Abstract
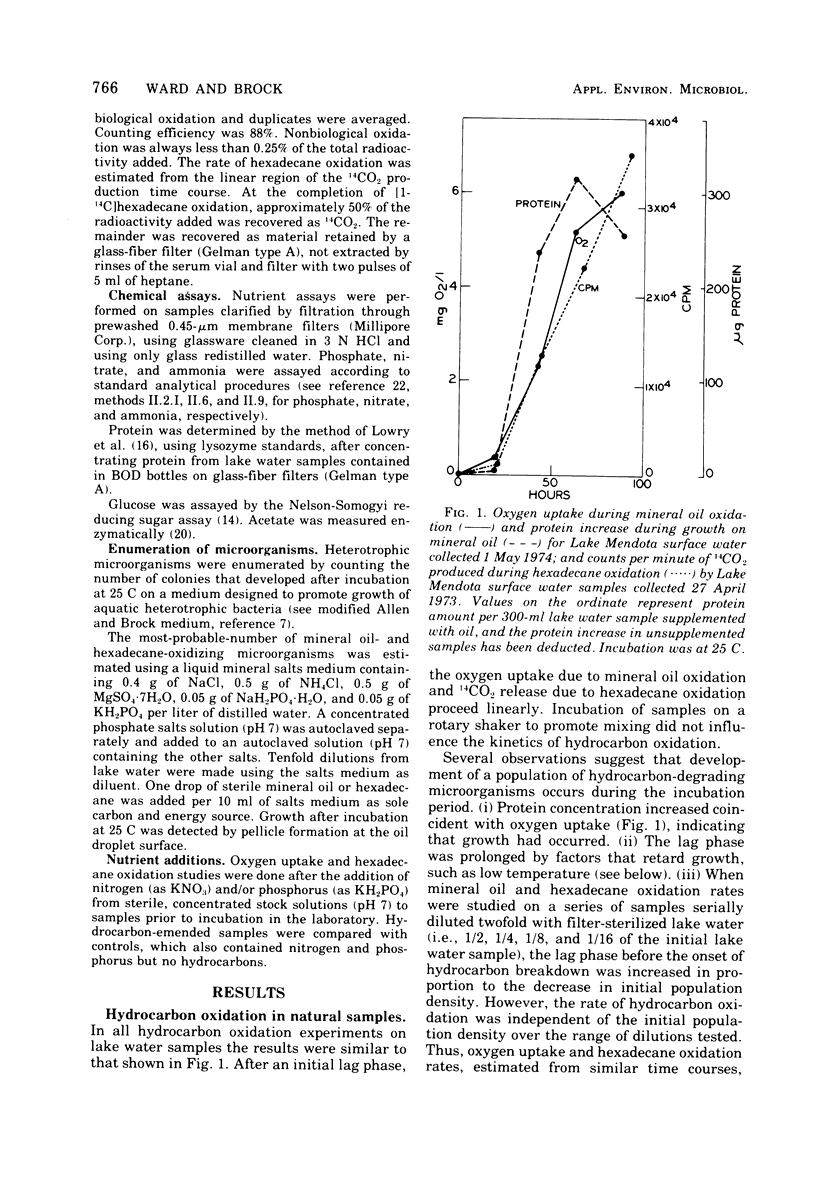
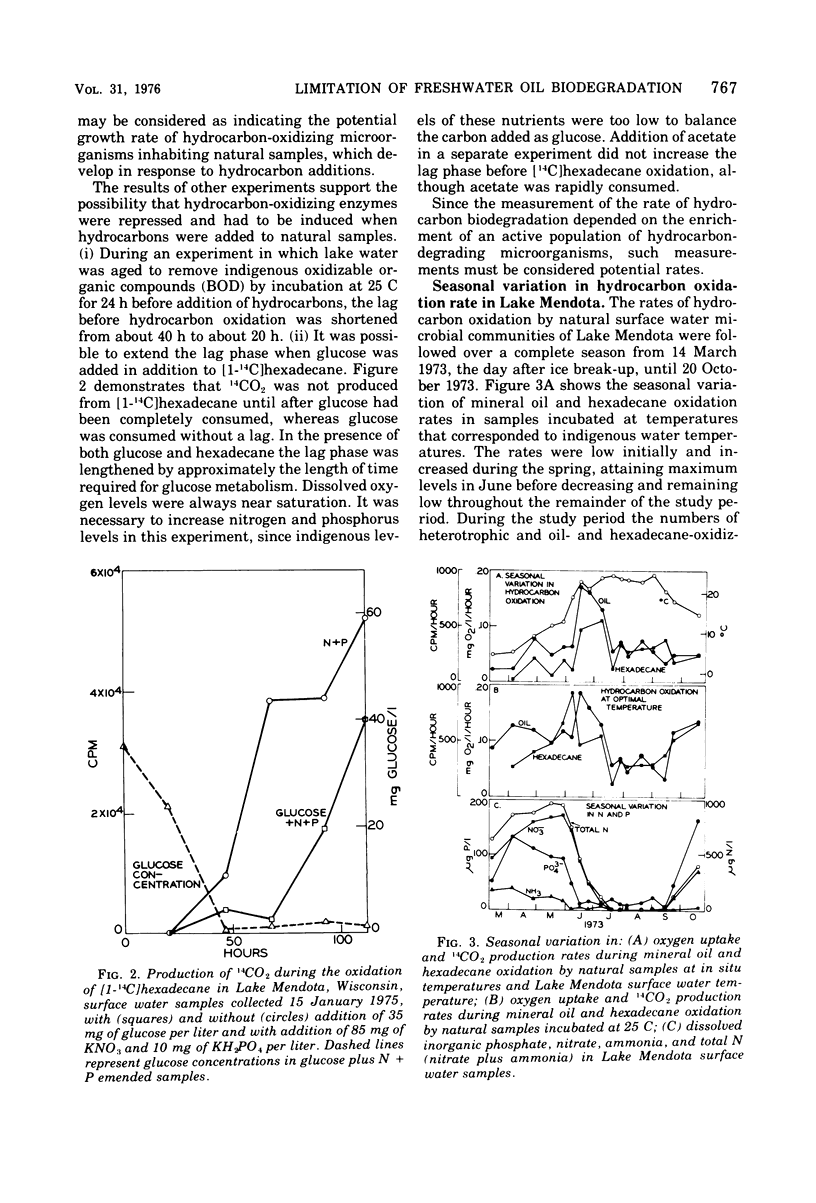
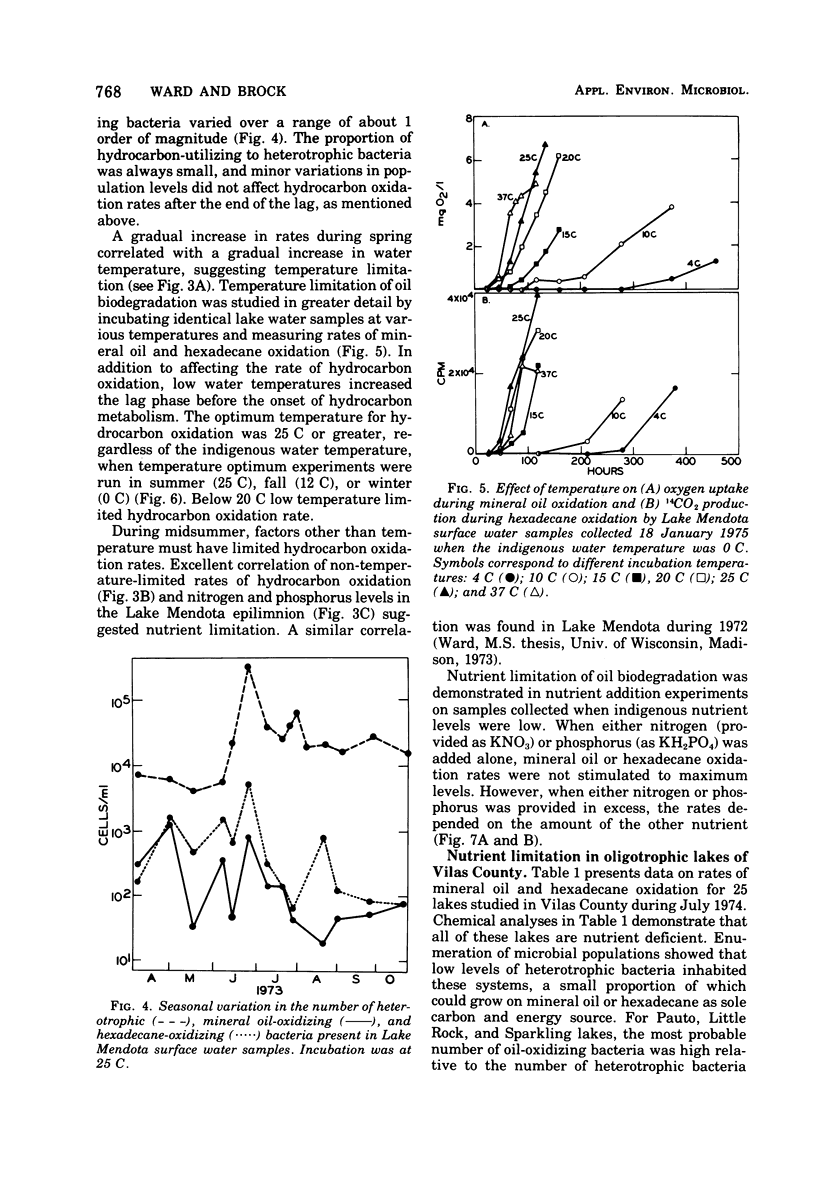
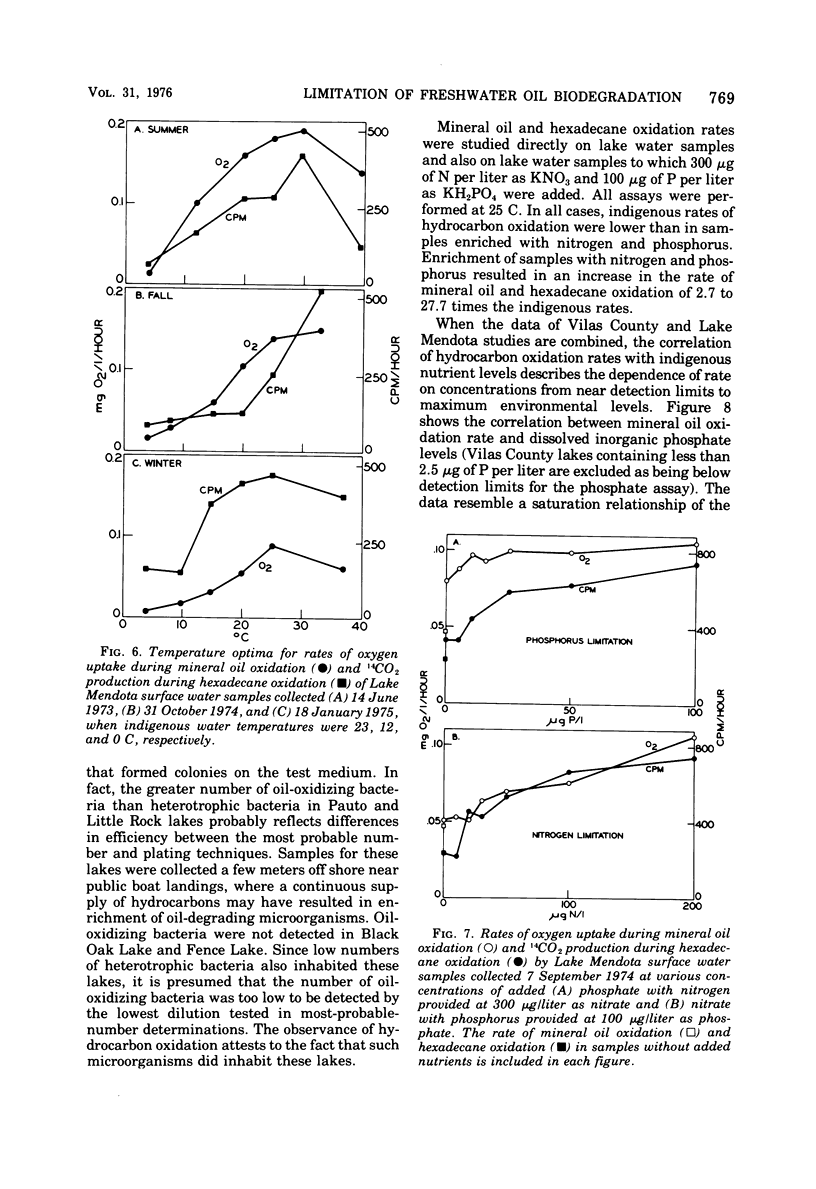
Rates of hydrocarbon biodegradation were estimated by following oxygen uptake during mineral oil oxidation or oxidation of [1-14C]hexadecane to 14CO2, when these substrates were added to natural water samples from Wisconsin lakes. A lag phase preceded hydrocarbon oxidation, the length of which depended on population density or on factors influencing growth rate and on the presence of nonhydrocarbon organic compounds. Hydrocarbon oxidation was coincident with growth and presumably represented the development of indigenous hydrocarbon-degrading microorganisms in response to hydrocarbon additions. In detailed studies in Lake Mendota, it was found that, despite the continued presence of hydrocarbon-degrading microorganisms in water samples, seasonal variations in the rates of mineral oil and hexadecane oxidation occurred which correlated with seasonal changes in temperature and dissolved inorganic nitrogen and phosphorus. The temperature optimum for oil biodegradation remained at 20 to 25 C throughout the year, so that temperature was the main limiting factor during winter, spring, and fall. During summer, when temperatures were optimal, nutrient deficiencies limited oil biodegradation, and higher rates could be obtained by addition of nitrogen and phosphorus. The rates of hydrocarbon biodegradation were thus high only for about 1 month of the ice-free period, when temperature and nutrient supply were optimal. Nutrient limitation of oil biodegradation was also demonstrated in 25 nutrient-poor lakes of northern Wisconsin, although in almost every case oil-degrading bacteria were detected. Knowledge of temperature and nutrient limitations thus will help in predicting the fate of hydrocarbon pollutants in freshwater.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlas R. M., Bartha R. Biodegradation of petroleum in seawater at low temperatures. Can J Microbiol. 1972 Dec;18(12):1851–1855. doi: 10.1139/m72-289. [DOI] [PubMed] [Google Scholar]
- Atlas R. M., Bartha R. Degradation and mineralization of petroleum in sea water: limitation by nitrogen and phosphorous. Biotechnol Bioeng. 1972 May;14(3):309–318. doi: 10.1002/bit.260140304. [DOI] [PubMed] [Google Scholar]
- Atlas R. M., Bartha R. Fate and effects of polluting petroleum in the marine environment. Residue Rev. 1973;49(0):49–85. doi: 10.1007/978-1-4613-9377-1_2. [DOI] [PubMed] [Google Scholar]
- Aurich H., Eitner G. Oxydation von n-Hexadecan durch Acinetobacter calco-aceticus. Bedingungen und Induktion beteiligeter Enzyme. Z Allg Mikrobiol. 1973;13(7):539–544. doi: 10.1002/jobm.3630130702. [DOI] [PubMed] [Google Scholar]
- Gibbs C. F., Pugh K. B., Andrews A. R. Quantitative studies on marine biodegradation of oil. II. Effect of temperature. Proc R Soc Lond B Biol Sci. 1975 Jan 21;188(1090):83–94. doi: 10.1098/rspb.1975.0004. [DOI] [PubMed] [Google Scholar]
- Gibbs C. F. Quantitative studies on marine biodegradation of oil. I. Nutrient limitation at 14 degrees C. Proc R Soc Lond B Biol Sci. 1975 Jan 21;188(1090):61–82. doi: 10.1098/rspb.1975.0003. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nyns E. J., Auquière J. P., Wiaux A. L. Adaptative or constitutive nature of the enzymes involved in the oxidation of n-hexadecane into palmitic acid by Candida lipolytica. Z Allg Mikrobiol. 1969;9(5):373–380. [PubMed] [Google Scholar]
- Smith D. W., Fliermans C. B., Brock T. D. Technique for measuring 14 CO 2 uptake by soil microorganisms in situ. Appl Microbiol. 1972 Mar;23(3):595–600. doi: 10.1128/am.23.3.595-600.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobell C. E. ACTION OF MICROORGANISMS ON HYDROCARBONS. Bacteriol Rev. 1946 Mar;10(1-2):1–49. [PMC free article] [PubMed] [Google Scholar]
- van Eyk J., Bartels T. J. Paraffin oxidation in Pseudomonas aeruginosa. I. Induction of paraffin oxidation. J Bacteriol. 1968 Sep;96(3):706–712. doi: 10.1128/jb.96.3.706-712.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



