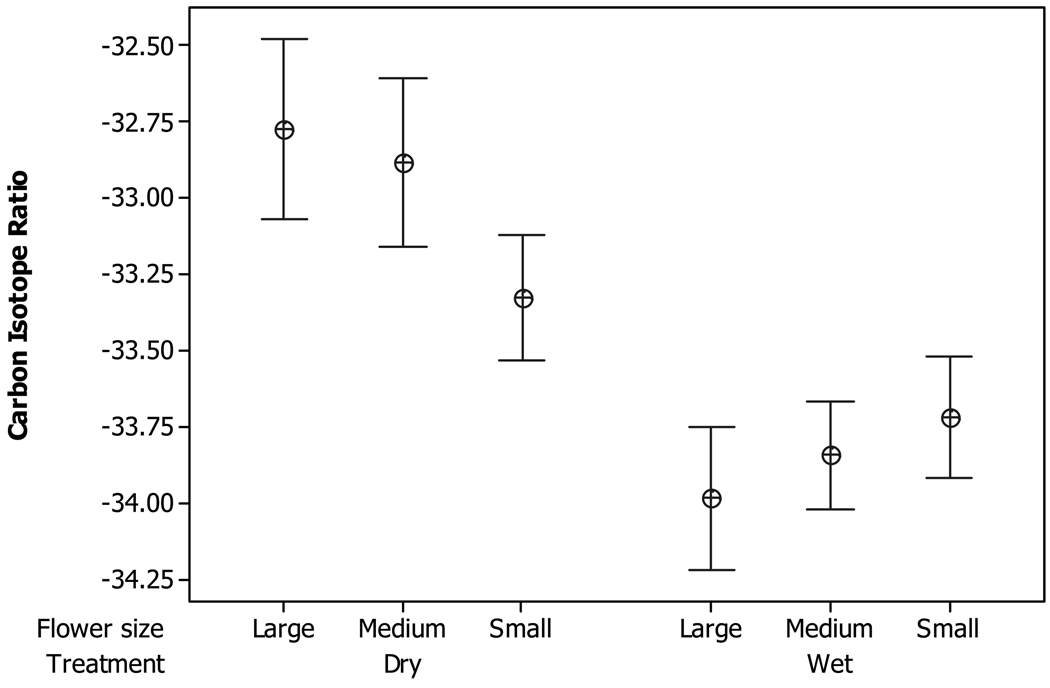
In total, 390 plants were scored for flower number and 315 plants for δ13C. The mean δ13C for each treatment/flower size category is given in Fig. 1. A two-way analysis of variance with treatment and flower size as fixed factors confirmed the statistical significance of the trends. Across flower sizes, plants in the dry treatment had higher δ13C values than those in the wet treatment (F1,309 = 49.8, P < 0.001). There was no average effect of flower size (F2,309 = 2.15, P > 0.05), but the interaction was significant (F2,309 = 9.05, P = 0.002), implying that the effect of flower size differed between the wet and dry treatments. Differences among flower size categories in the wet treatment were minimal. In the dry treatment, the estimated water use efficiency of the small-flower plants was substantially lower than that of the medium- and large-flower plants.
Fig. 1.
The mean carbon isotope ratio (δ13C value) is given for each flower size category in each experimental treatment. The bands define a 95% confidence interval on each mean.
DISCUSSION
What does it mean that small-flowered genotypes have lower water use efficiency? The differential response of M. guttatus genotypes to drought, which is inconsistent with our initial predictions, is most easily understood by considering what determines the carbon isotope ratio (δ13C). Variation in δ13C is caused mainly by variation in the concentration of CO2 within leaf tissue (ci), at least when atmospheric CO2 concentration (ca) is approximately constant (Farquhar et al., 1982, 1989). As CO2 concentration inside the leaf declines, discrimination against the heavier carbon isotope weakens and δ13C increases. Thus, the higher average δ13C of dry plants is expected because drought stress induces plants to close their stomata. This reduces ci and increases δ13C. Of course, closed stomata should also slow growth, at least insofar as it is limited by photosynthesis. In this experiment, the average number of flowers per plant in the dry treatment was only 60% that of plants in the wet treatment at harvest (F1,388 = 85.9, P < 0.001).
Thinking about what leaf CO2 concentration actually does mean, we suggest that the lower water use efficiency of small-flowered plants is consistent with the Galen hypothesis: δ13C is substantially elevated by drought in medium- and large-flowered plants, but not so much in small-flowered plants (Fig. 1). If small-flowered plants lose less water through corolla tissue as stipulated by Galen’s model, they may be able to have higher stomatal conductance through their leaf tissues. The latter should in turn allow greater photosynthesis and potentially greater growth under drought conditions. In other words, if small-flowered plants are more efficient in one respect (less loss through flowers), they could afford to be less efficient in another (greater loss through leaves).
Figure 1 demonstrates an association between flower size and δ13C, but not the proximate cause of that association. So it is premature to conclude that the Galen model explains our results. For example, flower size is also negatively related to development rate. Age at first flowering is lowest for small- and highest for large-flowered plants. In addition, flower size is positively related to vegetative size at the time of flowering (see Table 2 of Kelly, 2008). Thus vegetative correlates of flower size variation can provide an alternative explanation for Fig. 1: small-flowered plants may differ from larger-flowered plants in water use efficiency simply because the former also have less leaf area, and differences in leaf area are directly responsible for the differing responses to drought.
Instantaneous measurements of stomatal conductance, rates of photosynthesis and transpiration, and water potentials are necessary to isolate the physiological basis for the differential response of flower size genotypes. In fact, we have repeatedly attempted to collect these measurements using a Li-cor 6400 portable photosynthetic system (Lincoln, NB), but have failed due to technical difficulties. Annual M. guttatus is a small plant. At Iron Mountain, plants are typically less than 10 cm in height and have very small leaves with essentially no petioles. Very little gas exchange occurs in the small amount of leaf area that can be inserted into the standard cuvette of the Li-cor 6400 system. A second issue is the sufficiency of leaf-level measurements. Cells in the calyx might contribute as much to photosynthesis as do the leaves when plants reach flowering. A direct test of alternative proximate hypotheses will require the development of an experimental device/system suitable for whole-plant physiology of Mimulus (e.g. Tonsor and Scheiner, 2007).
Despite our uncertainty about mechanism, our results clearly demonstrate a genetic correlation between floral and physiological traits. Understanding the proximate causes for a genetic correlation is not essential for predicting immediate response to selection (Lande and Arnold, 1983), and for this reason, simply estimating these correlations (or covariances) is a primary objective in evolutionary genetics. Over longer time-scales, however, genetic correlations will themselves evolve. The nature and extent of modifications to genetic correlations are likely to depend on their physiological, developmental, and even ecological bases. The latter is clearly relevant here as the incidence and severity of drought is an ecological variable.
The genetic correlation between flower size and water loss might be easily modified if it just reflects overall size variation – that is, plants with greater total leaf area also have larger flowers. This only requires that there be variation in flower size given total vegetative size. In fact, we have already shown that selection can alter the allometric relationship between these two traits (see Figure 6 of Kelly, 2008). In contrast, modification of the genetic correlation may be more difficult if water loss occurs directly through the corolla. Mimulus corollas lack stomata and other leaf features that can regulate water loss. This example illustrates that some types of evolutionary constraint (genetic correlations) may be easier to overcome than others.
ACKNOWLEDGEMENTS
We thank J. Ward, T. Marriage, J. Mojica, S. Bodbyl, and C. Martin for comments on previous drafts of the manuscript. We acknowledge support from NSF grant DEB-0543052 and NIH grant GM073990.
REFERENCES
- Arnold S. Constraints on phenotypic evolution. Am. Nat. 1992;140:S85–S107. doi: 10.1086/285398. [DOI] [PubMed] [Google Scholar]
- Carroll AB, Pallardy SG, Galen C. Drought stress, plant water status, and floral trait expression in fireweed, Epilobium angustifolium (Onagraceae) Am. J. Bot. 2001;88:438–446. [PubMed] [Google Scholar]
- Ehleringer JR. Carbon and water relations in desert plants: an isotopic perspective. In: Ehleringer JR, Hall AE, Farquhar GD, editors. Stable Isotopes and Plant Carbon–Water Relations. San Diego, CA: Academic Press; 1993. pp. 155–172. [Google Scholar]
- Faegri K, Van der Pijl L. The Principles of Pollination Ecology. Oxford: Pergamon Press; 1979. [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. London: Prentice-Hall; 1996. [Google Scholar]
- Farquhar GD, O’Leary MH, Berry JA. On the relationship between carbon isotope discrimination and the intracellular carbon dioxide concentration in leaves. Aust. J. Plant Physiol. 1982;9:121–137. [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubrick KT. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Molec. Biol. 1989;40:503–537. [Google Scholar]
- Galen C. High and dry: drought stress, sex-allocation trade-offs, and selection on flower size in the alpine wildflower Polemonium viscosum (Polemoniaceae) Am. Nat. 2000;156:72–83. doi: 10.1086/303373. [DOI] [PubMed] [Google Scholar]
- Galen C, Sherry RA, Carroll AB. Are flowers physiological sinks or faucets? Costs and correlates of water use by flowers of Polemonium viscosum. Oecologia. 1999;118:461–470. doi: 10.1007/s004420050749. [DOI] [PubMed] [Google Scholar]
- Johnson SD. Pollinator-driven speciation in plants. In: Harder L, Barrett C, editors. Ecology and Evolution of Flowers. Oxford: Oxford University Press; 2006. pp. 295–309. [Google Scholar]
- Kelly JK. Testing the rare alleles model of quantitative variation by artificial selection. Genetica. 2008;132:187–198. doi: 10.1007/s10709-007-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JK, Arathi HS. Inbreeding and the genetic variance of floral traits in Mimulus guttatus. Heredity. 2003;90:77–83. doi: 10.1038/sj.hdy.6800181. [DOI] [PubMed] [Google Scholar]
- Lande R, Arnold S. The measurement of selection on correlated characters. Evolution. 1983;37:1210–1226. doi: 10.1111/j.1558-5646.1983.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Martin N. Flower size preferences of the honeybee (Apis mellifera) foraging on Mimulus guttatus (Scrophulariaceae) Evol. Ecol. Res. 2004;6:777–782. [Google Scholar]
- Ornduff R. Reproductive biology in relation to systematics. Taxon. 1969;18:121–133. [Google Scholar]
- Tonsor SJ, Scheiner SM. Plastic trait integration across a CO2 gradient in Arabidopsis thaliana. Am. Nat. 2007;169:E119–E140. doi: 10.1086/513493. [DOI] [PubMed] [Google Scholar]
- Willis JH. Partial self-fertilization and inbreeding depression in two populations of Mimulus guttatus. Heredity. 1993;71:145–154. [Google Scholar]
- Willis JH. Measures of phenotypic selection are biased by partial inbreeding. Evolution. 1996;50:1501–1511. doi: 10.1111/j.1558-5646.1996.tb03923.x. [DOI] [PubMed] [Google Scholar]