Abstract
Context: Pulmonary computed tomography angiography (CTA) and the Wells criteria both have interobserver variability in the assessment of pulmonary embolism (PE). Quantitative D-dimer assay findings have been shown to have a high negative predictive value in patients with low pretest probability of PE.
Objective: Evaluate roles for clinical probability and CTA in Emergency Department (ED) patients suspected of acute PE but having a low serum D-dimer level.
Design: Prospective observational study of ED patients with possible PE who underwent pulmonary CTA and had D-dimer levels ≤1.0 μg/mL.
Main Outcome: Clinical probability of PE determined by ED physicians using standard published criteria; pulmonary CTAs read by initial and study radiologists kept unaware of D-dimer results.
Results: In 16 months, 744 patients underwent pulmonary CTA, with 347 study participants who had a D-dimer level ≤ 1.0 μg/mL. In one participant, CTA showed a PE that was agreed on by both the initial and study radiologists. In six participants, the initial findings were reported as positive for PE but were not interpreted as positive by the study radiologist. In none of these participants was PE diagnosed on the basis of clinical probability, of findings on ancillary studies and three-month follow-up examination, or by another radiologist, unaware of findings, acting as a tiebreaker.
Conclusion: Pulmonary CTA findings positive for acute embolism should be viewed with caution, especially if the suspected PE is in a distal segmental or subsegmental artery in a patient with a serum D-dimer level of ≤1.0 μg/mL. Furthermore, the Wells criteria may be of limited additional value in this group of patients with low D-dimer levels because most will have low or intermediate clinical probability of PE.
Introduction
Pulmonary embolism (PE) is a common cause of mortality, with an overall incidence rate of >1 person per 1000 per year.1,2 Although pulmonary computed tomography angiography (CTA) has become the community standard for the evaluation of acute PE, it is subject to interobserver variability in the interpretation of findings, which increases when dealing with the determination of segmental or more distal PE.3,4 In addition, studies have also shown that pulmonary CTA is likely to be overused and not as highly sensitive or specific as once believed.5,6 This may result in additional exposure to radiation, injection of a potentially nephrotoxic agent, and costs to our patients.7–9 In this light, a screening process less reliant on pulmonary CTA for the diagnosis of PE in patients evaluated in the Emergency Department (ED) would be beneficial.
The Wells criteria (guideline questions for determining likelihood of PE) have been shown to be a reasonable clinical-assessment tool for acute PE.10–12 Clinical probability assessment of patients using the Wells criteria has been shown to classify patients’ risk of PE with reasonable accuracy.12,13 However, interpretation of the criteria also has interobserver variability.13
Several studies have investigated the clinical utility of the quantitative D-dimer assay in the evaluation and exclusion of PE12,14,15 and have found the assay to have a high negative predictive value in patients with low pretest probability of PE. Studies have indicated that negative findings on a quantitative D-dimer assay may preclude the necessity for pulmonary CTA in ruling out PE in an acute-care setting.13,16 Furthermore, a prospective study of patients seen in an acute-care setting for possible PE revealed that even a low but positive serum D-dimer level precludes the need to undergo pulmonary CTA.17
In light of this information, we queried whether the clinical probability estimate obtained with the use of the Wells criteria or a low but positive serum D-dimer level would increase the accuracy of the diagnosis of PE. We hypothesized that in cases of patients with a low but not necessarily negative level of serum D-dimer, there is limited utility for pulmonary CTA, irrespective of clinical assessment using the Wells criteria.
Methods
This was a prospective, observational study of all patients presenting to the ED of our facility with suspected PE who underwent pulmonary CTA and had a D-dimer level of ≤1.0 µg/mL. The study ran from February 2005 to June 2006 in the ED of a health maintenance organization (HMO) patient population. The protocol was approved by the hospital institutional review board with a waiver of informed consent.
Before study initiation, ED physicians were requested to obtain a serum D-dimer level for all patients for whom they ordered pulmonary CTA for PE. We had requested that during the study period, the ED physicians not consider the results of the D-dimer assay in their decision to order a pulmonary CTA. In addition, ED physicians were requested to fill out a worksheet detailing the clinical probability of PE using standard published Wells criteria for PE11,12 without knowledge of the pulmonary CTA and serum D-dimer assay results. According to this clinical-assessment model, the ED physician assigned points for the following: clinical signs and symptoms of deep vein thrombosis (DVT), 3.0 patients; heart rate >100 beats/min, 1.5 patients; immobilization or surgery in the preceding four weeks, 1.5 patients; previously diagnosed DVT or PE, 1.5 patients; hemoptysis, 1.0 patient; malignancy, 1.0 patient; and an alternate diagnosis that is less likely than PE, 3.0 patients. The weighted values for the Wells criteria were summated and trichotomized into low (<2), moderate (2–6), and high (>6) risk for PE. For those study participants for whom the worksheet had not been completed, the ED physician notes for the patient encounter and the patient's electronic medical record were reviewed and the data for the criteria were extracted. This was performed by a single research assistant and one of the study physicians together to reduce interobserver variability. Patients for whom the physician notes did not provide sufficient information to allow complete clinical assessment using the Wells criteria were excluded from the study.
The quantitative serum rapid D-dimer assays were performed using a latex agglutination technique (STA D-DI, Diagnostica Stago, Parsippany, NJ). The manufacturer reports that a serum D-dimer level <0.4 µg/mL fibrinogen equivalent units (FEUs) should be considered normal. However, for the purposes of our study, patients with a serum D-dimer level ≤1.0 µg/mL FEUs were included in the study. This cutoff level was based on the results of previous retrospective analyses16,17 and is also an easy number to remember.
Pulmonary CTA was done with a multislice computed tomography unit (GE Lightspeed QXi, Milwaukee, WI) with 1.25-mm collimation and a pitch of 1.5:1. Patients were injected with 120 mL of Omnipaque 300 (GE) at a rate of 3 mL/s.
During the study period, all pulmonary CTA studies ordered by the ED were reread by a single study radiologist who was unaware of the D-dimer assay results. The findings for the pulmonary studies were interpreted as positive, negative, or indeterminate. A positive finding resulted if a filling defect was present in one or more pulmonary arteries. A negative finding resulted if there was no filling defect and if there was normal enhancement of the pulmonary arteries. An indeterminate finding resulted if the pulmonary study findings could not be classified as positive or negative. The original radiology report for each pulmonary CTA study was also recorded as positive, negative, or indeterminate. Indeterminate study findings were considered if the report used such language as indeterminate, suboptimal visualization of the pulmonary arteries, or extensive imaging/motion artifact. In those cases in which PE was reported, the specific location of each PE was recorded. Interobserver variability between the initial clinical radiologist and the study radiologist was evaluated using the kappa statistic.
If there was a discrepancy between the original radiologist's interpretation and the study radiologist's interpretation in which one reader reported the study as positive for PE but other reader did not, a third radiologist was used as a tiebreaker. This radiologist was kept unaware of the interpretations of the first two radiologists and was also blinded to the D-dimer values.
Follow-up monitoring lasted a minimum of three months for all study participants to verify no new diagnosis of PE, lower-extremity venous thrombosis, or death from PE. The clinical monitoring was performed by a combination of telephone interview and electronic medical record (EMR) verification because all data for members of the HMO are available electronically.
Results
During the 16-month study period, a total of 744 patients from the ED underwent pulmonary CTA for suspected PE. Of these patients, 381 had a D-dimer value ≤1.0 µg/mL.
Clinical probability of PE could not be ascertained for 34 study participants who presented to the ED and received pulmonary CTA for assessment of PE. These participants were excluded from the study. Clinical parameters were ascertained for 347 participants. This was done through worksheets filled out by ED physicians (151 participants) and through EMRs (196 participants). There were 226 women and 121 men in the study cohort, with a mean age 58 ±18 years (range, 14–98 years). Using the Wells criteria for clinical probability of PE, 228 participants had a low clinical probability (score <2.0), 109 had moderate clinical probability (score 2–6, inclusive), and only 10 had high clinical probability (score >6). For the individual criteria, 33 participants had clinical signs and symptoms suggestive of DVT, 79 had an alternative diagnosis that was less likely than PE, 80 had a pulse rate >100 beats/min, 38 had immobilization or surgery in the preceding four weeks, 26 had a history of PE or DVT, 2 had hemoptysis, and 22 had recent or active malignancy (on treatment, treated in preceding six months, or palliative).
Table 1 shows the overall results of assessment against the Wells criteria, stratified according to D-dimer level: <0.4 µg/mL and 0.4 to 1.0 µg/mL. There was no significant difference in the clinical probability distribution of the PE between the two groups (χ2 test, p = 0.36).
Table 1.
Proportion of patients with low, moderate, and high Wells criteria scores for clinical probability of pulmonary embolism on the basis of D-dimer levels
| D-dimer level | Wells criteria | |||
| Low (<2) | Moderate (2–6) | High (>6) | Total | |
| <0.4 µg/mL | 34 | 20 | 3 | 57 |
| 0.4–1.0 µg/mL | 194 | 89 | 7 | 290 |
| Total | 228 | 109 | 10 | 347 |
According to the study radiologist's interpretation of the pulmonary CTAs, there were 43 studies with indeterminate findings, 303 with negative findings, and 1 study with positive findings. According to the initial clinical interpretation, there were 21 studies with indeterminate findings, 319 with negative findings, and 7 with positive findings.
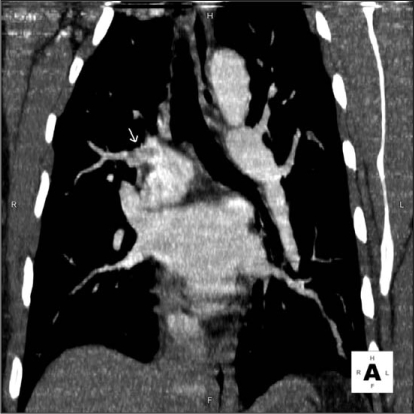
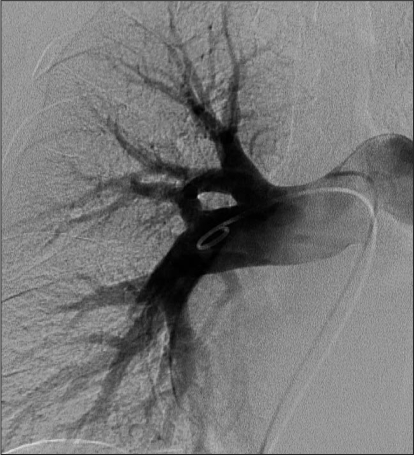
As shown in Table 2, there was a discrepancy in the CTA interpretation in 37 (10.7%) of the 347 studies (κ = 0.45). The study reader reported indeterminate findings for 43 pulmonary CTA studies versus 21 from the initial clinical reading. In addition, in 6 of 347 (1.7%) cases there was discrepancy between a positive finding on the initial clinical report versus a nonpositive finding on the other study interpretation. When presented to a tiebreaker radiologist, findings for three studies were considered indeterminate and findings for three were negative for PE. In four of six cases, the original radiology interpretation was made concerning a single embolus at a junction of a segmental and subsegmental branch, which was not reported by the study radiologist. In one patient, two emboli at junctions of segmental and subsegmental branches were reported. In the last patient, two emboli were reported in the proximal segmental branch pulmonary arteries (Figures 1 and 2). Table 3 details the findings in these six patients. None of these patients had a high clinical probability of PE according to the Wells criteria. Three patients underwent catheter pulmonary angiography within 24 hours of pulmonary CTA: two patients in whom the study radiologist believed that the study findings were indeterminate and one patient in whom the study radiologist believed that the findings were negative. All three pulmonary angiograms were interpreted as negative. These three patients had no clinical evidence of thromboembolism during the next three months. Regarding the other three patients, the study radiologist interpreted the study findings as negative in two and indeterminate in one. One of the patients was already taking warfarin for diagnosed atrial fibrillation at the time of the pulmonary CTA. No further diagnostic study or intervention was performed because the original pulmonary CTA report was of a small upper-lobe embolism and the primary care physician did not believe that additional therapy such as caval filtration was necessary. Despite the original reading of a small PE in one patient, the primary-care physician elected not to treat the patient and the patient did not have any other imaging studies. The final patient was treated with warfarin for six months. Neither patient had a report of thromboembolic disease at a three-month follow-up examination.
Table 2.
Discrepancies in initial clinical readings versus study readings of pulmonary computed tomography angiography studies
| Clinical reading | |||
| Study reading | Negative | Positive | Indeterminate |
| Negative | 294 | 3 | 6 |
| Positive | 0 | 1 | 0 |
| Indeterminate | 25 | 3 | 15 |
Figure 1.
Coronal image of the suspected embolus.
Figure 2.
Corresponding pulmonary angiogram, which shows no thrombus.
Table 3.
Clinical findings for six patients with discrepancies between clinical interpretation versus study interpretation
| Study participant | D-dimer (µg/mL) | Wells score | Study radiologist's findings | Findings on ancillary studies | Location of embolism |
| 48-year-old man | 0.61 | 2.5 | Indeterminate | Pulmonary angiography: negative | 1 LLL subsegmental |
| 70-year-old man (no treatment) | 0.73 | 0 | Indeterminate | None | 1 at LUL posterior segmental–subsegmental junction |
| 34-year-old woman | 0.4 | 3 | Negative | Pulmonary angiography and ultrasound of bilateral lower extremities: negative | 1 at RUL proximal segmental junction; 1 at LLL proximal segmental junction <2 mm |
| 71-year-old man (taking warfarin for atrial fibrillation) | 0.70 | 6 | Negative | None | 1 at LUL anterior segmental-subsegmental junction |
| 50-year-old woman (taking warfarin) | 0.49 | 0 | Negative | None | 1 LLL distal subsegmental; 1 at LLL segmental-subsegmental junction |
| 64-year-old man | 0.64 | 3 | Indeterminate | Pulmonary angiography: negative | 1 at LLL distal segmental-subsegmental junction |
LLL = left lower lobe; LUL = left upper lobe; RUL = right upper lobe.
… none of the 57 patients with a negative D-dimer level had positive findings on CTA, regardless of Wells criteria score.
Only one participant in this cohort of 347 with a D-dimer level of ≤1.0 µg/mL was noted on pulmonary CTA to have acute PE according to both the original radiology report and the study radiologist's interpretation. This patient was a man, age 50 years, who was examined because of mild dyspnea and sharp, stabbing chest pain. The attending ED physician believed that this patient presented none of the seven risk factors described in the Wells criteria for PE. Hence, his Wells score was 0 and the clinical probability for PE was low. His serum D-dimer level was 0.63 µg/mL. This patient's PE extended from the left main pulmonary artery through the lower-lobe pulmonary artery and along the entire length of the lateral segmental pulmonary artery, as well as extending cephalad into the proximal left upper lobe posterior segmental artery. The length of the embolus was approximately 5 cm.
Of the other 346 participants in the study who had a D-dimer level of ≤1.0 µg/mL and nonpositive pulmonary CTA findings, ten patients (2.9%) were lost to follow-up monitoring despite attempts to reach them by telephone and mail. According to their medical records, two of these patients were seen for follow-up examinations approximately two months after their pulmonary CTA, at which time neither was noted to have evidence of thromboembolic disease. For the remaining 336 patients, none were found to have PE or DVT during the three-month follow-up period. Nine patients died during the follow-up period, but none died of PE, according to their medical records. Causes of death were metastatic cancer (three participants), chronic obstructive pulmonary disease (two participants), heart failure, asystole with no report of PE, severe coronary artery disease, and acute myocardial infarction.
Discussion
Clinicians have become increasingly reliant on pulmonary CTA for the diagnosis of acute PE. However, the modality carries several negative consequences. Radiation exposure for a pulmonary CTA is on the order 5 millisieverts (mSv) or more.7,18,19 The most recent BEIR VII (Biologic Effects of Ionizing Radiation) report from the National Academy of Sciences estimates that there is a 1 in 1000 lifetime risk of inducing a nonfatal cancer and a 1 in 2000 chance of inducing a fatal cancer for every 10 mSv of radiation exposure.20 The use of an iodinated contrast agent entails a small but not insignificant risk for nephrotoxicity.8 This is especially true for patients with decreased renal function, a condition that is becoming increasing common as our society ages and the prevalence of diabetes continues to increase. Despite the use of low-osmolar and iso-osmolar contrast agents, the incidence of contrast-induced nephrotoxicity remains 4%.21 Many patients seen in EDs are saddled with large financial bills. At our facility, an uninsured patient would be charged $763 for a pulmonary CTA during the study period. Even those with health care insurance often have a substantial copayment for this study.
Despite these negative consequences, recent data suggest that pulmonary CTA is both overused and not as highly sensitive or specific as once believed.5,6 A recent multicenter trial, the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) II study, evaluated pulmonary CTA against a composite reference standard.5 That study found a sensitivity of only 83% and a specificity of 96%, even after excluding all indeterminate pulmonary CTA studies. Furthermore, although the prevalence of PE in the original PIOPED study was 33%,22 a recent study of 349 ED patients evaluated by pulmonary CTA revealed a prevalence of 5.7%.6 At our own institution, the annual prevalence of PE as detected by pulmonary CTA is <5%.
Like all imaging studies, pulmonary CTA is subject to interobserver variability in the interpretation of findings, especially in the determination of distal segmental or subsegmental pulmonary emboli.3,4 There was moderate interobserver agreement regarding pulmonary CTAs in our study (κ = 0.45). The study reader reported indeterminate findings on 43 pulmonary CTA studies versus indeterminate findings on 21 studies according to the initial clinical reading. The lower prevalence of indeterminate results reported by the initial clinical reader may be attributed in part to the pressure placed on the clinical reader to make a definitive diagnosis. An indeterminate result would require additional evaluation, which can be a hardship for the patient and taxes limited radiology resources. Furthermore, the radiologists who provided the initial clinical readings did not use a specific definition for an indeterminate result. In contrast, the sole study reader used a specific definition of indeterminate results: lack of uniform enhancement of the pulmonary arteries, and filling defects seen on only one axial slice that could not be confirmed by coronal or sagittal reconstruction.
In addition to the adverse effects that may occur with pulmonary CTA, treatment of PE itself has also been associated with negative consequences. Wysowski et al23 reported that warfarin was among the top ten drugs with the largest number of serious adverse event reports, according to the Food and Drug Administration's Adverse Event Reporting System. Bleeding complications associated with warfarin use have an annual rate of approximately 6% to 7%, although major hemorrhage occurs in only 2% of cases.24,25 Patients taking warfarin also require frequent monitoring to check the adequacy of therapy, which requires time and effort on both the part of the patient and their clinicians. Patients must also avoid many activities in which there is the possibility of bruising, such as most contact sports. Anticoagulation, like all medications, should not be instituted unless there is clear medical benefit. Eyer et al26 noted that most of their study participants with indeterminate or inconclusive pulmonary CTA findings did not receive anticoagulation and did not subsequently develop venous thromboembolism.
Our results also suggest that in the face of D-dimer levels of ≤1.0 µg/mL, the value of the Wells criteria in the evaluation of PE is limited. Studies have shown the presence of interrater variability regarding the Wells criteria.13 Even when clinical guidelines are present, their implementation can be problematic. A recent large multicenter study of 1529 consecutive patients seen in 117 EDs found that in 43% of patients, diagnostic management was inappropriate.27 In a busy ED, even the use of a short clinical decision tool such as the Wells criteria can be difficult. In a survey of ED physicians, Runyon et al28 found that only 57% of all respondents could correctly identify a specific component of the Wells criteria by spontaneous recall, and 53% reported not using an established clinical decision rule in more than half of their patients.
In our cohort, there were ten patients with a high probability of PE as determined by the Wells criteria. However, none of those patients had PE according to either the clinical or study radiologist reading, and none had evidence of PE at the three-month follow-up examination. Furthermore, the single patient in our cohort with a D-dimer level of ≤1.0 µg/mL that had PE according to CTA had a Wells criteria score of 0. None of the six patients mentioned previously with a discrepancy between the study CTA reading and clinical CTA reading had a high probability of PE as assessed using the Wells criteria.
A better method to evaluate acute PE would be helpful to clinicians seeking to avoid both excessive diagnostic imaging and its consequences. In our cohort, none of the 57 patients with a negative D-dimer level had positive findings on CTA, regardless of Wells criteria score. This mirrors the results of Wolf et al, who found no study participants with a negative serum D-dimer level to have PE in their cohort of ED patients.13 In addition, only one of the 347 participants with a D-dimer level of ≤1.0 µg/mL was found to have definitive PE by pulmonary CTA; whereas, in six patients, the diagnosis of acute PE might have been incorrect. These results suggest that errors in the proper interpretation of PE by CTA, especially in the distal segmental or subsegmental pulmonary arteries, may be greater than the likelihood of missing a PE by using a D-dimer cutoff level of ≤1.0 µg/mL to exclude the need for pulmonary CTA. Conversely, when presented with a reported finding of PE in the distal segmental or subsegmental arteries on pulmonary CTA in a patient with a serum D-dimer level of ≤1.0 µg/mL, consideration should be given to additional evaluation rather than initiation of anticoagulation.
The increasing reliance on pulmonary CTA in diagnosing PE has led to increased imaging and its consequences.
That commercially available D-dimer assays are not standardized was an important limitation to our study. The assay in our study used a latex agglutination technique (STA D-DI). Other assays differ in their measurement of D-dimer levels. For example, the rapid enzyme-linked immunosorbent assay D-dimer assay (VIDAS D-dimer, bioMérieux, Marcy l’Étoile, France) uses a normal cutoff of 0.5 µg/mL FEU. Therefore, the cutoff point of 1.0 µg/mL used in our study cannot be applied to other commercial assays. We chose a serum D-dimer level of 1.0 µg/mL as a cutoff in the evaluation of PE even though the true negative for this commercial assay is 0.4 µg/mL. This cutoff level of ≤1.0 µg/mL was based on the results of previous analyses16,17 and is also an easy number to remember.
The increasing reliance on pulmonary CTA in diagnosing PE has led to increased diagnostic imaging and its consequences. Our study demonstrates that in most patients with a D-dimer level of ≤1.0 µg/mL, pulmonary CTA may not be of added utility because of its limited sensitivity and its interobserver variability, especially for detection of distal segmental or subsegmental embolus. Furthermore, the Wells criteria may be of limited additional value in predicting PE in this patient group (low D-dimer level).
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Acknowledgment
Katharine O'Moore-Klopf, ELS, of KOK Edit provided editorial assistance.
References
- 1.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998 Mar 23;158(6):585–93. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 2.Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) Lancet. 1999 Apr 24;353(9162):1386–9. doi: 10.1016/s0140-6736(98)07534-5. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz Y, Caballero P, Caniego JL, et al. Prospective comparison of helical CT with angiography in pulmonary embolism: global and selective vascular territory analysis. Interobserver agreement. Eur Radiol. 2003 Apr;13(4):823–9. doi: 10.1007/s00330-002-1588-7. [DOI] [PubMed] [Google Scholar]
- 4.Chartrand-Lefebvre C, Howarth N, Lucidarme O, et al. Contrast-enhanced helical CT for pulmonary embolism detection: inter- and intraobserver agreement among radiologists with variable experience. AJR Am J Roentgenol. 1999 Jan;172(1):107–12. doi: 10.2214/ajr.172.1.9888748. [DOI] [PubMed] [Google Scholar]
- 5.Stein PD, Fowler SE, Goodman LR, et al. PIOPED II Investigators. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006 Jun 1;354(22):2317–27. doi: 10.1056/NEJMoa052367. [DOI] [PubMed] [Google Scholar]
- 6.Prologo JD, Gilkeson RC, Diaz M, Asaad J. CT pulmonary angiography: a comparative analysis of the utilization patterns in emergency department and hospitalized patients between 1998 and 2003. AJR Am J Roentgenol. 2004 Oct;183(4):1093–6. doi: 10.2214/ajr.183.4.1831093. [DOI] [PubMed] [Google Scholar]
- 7.Hurwitz LM, Reiman RE, Yoshizumi TT, et al. Radiation dose from contemporary cardiothoracic multidetector CT protocols with an anthropomorphic female phantom: implications for cancer induction. Radiology. 2007 Dec;245(3):742–50. doi: 10.1148/radiol.2453062046. [DOI] [PubMed] [Google Scholar]
- 8.Lufft V, Hoogestraat-Lufft L, Fels LM, et al. Contrast media nephropathy: intravenous CT angiography versus intraarterial digital subtraction angiography in renal artery stenosis: a prospective randomized trial. Am J Kidney Dis. 2002 Aug;40(2):236–42. doi: 10.1053/ajkd.2002.34501. [DOI] [PubMed] [Google Scholar]
- 9.Ost D, Khanna D, Shah R, et al. Impact of spiral computed tomography on the diagnosis of pulmonary embolism in a community hospital setting. Respiration. 2004 Sep-Oct;71(5):450–7. doi: 10.1159/000080628. [DOI] [PubMed] [Google Scholar]
- 10.Penaloza A, Mélot C, Dochy E, et al. Assessment of pretest probability of pulmonary embolism in the emergency department by physicians in training using the Wells model. Thromb Res. 2007;120(2):173–9. doi: 10.1016/j.thromres.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Wells PS, Ginsberg JS, Anderson DR, et al. Use of a clinical model for safe management of patients with suspected pulmonary embolism. Ann Intern Med. 1998 Dec 15;129(12):997–1005. doi: 10.7326/0003-4819-129-12-199812150-00002. [DOI] [PubMed] [Google Scholar]
- 12.Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001 Jul 17;135(2):98–107. doi: 10.7326/0003-4819-135-2-200107170-00010. [DOI] [PubMed] [Google Scholar]
- 13.Wolf SJ, McCubbin TR, Feldhaus KM, Faragher JP, Adcock DM. Prospective validation of Wells Criteria in the evaluation of patients with suspected pulmonary embolism. Ann Emerg Med. 2004 Nov;44(5):503–10. doi: 10.1016/j.annemergmed.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Kearon C, Ginsberg JS, Douketis J, et al. CanadianPulmonary Embolism Diagnosis Study (CANPEDS) Group. An evaluation of D-dimer in the diagnosis of pulmonary embolism: a randomized trial. Ann Intern Med. 2006 Jun 6;144(11):812–21. doi: 10.7326/0003-4819-144-11-200606060-00007. [DOI] [PubMed] [Google Scholar]
- 15.Kruip MJ, Slob MJ, Schijen JH, van der Heul C, Büller HR. Use of a clinical decision rule in combination with D-dimer concentration in diagnostic workup of patients with suspected pulmonary embolism: a prospective management study. Arch Intern Med. 2002 Jul 22;162(14):1631–5. doi: 10.1001/archinte.162.14.1631. [DOI] [PubMed] [Google Scholar]
- 16.Abcarian PW, Sweet JD, Watabe JT, Yoon HC. Role of a quantitative D-dimer assay in determining the need for CT angiography of acute pulmonary embolism. AJR Am J Roentgenol. 2004 Jun;182(6):1377–81. doi: 10.2214/ajr.182.6.1821377. [DOI] [PubMed] [Google Scholar]
- 17.Hirai LK, Takahashi JM, Yoon HC. A prospective evaluation of a quantitative D-dimer assay in the evaluation of acute pulmonary embolism. J Vasc Interv Radiol. 2007 Aug;18(8):970–4. doi: 10.1016/j.jvir.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Huda W. Radiation doses and risks in chest computed tomography examinations. Proc Am Thorac Soc. 2007 Aug 1;4(4):316–20. doi: 10.1513/pats.200611-172HT. [DOI] [PubMed] [Google Scholar]
- 19.Kuiper JW, Geleijns J, Matheijssen NA, Teeuwisse W, Pattynama PM. Radiation exposure of multi-row detector spiral computed tomography of the pulmonary arteries: comparison with digital subtraction pulmonary angiography. Eur Radiol. 2003 Jul;13(7):1496–1500. doi: 10.1007/s00330-002-1753-z. [DOI] [PubMed] [Google Scholar]
- 20.Higson D. BEIR VII-2. J Radiol Prot. 2005 Sep;25(3):324–5. [PubMed] [Google Scholar]
- 21.Barrett BJ, Katzberg RW, Thomsen HS, et al. Contrast-induced nephropathy in patients with chronic kidney disease undergoing computed tomography: a double-blind comparison of iodixanol and iopamidol. Invest Radiol. 2006 Nov;41(11):815–21. doi: 10.1097/01.rli.0000242807.01818.24. Erratum in: Invest Radiol 2007Feb,42(2):94. [DOI] [PubMed] [Google Scholar]
- 22.Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). The PIOPED Investigators. JAMA. 1990 May 23-30;263(20):2753–9. doi: 10.1001/jama.1990.03440200057023. [DOI] [PubMed] [Google Scholar]
- 23.Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: a prevalent adverse effect resulting in regulatory action. Arch Intern Med. 2007 Jul 9;167(13):1414–9. doi: 10.1001/archinte.167.13.1414. [DOI] [PubMed] [Google Scholar]
- 24.Wells PS, Forgie MA, Simms M, et al. The outpatient bleeding risk index: validation of a tool for predicting bleeding rates in patients treated for deep venous thrombosis and pulmonary embolism. Arch Intern Med. 2003 Apr 28;163(8):917–20. doi: 10.1001/archinte.163.8.917. [DOI] [PubMed] [Google Scholar]
- 25.Palareti G, Leali N, Coccheri S, et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet. 1996 Aug 17;348(9025):423–8. doi: 10.1016/s0140-6736(96)01109-9. [DOI] [PubMed] [Google Scholar]
- 26.Eyer BA, Goodman LR, Washington L. Clinicians’ response to radiologists’ reports of isolated subsegmental pulmonary embolism or inconclusive interpretation of pulmonary embolism using MDCT. AJR Am J Roentgenol. 2005 Feb;184(2):623–8. doi: 10.2214/ajr.184.2.01840623. [DOI] [PubMed] [Google Scholar]
- 27.Roy PM, Meyer G, Vielle B, et al. EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006 Feb 7;144(3):157–64. doi: 10.7326/0003-4819-144-3-200602070-00003. [DOI] [PubMed] [Google Scholar]
- 28.Runyon MS, Richman PB, Kline JA. Pulmonary Embolism Research Consortium Study Group. Emergency medicine practitioner knowledge and use of decision rules for the evaluation of patients with suspected pulmonary embolism: variations by practice setting and training level. Acad Emerg Med. 2007 Jan;14(1):53–7. doi: 10.1197/j.aem.2006.07.032. [DOI] [PubMed] [Google Scholar]