Introduction
Acute phosphate nephropathy (APN) is an underrecognized cause of both acute and chronic renal failure.1 Individuals with decreased renal function who are exposed to high doses of phosphorous are susceptible to developing APN.2 The risk for APN is increased in patients with underlying chronic kidney disease, older age, and female sex and in patients taking angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, diuretics, or nonsteroidal anti-inflammatory drugs (NSAIDs).2 APN has been reported to occur after exposure to sodium-phosphate (NaP) bowel-cleansing solutions. Clinically, some patients may present acutely with severe elevations of serum phosphorous and acute kidney injury (AKI); however, the injury may take weeks after exposure to occur, or the finding of an elevated creatinine level may be discovered incidentally weeks or months after the ingestion of these bowel preparations.3 Therefore, a careful history is important for making this diagnosis. Renal biopsy demonstrates deposition of calcium and phosphorous with damage in the tubulointerstitium.4 Patients may have variable outcomes, with some having no recovery of renal function and others recovering some function. There is no particular intervention that can be instituted once the nephropathy occurs. Avoidance of NaP-based bowel-cleansing solutions, particularly by high-risk individuals, is key to preventing APN.
Case Example
Your patient calls to inquire about taking a bowel preparation called Visicol. The patient is due to have her routine screening colonoscopy done and was instructed to take Colyte beforehand, but she states that many of her friends have had difficulties with Colyte because of its taste and that she had heard that Visicol was much easier to take. She is concerned that she will not be able to tolerate taking Colyte. The patient is a Caucasian woman, age 51 years, with hypertension, hypercholesterolemia and type 2 diabetes mellitus. Results of her most recent laboratory test two months earlier reveal a serum creatinine level of 1.4 mg/dL. Her estimated glomerular filtration rate (GFR), based on the abbreviated modifications of diet in renal disease formula, is 42 mL/min/m2. Her medications include lisinopril, hydrochlorothiazide, and glipizide. At her most recent office visit, her blood pressure, diabetes, and cholesterol were noted to be in good control. She occasionally takes over-the-counter ibuprofen for headaches.
Discussion
Protocol Choices
There are many protocols used in preparing patients for colonoscopy. Stimulant laxatives had been used in the past but are not commonly used today because of their potential adverse effects, such as upset stomach, vomiting, irritation, stomach cramping, and rectal bleeding. Hyperosmotic laxatives such as mannitol or sorbitol have also been used in the past. There is a theoretical risk of explosion with these preparations because of the hydrogen gas produced by the fermentation of the unabsorbed carbohydrates in the bowel and are thus used less routinely.5
Currently, the method most commonly used is a balanced-electrolyte solution, such as the polyethylene glycol (PEG) in Colyte.5 The main complaint from patients in using this preparation has been its taste and the large volume of the solution used in the preparation for their procedures. There have been variations on this preparation in an attempt to improve the taste and thus improve patient compliance with these protocols. They remain the most commonly used preparation for endoscopic bowel procedures.5
The other type of bowel preparation includes saline laxatives. They contain magnesium or phosphate ions, which are hyperosmotic, causing water to shift into the bowel lumen and stimulating peristalsis.5 The most commonly used formulation in this category is Fleet Phospho Soda, which is in a liquid form compared to Visicol which is a tablet formulation. Some studies have shown saline laxatives may be superior compared to balanced-electrolyte solutions such as PEG because patients comply more with saline laxatives and incur less nausea, vomiting, and bloating.5 The protocol entails taking 3 tablets with 8 ounces of clear liquid every 15 minutes, for a total of 20 tablets 12 hours before the procedure. This is repeated at three and five hours before the procedure.5
The main concern with the use of laxatives containing sodium phosphate is an acute increase in serum phosphate levels, which may result in an acute calcium phosphate deposition, followed by AKI.6 Other possible acute problems include volume depletion, hypocalcemia, and hypernatremia. The increase in serum phosphate levels is clinically insignificant in most patients, but patients with preexisting renal disease may be at greater risk for its consequences.7 Patients at risk are those with GFR < 50 mL/min. These protocols can also cause significant electrolyte abnormalities and may lead to hypocalcemia, hypernatremia, and hypomagnesemia.1 They also have been associated with seizures and alteration in colonic mucosa that can mimic changes seen with NSAIDs or inflammatory bowel disease.
Renal Manifestations
The type of renal injury caused by these agents has been termed acute phosphate nephropathy (APN). Although patients at highest risk remain those with preexisting renal disease, it is important to realize that APN can occur in patients with normal renal function as well.
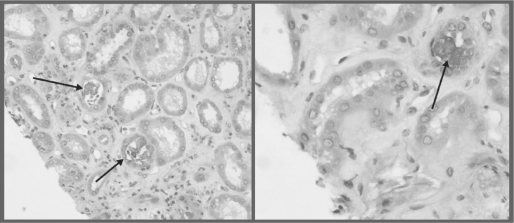
The histology in APN reveals diffuse renal deposition of calcium phosphate precipitants along with tubulointerstitial damage4 (Figure 1). The deposition occurs in the tubular lumen, interstitium, or both. Clinically, patients may present with low-grade proteinuria (usually <1g/d). Elevated serum creatinine may be seen immediately after bowel prep, along with acute elevations in serum phosphate levels. However, some patients may develop AKI weeks after exposure, thus making the diagnosis more difficult and likely underrecognized.
Figure 1.
Some tubules are irregularly flattened with loss of brush border staining. Calcifications are identified in the lumina of some tubules and focally in the interstitium.
The outcome of APN is highly variable and depends mostly on baseline renal function. There has been speculation that genetic factors may also play a role in susceptibility to APN. The disorder may be more common in women, Caucasians, patients with diabetes, and individuals age 55 and greater.1 In May 2006, the US Food and Drug Administration (FDA) issued an alert cautioning against using oral NaP in high-risk patients.8 Clinicians should weigh the risks of using NaP bowel preparation agents against the benefits in the following subgroup of high-risk patients: those with hepatic or renal insufficiency, patients with congestive heart failure, patients older than 55 years, patients with volume depletion or hypercalcemia, and patients taking drugs that affect renal perfusion, such as NSAIDs, angiotensin-converting enzyme inhibitors, angiotensin-receptor blockers, and diuretics.8 Withholding these medications immediately before and after the procedure should be considered to minimize risk of APN.
The outcome of APN is highly variable and depends mostly on baseline renal function.
In December 2008, the FDA issued another alert regarding the use of oral NaP products. It required that the manufacturers of Visicol and OsmoPrep add a boxed warning to the labeling of their products and recommended against the use of over-the-counter oral NaP products (such as Fleet Phospho Soda) for bowel cleansing in preparation for endoscopic procedures unless the products are directly prescribed by a health care professional.9
Case Resolution
The patient under consideration here has many potential risk factors for APN. Her primary risk factors are age, chronic kidney disease, and sex.10 Furthermore, she is taking medications that may increase her risk of APN: specifically ibuprofen and lisinopril/hydrochlorothiazide. For these reasons, she should be advised against taking phosphate-containing bowel preparations and be counseled to use a standard PEG solution. If she refuses, an informed decision should be made regarding phosphate-containing solutions after discussing the risks with the patient. The use of NSAIDs, diuretics, and angiotensin-converting enzymes should be suspended temporarily and other medications should be substituted for controlling blood pressure as needed to minimize risk.
APN is a potentially irreversible consequence of phosphate-containing bowel preparations. The prevalence of APN is likely to be higher than what is reported because many of these patients are not diagnosed or recognized. Given the fact that there are safer alternatives to phosphorous-containing formulations, using phosphorous formulations does not warrant the risk of APN in high-risk patients, particularly in patients with chronic kidney disease.
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Acknowledgments
Katharine O'Moore-Klopf, ELS, of KOK Edit provided editorial assistance.
Filtration
The amount of creatinine present in the urine of man after ingestion of this substance is so large that it requires a filtration of up to 200 cc per min to explain it … The result is taken to be in favour of the filtration theory.
— Studies of Kidney Function, 1926, Poul Brandt Rehberg, 1895–1989, Danish physiologist and researcher
References
- 1.Markowitz GS, Stoke MB, Radhakrishnan J, D'Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005 Nov;16(11):3389–96. doi: 10.1681/ASN.2005050496. [DOI] [PubMed] [Google Scholar]
- 2.Makkar R, Shen B. What are the caveats to using sodium phosphate agents for bowel preparation? Cleve Clin J Med. 2008 Mar;75(3):173–6. doi: 10.3949/ccjm.75.3.173. [DOI] [PubMed] [Google Scholar]
- 3.Khurana A, McLean L, Atkinson S, Foulks CJ. The effects of oral sodium phosphate drug products on renal function in adults undergoing bowel endoscopy. Arch Intern Med. 2008 Mar 24;168(6):593–7. doi: 10.1001/archinte.168.6.593. [DOI] [PubMed] [Google Scholar]
- 4.Aasebø W, Scott H, Ganss R. Kidney biopsies taken before and after oral sodium phosphate bowel cleansing. Nephrol Dial Transplant. 2007 Mar;22(3):920–2. doi: 10.1093/ndt/gfl694. [DOI] [PubMed] [Google Scholar]
- 5.DiPalma JA, Brady CE, 3rd, Stewart DL, et al. Comparison of colon cleansing methods in preparation for colonoscopy. Gastroenterology. 1984 May;86(5 Pt 1):856–60. [PubMed] [Google Scholar]
- 6.Markowitz G, Nasr SH, Klein P, et al. Renal failure due to acute nephrocalcinosis following oral sodium phosphate bowel cleansing. Hum Pathol. 2004 Jun;35(6):675–84. doi: 10.1016/j.humpath.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Russmann S, Lamerato L, Motsko SP, Pezzullo JC, Faber MD, Jones JK. Risk of further decline in renal function after the use of oral sodium phosphate or polyethylene glycol in patients with a preexisting glomerular filtration rate below 60 mL/min. Am J Gastroenterol. 2008 Nov;103(11):2707–16. doi: 10.1111/j.1572-0241.2008.02201.x. [DOI] [PubMed] [Google Scholar]
- 8.Center of Drug Evaluation Research. FDA Alert: Oral sodium phosphate (OSP) products for bowel cleansing [monograph on the Internet]. Silver Spring (MD): US Food and DrugAdministration. 2006 Mar. updated 2009 Apr 30 [cited2009 May 28]. Available from: www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm085308.htm.
- 9.Center of Drug Evaluation Research. FDA Alert: Oral sodium phosphate (OSP) products for bowel cleansing (marketed as Visicol and OsmoPrep), and oral sodium phosphate products available without a prescription [monograph on the Internet]. Silver Spring (MD): US Food and Drug Administration; 2008 Dec 11 [cited 2009 May 28]. Available from: www.fda.gov/cder/drug/infopage/OSP_solution/default.htm.
- 10.Gumurdulu Y, Serin E, Ozer B, Gokcel A, Boyacioglu S. Age as a predictor of hyperphosphatemia after oral phosphosoda administration for colon preparation. J Gastroenterol Hepatol. 2004 Jan;19(1):68–72. doi: 10.1111/j.1440-1746.2004.03253.x. [DOI] [PubMed] [Google Scholar]