Abstract
West Nile virus (WNV) is one of the leading causes of insect-borne encephalitis and acute flaccid paralysis in the US. Acute flaccid paralysis is a potentially serious illness, which manifests itself as a Guillain-Barré-like syndrome with generalized weakness and shortness of breath. We report a case involving a patient who presented with acute flaccid paralysis due to WNV infection and was successfully treated with intravenous immunoglobulin from Israeli donors.
Introduction
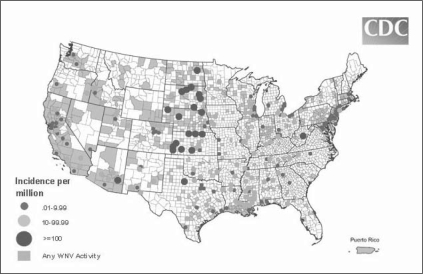
West Nile virus (WNV) is one of the leading causes of insect-borne encephalitis in the US. WNV first outbreak happened in New York City during the sweltering summer of 1999. Since then, seasonal outbursts of febrile illness and ne urologic disease caused by WNV have struck across the US. WNV is classified as a member of Flaviviridae, the same family that includes hepatitis C, dengue, and yellow fever. Mosquitoes act as the transmitting vector for the virus, and infected birds, especially corvids (crows and jays), are the reservoir of the disease. They are responsible for spreading the disease across the country by traveling along migration routes. WNV tends to cause severe mortality in birds when it reaches a new area. Humans appear to be incidental hosts; that is, humans are not necessary to complete the disease cycle. South Dakota consistently has one of the highest rates of WNV neuroinvasive disease in the US, as do North Dakota, Nebraska, Kansas, Texas, Arizona, and West Virginia (Figure 1).1
Figure 1.
Incidence of West Nile virus human neuroinvasive disease in 2008. The map reflects surveillance findings occurring January 1, 2008 through December 31, 2008.1
Reprinted from: Final 2008 West Nile Virus human neuroinvasive disease incidence in the United States [map on the Internet]. Atlanta, GA: Centers for Disease Control and Prevention: Division of Vector-Borne Infectious Diseases; last modified 2009 Apr 10 [cited 2009 Jun 1]. Available from: www.cdc.gov/ncidod/dvbid/westnile/mapsincidence/survScontrol08IncidMaps.htm.
In 2007, a total of 3630 cases were reported from 775 of the 3142 counties in the US.2 Of these cases, 65% were West Nile fever, 34% were WNV neuroinvasive disease, and 1% were unspecified clinical syndromes. About 1 of 150 people infected with WNV develops severe illness. In the US, most deaths from the disease have occurred in elderly patients. WNV infection can cause severe, potentially fatal neurologic illnesses, including encephalitis, meningitis, and acute flaccid paralysis (AFP). AFP has been attributed to anterior myelitis.3 A 2002 Morbidity and Mortality Weekly Report from the Centers for Disease Control and Prevention described six cases of WNV-associated AFP in which clinical and electrophysiologic findings suggested a pathologic process involving anterior horn cells and motor axons similar to that seen in acute poliomyelitis.4 We report the case of a patient with AFP secondary to WNV that was successfully treated with intravenous immunoglobulin (IVIG) at the recommendation of an infectious diseases specialist.
On electromyography … patients often exhibit nerve-conduction velocities consistent with both axonal and demyelinating lesions.9
Case Presentation
A white man, age 55 years, with a medical history of diabetes mellitus and hypothyroidism presented in August 2005 to Sioux Valley University Medical Center in Sioux Falls, South Dakota, complaining of progressive muscle weakness and numbness in all four extremities for the preceding three days. The patient's cognition was not impaired, and he responded appropriately to questions. Full neurologic examination revealed muscle weakness (Table 1) and hyporeflexia. Laboratory studies revealed a total leukocyte count of 9.2 × 103/µL; neutrophils, 77%; hemoglobin, 13.5 g/µL; and a platelet count, 208 × 103/pL. Findings from renal and hepatic panels were unremarkable. Lumbar puncture revealed a leukocyte count of 3 leukocytes/mm3 (16% neutrophils, 45% lymphocytes, and 37% monocytes), a slightly elevated glucose level (133 mg/µL), and a normal protein level (47 mg/dL). Cerebrospinal fluid Gram stain and cultures were negative. Magnetic resonance images of the spine showed some degenerative changes from C4 to C6, with mild impingement of the cord that did not explain the quickly developing muscle weakness. Findings on both computed tomography and magnetic resonance imaging scans of the brain were negative.
Table 1.
Muscle strength and reflexes before and after IVIG therapy
| D1 | D2 | D3a | D4 | D5 | D8b | D9 | D10 | D11 | D12 | D28 | ||
| RUE | MS | 4/5 | 2/5 | 1/5 | 1/5 | 0/5 | 0/5 | 1/5 | 1/5 | 3/5 | 3/5 | 4/5 |
| REF | +1 | +1 | 0 | 0 | 0 | 0 | 0 | + 1 | +1 | +1 | +2 | |
| LUE | MS | 3/5 | 3/5 | 1/5 | 1/5 | 0/5 | 0/5 | 1/5 | 1/5 | 3/5 | 3/5 | 4/5 |
| REF | +1 | +1 | 0 | 0 | 0 | 0 | 0 | + 1 | +1 | +1 | +2 | |
| RLE | MS | 4/5 | 3/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 2/5 | 4/5 | 4/5 | 4/5 |
| REF | +1 | 0 | 0 | 0 | 0 | 0 | 0 | + 1 | +1 | +1 | +1 | |
| PLA | No | No | No | No | No | No | No | No | WNL | WNL | WNL | |
| LLE | MS | 4/5 | 2/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 2/5 | 4/5 | 4/5 | 4/5 |
| REF | +1 | 0 | 0 | 0 | 0 | 0 | 0 | + 1 | +1 | +1 | +1 | |
| PLA | No | No | No | No | No | No | No | No | WNL | WNL | WNL |
aPlasmapheresis was started on day 3 after admission and stopped on day 6.
bIVIG was started on day 8 after admission.
IVIG = intravenous immunoglobulin; LLE = left lower extremity; LUE = left upper extremity; RLE = right lower extremity; RUE = right upper extremity; MS = muscle strength; PLA = plantar reflex; REF = tendon reflexes; WNL = within normal limits.
The weakness continued to progress until the patient developed difficulty swallowing and shortness of breath on the third day. The patient was transferred to the intensive care unit and placed on ventilator. Neurologic examination revealed worsening muscle strength and absence of reflexes in all four extremities. Guillain-Barré syndrome was suspected, given the progressive nature of the patient's muscle weakness, dysphagia, and hypoxia. Plasmapheresis and dexamethasone were administered. Nerve-conduction studies revealed severe, diffuse, sensorimotor mixed polyneuropathy that was predominantly axonal in nature. Despite plasmapheresis and corticosteroid therapy, the patient's condition continued to deteriorate, with no improvement in muscle strength. By the sixth day, immunoglobulin M antibodies for WNV were detected in the serum. AFP secondary to WNV infection was considered; corticosteroids and plasmapheresis were stopped by the infectious diseases specialist who instead recommended a trial of IVIG therapy based on reports of positive results with it.5–7 On day 8, IVIG with high titers of antibodies to WNV (Omr-IgG-am; OMRIX Biopharmaceuticals Ltd, Israel) was started at a dosage of 0.4 g/kg per day for seven days. Dramatic improvement in muscle strength ensued during the days after the administration of IVIG (Table 1). The patient was weaned off the ventilator on day 11. On day 28 the patient was transferred to inpatient rehabilitation.
Discussion
WNV is a potentially serious illness. It can present itself clinically in a way indistinguishable from Guillain-Barré with generalized weakness and shortness of breath.8 On electromyography, however, patients often exhibit nerve-conduction velocities consistent with both axonal and demyelinating lesions.9 Axonal changes are usually more prominent, findings unusual for Guillain-Barré syndrome. Our patient's nerve-conduction studies revealed severe, diffuse, mixed polyneuropathy that was predominantly axonal in nature. Moreover, it should be noted that in differentiating our patient's condition from Guillain-Barré, we found the cerebrospinal protein level to be normal.
Treatment for WNV infection is mainly supportive. Ribavirin in high doses and interferon-α-2b were shown to inhibit WNV replication in vitro, but inconsistent results have been shown in vivo.10,11 The success of IVIG in other viral diseases made it the best new option to consider in our case.12 Animal studies show that IVIG provides full protection and recovery from WNV infection by antagonizing the WNV glycoproteinous envelope that mediates virus-cell contact.13 Being the most immunologically provocative structural protein, the viral envelope triggers virus-neutralizing antibodies that prevent the viral infection of the host's cells by targeting the epitopes included in the glycoproteinous envelope.
There are few reports in the literature about the use of IVIG in cases of WNV encephalitis.5–7 IVIG contains a high titer of anti-WNV antibodies (1:1600). Recently, OMRIX Biopharmaceuticals of Israel developed a strategy for the selection of plasma units from the 10% fraction of blood donors containing WNV antibodies. Positive units were processed into the pharmaceutical grade WNV IVIG (WNIG). WNIG is at least 5- to 10-fold more potent than regular Israeli IVIG.14
Remarkable improvement resulted from the use of IVIG despite its late administration (eight days after admission). Intrathecal administration of immunoglobulin has been reported in other infections with no complications and should probably be considered in patients with WNV as well.15 Currently, a multicenter phase I/II clinical study is being conducted by OMRIX Biopharmaceuticals and the National Institutes of Health with WNIG, which has been given the “orphan drug” status by the Food and Drug Administration. More research is needed to study the optimal timing, dosage, and route of administration of this immunoglobulin in serious cases of WNV infection.
Immunoglobulin may be the first line of treatment in any patient with AFP suspected of having WNV infection, which should be differentiated from Guillain-Barré syndrome with the help of nerveconduction tests, lumbar puncture and serological studies.
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Acknowledgments
Katharine O'Moore-Klopf, ELS, of KOK Edit provided editorial assistance.
Primary Infection
What seems to be clear to me is that after the primary infection most of the cells die indirectly, but at the later stage, when the viral load is very high, the virus kills a lot of cells directly.
— Luc Montagnier, b 1932, French virologist, 2008 Nobel Prize recipient in Physiology or Medicine for his co-discovery of Human Immunodeficiency Virus
References
- 1. Final 2008 West Nile Virus human neuroinvasive disease incidence in the United States [map on the Internet]. Atlanta, GA: Centers for Disease Control and Prevention: Division of Vector-Borne Infectious Diseases; last modified 2009 Apr 10 [cited 2009 Jun 1]. Available from: www.cdc.gov/ncidod/dvbid/westnile/mapsincidence/surv&control08IncidMaps.htm.
- 2.Centers for Disease Control and Prevention (CDC) West Nile Virus Activity—United States, 2007. MMWR Morb Mortal Wkly Rep. 2008 Jul 4;57(26):720–3. [PubMed] [Google Scholar]
- 3.Ohry A, Karpin H, Yoeli D, Lazari A, Lerman Y. West Nile virus myelitis. Spinal Cord. 2001 Dec;39(12):662–3. doi: 10.1038/sj.sc.3101228. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Acute flaccid paralysis syndrome associated with West Nile Virus infection—Mississippi and Louisiana, July-August 2002. MMWR Morb Mortal WklyRep. 2002 Sep 20;51(37):825–8. [PubMed] [Google Scholar]
- 5.Hamdan A, Green P, Mendelson E, Kramer MR, Pitlik S, Weinberger M. Possible benefit of intravenous immunoglobulin therapy in a lung transplant recipient with West Nile virus encephalitis. Transpl Infect Dis. 2002 Sep;4(3):160–2. doi: 10.1034/j.1399-3062.2002.01014.x. [DOI] [PubMed] [Google Scholar]
- 6.Haley M, Retter AS, Fowler D, Gea-Banacloche J, O'Grady NP. The role for intravenous immunoglobulin in the treatment of West Nile virus encephalitis. Clin InfectDis. 2003 Sep 15;37(6):e88–90. doi: 10.1086/377172. [DOI] [PubMed] [Google Scholar]
- 7.Shimoni Z, Niven MJ, Pitlick S, Bulvik S. Treatment of West Nile virus encephalitis with intravenous immunoglobulin. Emerg Infect Dis. 2001 Jul-Aug;7(4):759. doi: 10.3201/eid0704.010432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed S, Libman R, Wesson K, Ahmed F, Einberg K. Guillain-Barré syndrome: An unusual presentation of West Nile virus infection. Neurology. 2000 Jul 12;55(1):144–6. doi: 10.1212/wnl.55.1.144. [DOI] [PubMed] [Google Scholar]
- 9.Weiss D, Carr D, Kellachan J, et al. West Nile Virus Outbreak Response Working Group. Clinical findings of West Nile virus infection in hospitalized patients, New York and New Jersey, 2000. Emerg Infect Dis. 2001 Jul-Aug;7(4):654–8. doi: 10.3201/eid0704.010409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson JF, Rahal JJ. Efficacy of interferon alpha-2b and ribavirin against West Nile virus in vitro. Emerg Infect Dis. 2002 Jan;8(1):107–8. doi: 10.3201/eid0801.010252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan-Tack KM, Forrest G. Failure of interferon alpha-2b in a patient with West Nile virus meningoencephalitis and acute flaccid paralysis. Scand J Infect Dis. 2005;37(11-12):944–6. doi: 10.1080/00365540500262690. [DOI] [PubMed] [Google Scholar]
- 12.Deresinski SC. Hyperimmune products in the prevention and therapy of infectious disease: a report of a hyperimmune products expert advisory panel. BioDrugs. 2000 Sep;14(3):147–58. doi: 10.2165/00063030-200014030-00002. [DOI] [PubMed] [Google Scholar]
- 13.Ben-Nathan D, Lustig S, Tam G, Robinzon S, Segal S, Rager-Zisman B. Prophylactic and therapeutic efficacy of human intravenous immuno- globulin in treating West Nile virus infection in mice. J Infect Dis. 2003 Jul 1;188(1):5–12. doi: 10.1086/376870. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Nathan D, Gershoni-Yahalom O, Samina I, et al. Using high titer West Nile intravenous immunoglobulin from selected Israeli donors for treatment of West Nile virus infection. BMC Infect Dis 2009 Feb. 17;9:18. doi: 10.1186/1471-2334-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geeta MG, Krishnakumar P, Mathews L. Intrathecal tetanus immunoglobulins in the managementof tetanus. Indian J Pediatr. 2007 Jan;74(1):43–5. doi: 10.1007/s12098-007-0025-y. [DOI] [PubMed] [Google Scholar]