Abstract
Purpose
Pharmacokinetic (PK) studies provide critical information about the disposition of anticancer drugs in children. In the Children’s Oncology Group (COG) Phase 1 Consortium, pharmacokinetic studies are usually optional. We surveyed the attitudes towards PK studies among subjects in phase 1 trials at COG institutions.
Methods
Subjects were eligible if they participated in a phase 1 anticancer drug study with optional PK studies within the 4 weeks, regardless of whether they agreed to participate in the PK studies. Staff provided demographics; subjects/parents completed a questionnaire.
Results
Fifty eligible subjects enrolled. Thirty-six (72%) of the 50 eligible subjects consented to participate in PK studies; 14 (25%) declined. The most common reasons for participating were “the results might help researchers learn more about the drug” and “results from the pharmacokinetic studies might help other children.” The most common reasons for not participating were “having the samples drawn would mean spending extra time in the hospital,” and “my child might have needed a separate IV catheter in order to participate.”
Conclusions
The majority of subjects identified altruistic motives for participation in PK studies. Subjects who did not participate in PK studies identified extra time and need for an extra IV as important concerns. Simple interventions like sending staff to the subjects’ home to draw PK samples or drawing samples from existing catheters could increase the number of subjects who are willing to participate in PK studies.
Keywords: pharmacokinetics, cancer, children, phase 1 studies, ethics
Introduction
Pharmacokinetic (PK) studies provide critical information about the disposition of anticancer drugs in children. In the Children’s Oncology Group (COG) Phase 1 Consortium, almost all phase 1 studies include secondary PK goals. Participation in the PK portion of these COG studies is usually optional when there is no prospect of direct benefit to the child from the PK studies; the rate of participation has been approximately 60–70% for the past several years. A higher rate of participation would be desirable from a scientific standpoint, since the opportunities to collect PK data in children are limited and phase 1 is the setting in which intensive collection of PK samples is most feasible.
Many early phase anticancer drug studies contain procedures that are required to minimize risk or maximize potential benefit to the individual subject, such as blood tests to evaluate the impact of the drug on organ function, or imaging studies to evaluate tumor response to treatment. Since these procedures have a direct impact on the subject’s safety and appropriateness of continuation in the study, there is rarely argument that they should be viewed as optional. However, most clinical trials also include procedures that present no prospect of benefit to the individual subject, but may be very important for further development of the drug, such as blood draws for pharmacokinetic analysis, tumor sampling for biomarker expression, or investigational imaging studies.
Whether such nontherapeutic procedures should be optional or required is a topic of considerable debate. In favor of requiring these procedures is the compelling scientific argument that in early phase studies, all information is precious and loss of data from any subject could significantly hinder drug development efforts, thus reducing the potential benefits of the study to society. Furthermore, there is no legal right to access to investigational drugs [1]. For this reason, nontherapeutic procedures may be required in some views; since unproven agents being explored in early phase studies do not constitute medically indicated treatment, it is not coercive or unfair to make procedures mandatory that are necessary for evaluation of the drug [2].
The counterargument is that, as evidenced by the lack of 100% participation when nontherapeutic studies are optional, requiring subjects to consent to such studies is by definition coercing them into doing something not all of them would want to do, as a condition for their obtaining the potential personal benefits, however small, of receiving the investigational intervention. When the subjects of early phase studies are children, whose capacity to provide informed consent is limited, an additional argument can be made that research procedures that do not directly affect their safety or treatment usually should be optional, because such vulnerable subjects require extra protections from research related risks [3].
Although there is a relatively large literature pertaining to informed consent in general and informed consent for pediatric oncology studies in particular, there are fewer publications specifically addressing the issue of how families view nontherapeutic components of research. Understanding families’ attitudes could reveal barriers to participation that could be overcome while avoiding coercion. Thus we surveyed the attitudes towards optional PK sampling among subjects participating in phase 1 trials at COG institutions.
Methods
Informed consent was obtained according to federal and institutional guidelines. Subjects were eligible if they had consented to participate in a phase 1 anticancer drug study that included optional PK sampling at a COG Phase 1 Consortium center within the 4 weeks prior to enrollment on this questionnaire study. The study did not have to be a Children’s Oncology Group study. If the subject was a minor and a parent or legally authorized representative provided consent, the person who provided that consent completed the survey. For simplicity, the parents/children are referred to interchangeably as “the subject” throughout this paper. Subjects were eligible regardless of whether they initially agreed to participate in the optional pharmacokinetic studies and regardless of whether they actually completed the PK sampling, as long as the reason for not completing the sampling was not withdrawal of consent. There was no limit to how many previous phase 1 or other studies the subjects had participated in, but each subject could participate only once in the survey.
Study staff provided the following information for each subject: demographics; whether the subject consented to optional PK studies; the study drug and route of administration (oral, PO or intravenous, IV); whether PK sampling would have required an placement of an additional IV; and whether PK sampling would have required additional time in clinic above that required by study/clinical care, and if so, into what range the additional time would fall (1–4 hours; 4–8 hours; 8–12 hours; overnight; an additional visit to clinic not otherwise required).
Results
Fifty-three subjects were enrolled (Table I) at 13 different sites; 3 subjects were not eligible because the chemotherapy study in which they were participating did not include optional pharmacokinetic studies (all 3 indicated consent for pharmacokinetic studies in the survey). Twenty-six subjects (52%) were enrolled onto COG studies and 24 (48%) were enrolled onto other phase 1 studies. The survey was completed by 8 adult subjects, 4 adolescents, and 38 parent/legally authorized representatives. Although the questionnaire was available in Spanish, all subjects completed the English version. No subjects indicated that they were unsure of whether they had consented to participate in optional pharmacokinetic studies. Twenty-nine (58%) enrolled onto studies where study drug was given PO and 21 (42%) onto studies where study drug was administered IV. Additional details about the studies are contained in table II. Thirty-six (72%) of the 50 eligible subjects consented to participate in optional pharmacokinetic studies; 14 (25%) declined. There were no differences between the consenting and non-consenting groups by institution, route of study drug administration, requirement for additional IV for pharmacokinetic sampling, additional time required, race, gender, ethnicity, or age. Reasons for consenting or not consenting to optional pharmacokinetic are shown in Figures 1 and 2.
Table I.
Subject demographics (n=50)
| Characteristic | Number (%) |
|---|---|
| Age (years) | |
| Median | 12.5 |
| Range | 2–21 |
| Gender | |
| Male | 31 (62) |
| Female | 19 (38) |
| Race | |
| White | 39 (78) |
| Black or African American | 9 (18) |
| Other | 2 (4) |
| Ethnicity | |
| Non-Hispanic | 44 (88) |
| Hispanic | 4 (8) |
| Unknown | 2 (4) |
Table II.
Characteristics of the studies and number of subjects consenting to optional pharmacokinetic
| Number of Studies | Number (percent) of subjects agreeing to pharmacokinetic sampling | Number (percent) of subjects not agreeing to pharmacokinetic sampling | ||
|---|---|---|---|---|
| Route of study drug administration | IV | 21 | 16 (76) | 5 (24) |
| PO | 29 | 20 (69) | 9 (31) | |
| Additional IV required for pharmacokinetic sampling | No | 23 | 17 (74) | 6 (26) |
| Yes | 27 | 19 (70) | 8 (30) | |
| Additional time required for pharmacokinetic sampling | No | 5 | 4 (80) | 1 (20) |
| Yes | 45 | 32 (71) | 13 (29) | |
| 1–4 hrs (n=11) | 11 (100) | - | ||
| 4–8 hrs (n=9) | 6 (67) | 3 (33) | ||
| 8–12 hrs (n=5) | 4 (80) | 1 (20) | ||
| Overnight (n=1) | 1 (100) | - | ||
| Extra visit (n=19) | 10 (53) | 9 (47) |
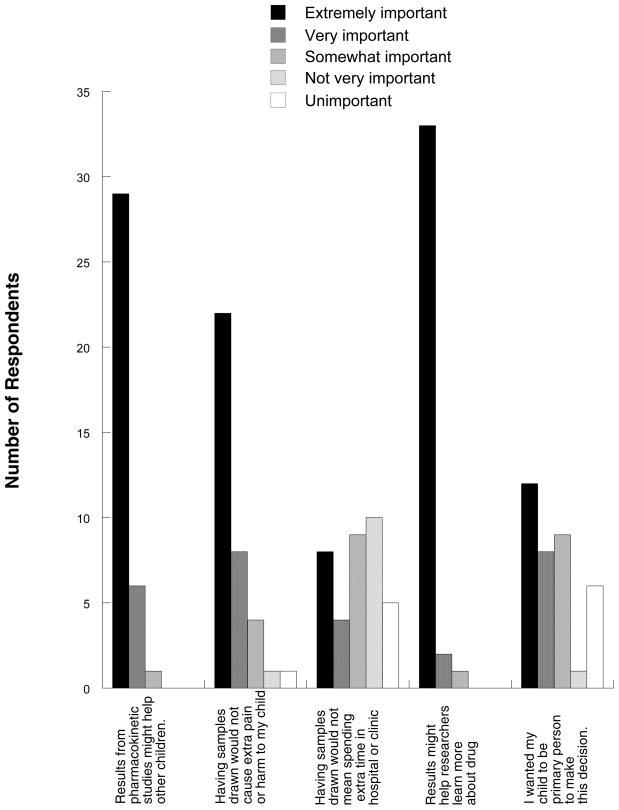
Figure 1.
Responses from parents/subjects who consented to participate in optional pharmacokinetic studies (n=36).
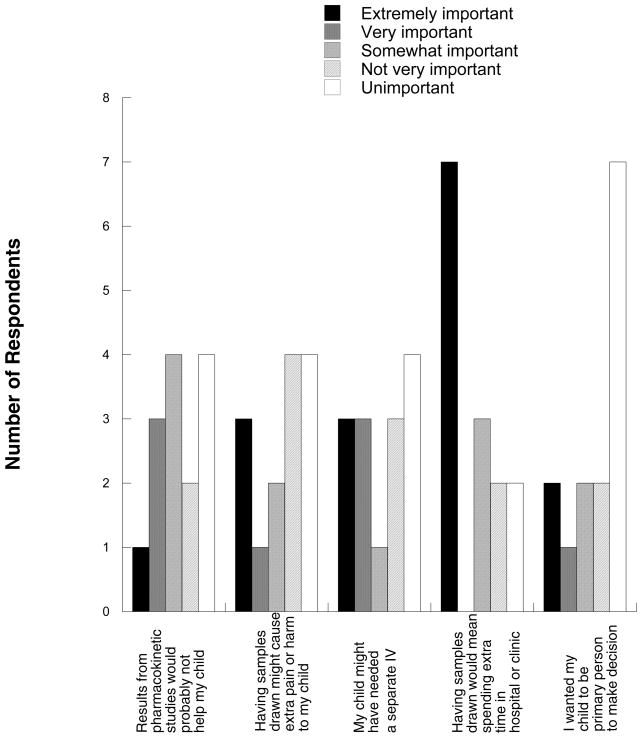
Figure 2.
Responses from parents/subjects who did not consent to participate in optional pharmacokinetic studies (n=14).
Of the 14 subjects who declined, 7 included additional comments in the survey: “the most important factors in choosing not to do the pharmacokinetics were a slight delay in getting the drug because of pharmacokinetic timing, hospitals hours”; “could not get a ride to come here”; “The holidays are coming up and therefore I chose not to participate”; “Limiting time at the hospital for my son is extremely important to our family. Unnecessary blood draws also would not benefit him and only expose him to additional risks and potential discomfort”; “We live 1 hour and 15 mins away I'm a single parent with 2 kids”; “I would have participated if I had a longer notice and available time”; “Not a concern for us at this time. Thank you.”
Eight subjects who consented included additional comments: “We hope and pray that this study will lead to a breakthrough in curing solid tumor cancers for this, as well as future generations of children”; “Anything we can do to help further research, which helps cure this disease is very important to our family. We want to be active participants in the research. We want to help others”; “We volunteered for this study in order to assist in finding out more information with this medicine to hopefully help in finding a cure for other patients with Ewing's Sarcoma”; “Anything will help people with this illness we will participate”; “If it is at all possible for my daughter's condition to be used to help another child with cancer, we felt it was very important to participate”; “[he] had to stay at [the hospital] all day to do the pharmacokinetic sampling. That is the only thing that made me think twice about participating in the study”; “My only concern at the time I was asked was How much blood would they be taking? And if it would harm her or cause her to complain?”; “Some parents think this is going to be too much of draw blood. I think they need to have more information about this.”
Discussion
Our study comprised a sample of 50 subjects at the COG Phase 1 Consortium institutions who were enrolled in phase 1 protocols that included optional pharmacokinetic studies. Seventy-two percent had consented to participate in the optional studies. While we made no attempt to control for demographic factors or otherwise ensure that the survey population comprised a representative sample of the phase 1 population, we are not aware of overt biases in selection of the survey population. Although investigators were encouraged to approach subjects regardless of whether they had agreed to participate in optional PK studies, it is possible that participation in the questionnaire study was biased towards subjects who agreed to participate in optional PK studies if subjects who did not want to participate in optional PK studies also did not want to participate in the survey. For comparison, during the period that this questionnaire study was accruing subjects, 62 subjects enrolled onto COG phase 1 consortium studies that included optional PK. Thirty-six (58%) of those subjects consented to the optional PK studies.
The extent to which the therapeutic misconception [4] affects subjects’ consent to participate in phase 1 studies [5–9] is beyond the scope of this discussion and does not directly bear on why subjects agree to participate in optional procedures within the studies. It has been suggested that subjects whose research protocol includes nontherapeutic tumor biopsies often believe incorrectly that the biopsies will benefit them [10]. Consent forms do not always explicitly differentiate research studies from those that are clinically indicated [11]. However, of the 11 phase 1 studies on which subjects of this survey were enrolled, 9 utilized specific opt-in/opt-out checkboxes in the consent form to document permission for the optional studies. While our study was not designed to assess whether subjects who participated in pharmacokinetic studies truly understood that the studies were optional, the answers do not suggest that subjects were confused on this topic. Thirty-five of 36 (97%) subjects who consented ranked as very or extremely important that results from the pharmacokinetic studies might help other children and that the results might help researchers learn more about the drug.
The proper role of altruism in consent for research participation, especially for enrollment of children onto cancer clinical trials, is controversial, as is the extent to which altruism actually affects subject choices [12–17]. It is likely that the relatively minor risks of participation in the PK studies played an important role in subjects’ decisions to participate: 30 (83%) ranked it as very or extremely important that having the samples drawn would not cause pain or harm to the child. Nonetheless, subjects in this survey clearly identify altruistic motives for participation in the optional pharmacokinetic studies.
Conversely, subjects who did not consent did not seem to reject participation because it would not help them or because they overestimated the risks: only 4 of 14 (29%) not participating indicated that it was very or extremely important that results from the pharmacokinetic studies would probably not help their child or that having the samples drawn might cause extra pain or harm. As mentioned above, several subjects commented that safety of the amount of blood to be drawn for PK studies was important to them. Although consent forms usually include information such as “the volume of blood to be drawn is safe,” it is possible that this particular point needs to be addressed in more detail with prospective subjects.
In contrast to concerns about harm, spending extra time in the hospital or clinic was ranked as extremely important by half the respondents who did not consent. In addition, 6 of 14 (43%) indicated that it was very or extremely important that the child might have needed a separate IV catheter in order to participate, suggesting that the concrete risk of distress during IV placement, rather than an overall perception of pain or harm, was a major factor.
Although the overall risks of participating in optional pharmacokinetic studies are low, the logistical burdens of clinical trial participation in general can affect quality of life [18]. In addition, that the risk is low does not necessarily mitigate the potentially coercive aspect of requiring a nontherapeutic procedure. Based on the results of our survey, the major barriers to children’s voluntary participation are the time and inconvenience of being at the clinic for prolonged sampling, and having to have an IV placed for the sampling. Therefore, we recommend introducing simple interventions, such as sending research staff to the subjects’ home to draw pharmacokinetic samples in order to reduce extra clinic time and visits, and validating methods that permit samples to be drawn from indwelling central lines without risk of contamination by drug administered through the line. In addition, increased use of limited sampling strategies that require fewer samples to be obtained from individual subjects could also be helpful. These measures could result in the ethically just, and scientifically desirable, outcome of increasing the number of subjects who are willing to participate in optional pharmacokinetic studies, while avoiding coercion of those who truly wish to decline.
Acknowledgments
The authors would like to thank Sara Germann and Elizabeth O’Connor for their capable assistance. Research Funding: Supported by National Cancer Institute Grant No. U01 CA97452
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to disclose.
References
- 1.Constitutional law--substantive due process--en banc D.C. Circuit rejects fundamental right to experimental medications.--Abigail Alliance for Better Access to Developmental Drugs v. von Eschenbach, 495 F.3d 695 (D.C. Cir. 2007)(en banc), cert. denied, 128 S. Ct. 1069 (2008) Harv Law Rev. 2008;121(6):1685–1692. [PubMed] [Google Scholar]
- 2.Brown AP, Wendler DS, Camphausen KA, et al. Performing nondiagnostic research biopsies in irradiated tissue: a review of scientific, clinical, and ethical considerations. J Clin Oncol. 2008;26(24):3987–3994. doi: 10.1200/JCO.2008.16.9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson BD, Adamson PC, Weiner SL, et al. Tissue collection for correlative studies in childhood cancer clinical trials: ethical considerations and special imperatives. J Clin Oncol. 2004;22(23):4846–4850. doi: 10.1200/JCO.2004.02.138. [DOI] [PubMed] [Google Scholar]
- 4.Appelbaum PS, Roth LH, Lidz C. The therapeutic misconception: informed consent in psychiatric research. Int J Law Psychiatry. 1982;5(3–4):319–329. doi: 10.1016/0160-2527(82)90026-7. [DOI] [PubMed] [Google Scholar]
- 5.Agrawal M, Grady C, Fairclough DL, et al. Patients' decision-making process regarding participation in phase I oncology research. J Clin Oncol. 2006;24(27):4479–4484. doi: 10.1200/JCO.2006.06.0269. [DOI] [PubMed] [Google Scholar]
- 6.Hutchison C. Phase I trials in cancer patients: participants' perceptions. Eur J Cancer Care (Engl) 1998;7(1):15–22. doi: 10.1046/j.1365-2354.1998.00062.x. [DOI] [PubMed] [Google Scholar]
- 7.Miller M. Phase I cancer trials. A collusion of misunderstanding Hastings. Cent Rep. 2000;30(4):34–43. [PubMed] [Google Scholar]
- 8.Weinfurt KP, Castel LD, Li Y, et al. The correlation between patient characteristics and expectations of benefit from Phase I clinical trials. Cancer. 2003;98(1):166–175. doi: 10.1002/cncr.11483. [DOI] [PubMed] [Google Scholar]
- 9.Weinfurt KP, Sulmasy DP, Schulman KA, et al. Patient expectations of benefit from phase I clinical trials: linguistic considerations in diagnosing a therapeutic misconception. Theor Med Bioeth. 2003;24(4):329–344. doi: 10.1023/a:1026072409595. [DOI] [PubMed] [Google Scholar]
- 10.Agulnik M, Oza AM, Pond GR, et al. Impact and perceptions of mandatory tumor biopsies for correlative studies in clinical trials of novel anticancer agents. J Clin Oncol. 2006;24(30):4801–4807. doi: 10.1200/JCO.2005.03.4496. [DOI] [PubMed] [Google Scholar]
- 11.Horng S, Emanuel EJ, Wilfond B, et al. Descriptions of benefits and risks in consent forms for phase 1 oncology trials. N Engl J Med. 2002;347(26):2134–2140. doi: 10.1056/NEJMsa021182. [DOI] [PubMed] [Google Scholar]
- 12.Jacoby L. The role of altruism in parental decision-making for childhood cancer clinical trials--further questions to explore. Am J Bioeth. 2006;6(5):52. doi: 10.1080/15265160600862528. [DOI] [PubMed] [Google Scholar]
- 13.Joffe S. Altruistic discourse and therapeutic misconception in research informed consent. Am J Bioeth. 2006;6(5):53–54. doi: 10.1080/15265160600862924. [DOI] [PubMed] [Google Scholar]
- 14.Ladd RE, Forman E. Altruistic motives reconsidered. Am J Bioeth. 2006;6(5):55–56. doi: 10.1080/15265160600862940. [DOI] [PubMed] [Google Scholar]
- 15.Simon C, Eder M, Kodish E, et al. Altruistic discourse in the informed consent process for childhood cancer clinical trials. Am J Bioeth. 2006;6(5):40–47. doi: 10.1080/15265160600862395. [DOI] [PubMed] [Google Scholar]
- 16.Spriggs M. Can children be altruistic research subjects? Am J Bioeth. 2006;6(5):49–50. doi: 10.1080/15265160600862445. [DOI] [PubMed] [Google Scholar]
- 17.Wasson K. Altruism and pediatric oncology trials: it does not tip the decision-making scales. Am J Bioeth. 2006;6(5):48. doi: 10.1080/15265160600862429. [DOI] [PubMed] [Google Scholar]
- 18.Cohen MZ, Slomka J, Pentz RD, et al. Phase I participants' views of quality of life and trial participation burdens. Support Care Cancer. 2007;15(7):885–890. doi: 10.1007/s00520-007-0216-0. [DOI] [PubMed] [Google Scholar]