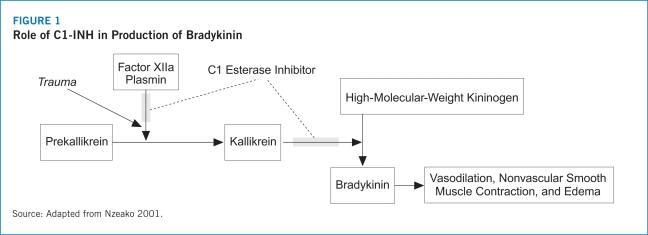
As a human C1 esterase inhibitor, the proposed mechanism of action of Berinert® is the replacement of the missing or dysfunctional human protein that contributes to the pathogenesis of HAE (Berinert® Prescribing Information 2009). Berinert® has been approved as an on-demand therapy for patients experiencing acute abdominal or facial HAE attacks (Berinert® Prescribing Information 2009). Berinert® employs weight-based dosing with a recommended dose of 20 U/kg of body weight (Berinert® Prescribing Information 2009).
Education of patients in recognizing prodromal symptoms prior to an acute attack, such as flu-like symptoms, headache, nausea, non-itchy rash, tingling sensation, and diarrhea, may allow healthcare professionals to administer Berinert® for on-demand use. Berinert® is intended for administration by intravenous infusion by healthcare providers in a variety of settings including hospitals, physicians’ offices, or home healthcare settings (Berinert® Prescribing Information 2009). Berinert® does not require refrigeration, which increases patients’ ability and convenience in storing Berinert® in the home setting or taking it with them to have available as quickly as possible following the onset of acute facial or abdominal HAE attack (Berinert® Prescribing Information 2009).
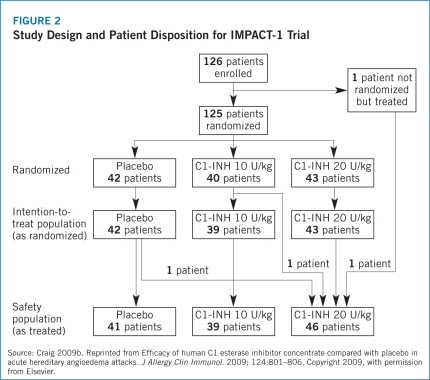
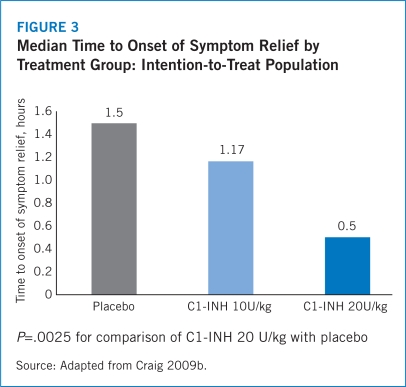
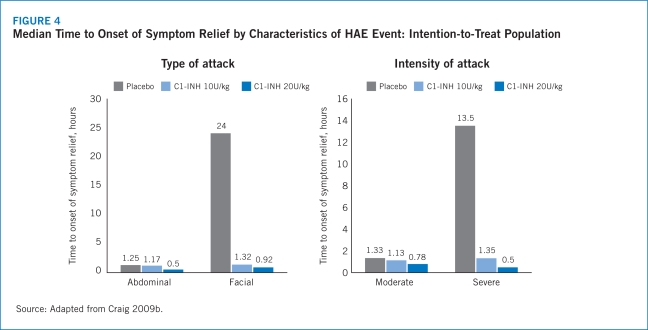
The IMPACT-1 trial established the efficacy of Berinert® for the treatment of acute onset of facial and abdominal HAE attacks with median time to onset of symptom relief reported as 0.50 (range, 0.17–24.00) hours for Berinert® 20 U/kg compared with 1.50 (range, 0.20–24.00) hours for the placebo group (P=.0025) (Craig 2009b). Median time to symptom relief for abdominal attacks was 0.5 hours for Berinert® 20 U/Kg compared with 1.25 hours for placebo and 0.92 hours for Berinert® 20 U/kg compared with 24 hours for placebo among patients who experienced facial attacks. Further, the IMPACT-1 trial demonstrated that 75% of patients treated with Berinert® 20 U/kg experienced symptom relief within 1 hour following initiation of treatment compared with 40% of those treated with placebo (Craig 2009b). There was no statistically significant difference in median time to onset of symptom relief between moderate and severe attacks with Berinert® 20 U/kg (0.78 [range, 0.17–24.00] hours vs. 0.50 [range, 0.17–24.00] hours, respectively; P=.463). Additionally, there was no statistically significant difference in median time to onset of symptom relief between abdominal and facial attacks (0.50 [range, 0.17–24.00] hours vs. 0.92 [range, 0.25–24.00] hours, respectively). This suggests that the efficacy of Berinert® 20 U/kg is equivalent between affected body locations and for attacks of varying severity (Craig 2009b).
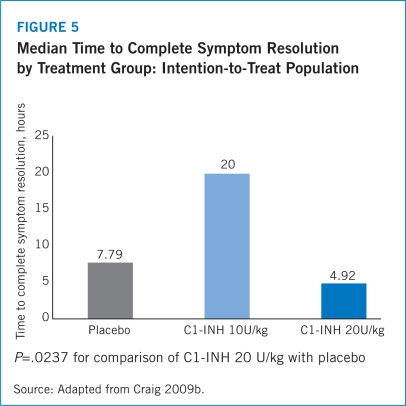
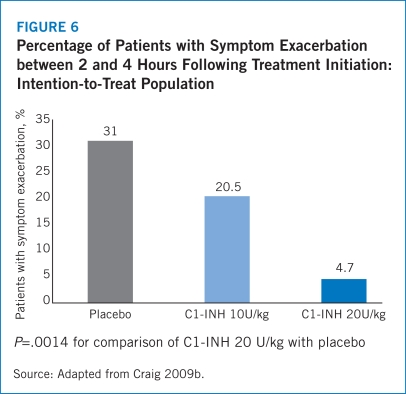
The half-life of Berinert® is 21.9 hours (16.5 to 24.4 hours) with a median residence time of 31.5 hours (23.7 to 35.2 hours), which may potentially reduce the need for a second dose in order to achieve 24-hour symptom control (Berinert® Prescribing Information 2009). Results from IMPACT-1 revealed that the median time to complete resolution of all symptoms was significantly shorter for patients treated with Berinert® 20 U/kg at 4.92 hours compared with 7.79 hours for the placebo group (P=.0237) (Craig 2009b). The IMPACT-1 trial also found that 31.0% of patients who received placebo experienced symptom exacerbations between 2 and 4 hours following treatment compared with 4.7% of patients treated with Berinert® 20 U/kg (P=.0014). Among patients who received Berinert® 20 U/kg, no new attacks occurred before the complete resolution of the previous attack, which demonstrates that Berinert® is not associated with rebound angioedema (Craig 2009b).
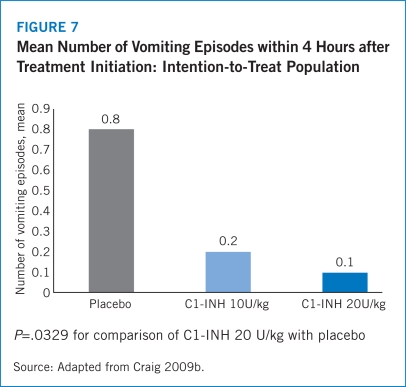
The safety results from the IMPACT-1 trial demonstrated that Berinert® 20 U/kg was well-tolerated. Notably, no patients experienced increased severity of HAE symptoms in the Berinert® 20 U/kg arm while 28.6% of patients treated with placebo experienced increased intensity of HAE symptoms between 2 and 4 hours following treatment (Craig 2009b). The IMPACT-1 trial also revealed significantly lower rates of AEs within 4 hours of treatment initiation for patients administered Berinert® 20 U/kg (19.6%) compared with placebo (43.9%). The most frequent AEs observed in the IMPACT-1 trial were nausea, diarrhea, abdominal pain, and muscle spasms with lower frequencies for each of these events among patients treated with Berinert® 20 U/kg compared with those in the placebo arm (Craig 2009b).
The need for new therapeutic options to treat patients with HAE, particularly those experiencing acute attacks, was recently reported in results from a recent voluntary, web-based survey conducted by the U.S. Hereditary Angioedema Association. The survey was conducted to assess the economic burden imposed by the treatment of acute HAE attacks and long-term treatment of patients with HAE (Wilson 2010). Demographic and clinical questions assessed the severity, location, duration, and treatment of patients’ most recent HAE event as well as the average severity of HAE attacks, medical resource utilization, indirect costs associated with HAE due to travel and childcare, missed time from work, and average frequency of attacks in the year preceding the survey. In addition, the Work Productivity and Impairment–General Health (WPAI-GH) questionnaire was included to determine the impact of HAE on work productivity and activity impairment during the preceding week with results expressed as percentages. Higher percentages were associated with increased work impairment and decreased productivity.
Eligible respondents (N=457) were 18 years old (19 years old in Alabama or Nebraska), had a diagnosis of HAE, and were able to complete an online survey in English. Patients reported a mean of 26.9 HAE attacks per year and 94% had at least 1 HAE attack in the year prior to completing the survey with 56.5% of these being abdominal episodes and 24.5% experiencing a laryngeal attack. The majority (69.4%) of patients did not seek medical interventions for their most recent attack although 12.5% were seen at emergency departments (EDs) and 58.9% of these were admitted to the hospital. Slightly more than half (50.8%) of respondents indicated that they received long-term care from allergists or immunologists for their HAE with 26.3% receiving such care from family practitioners, internists, or primary care physicians, and 10.5% relied on emergency medical physicians for their care. More than two-thirds of respondents (68.3%) required treatment with medications in the previous year to manage their HAE episodes.
The average annual direct medical cost per patient was $25,884 with $21,339 (82.4%) of this total attributed to care for acute HAE attacks. Hospitalization for treatment of acute attacks was the primary component of all direct medical costs at 67.0% with 10.1% of total direct costs for acute attacks associated with ED visits, and 9.8% for routine visits.
Respondents indicated that HAE significantly affected their ability to work with 50.6% reporting they had missed at least 1 day of work during their most recent attack and lost an average of 3.3 workdays. This was estimated to be associated with approximately $525 in lost wages for each HAE attack. The average annual cost of missed work due to acute HAE attacks was estimated at $3,402. Responses to the WPAI-GH indicated a mean impairment of 33.5% while working, and it was estimated that an average cost of $5,750 was incurred per patient year due to work impairments. Inability to maintain full-time employment due to HAE was reported by 16.4% of respondents. The annual average per-patient cost of lost wages for not being able to work full-time was estimated at $6,512. The largest proportion of indirect costs for the average patient with HAE was attributed to the inability to work full-time (40.4%) while an additional 37.5% of indirect costs were associated with decreased work productivity. Direct medical costs increased with the severity of HAE events ranging from total annual costs of $14,379 for mild attacks to $26,914 and $95,460 for moderate and severe attacks, respectively. A summary of direct and indirect costs for acute HAE attacks is presented in Table 13.
Table 13.
Direct and Indirect Economic Costs (U.S. Dollars) for Acute Hereditary Angioedema Attacks by Severity of Attack
| Costs |
Overall population |
Severity of most recent HAE attack |
| mild |
moderate |
severe |
| Direct medical costs |
| Number of patients |
419 |
118 |
189 |
112 |
| Clinical care*
|
1,166 |
427 |
469 |
3,220 |
| ED visits/ hospitalizations |
19,938 |
570 |
5,041 |
65,482 |
| Medications |
235 |
44 |
254 |
404 |
| Indirect medical costs |
| Number of patients |
419 |
118 |
189 |
112 |
| Childcare and travel†
|
444 |
19 |
97 |
1,549 |
| Missed work |
3,402 |
940 |
3,987 |
5,109 |
This study provides a comprehensive assessment of the direct and indirect medical and economic burden imposed by HAE with substantial costs associated with acute attacks as well as long-term management. The total annual cost for an average patient with HAE was estimated at $42,000 including both indirect and direct expenditures. Notably, costs associated with the treatment of severe attacks were almost three times greater than the costs of medical care for patients who experienced attacks of moderate severity, and treatment costs for moderate HAE episodes were almost double those for care of patients who experienced mild attacks. ED visits and hospitalizations accounted for about 48% of the total annual cost of care for patients with acute HAE attacks.
These results confirm that HAE imposes a significant economic burden on patients, their families, employers, and the healthcare delivery system in the U.S. Acute HAE episodes were associated with the highest direct medical costs (particularly for patients with moderate and severe acute attacks) as well as indirect costs associated with lost time at work, decreased productivity, and an inability to maintain full-time employment. These results underscore the need for new therapies to improve the management of patients experiencing acute HAE attacks in order to reduce the overall economic burden of this disease for both the patient and the healthcare system.
Another recent online survey of 80 U.S. physicians including 94% who were self-identified as allergists and 6% as immunologists assessed their perceptions of HAE and the need to screen patients and their family members with suspected HAE (Lunn 2010). The investigators also conducted an e-mail and postal survey of U.S. and European patients with HAE to assess healthcare utilization from presentation to diagnosis, identify treatment differences between countries, and assess patients’ perceptions of the effectiveness of prevention and acute treatment for HAE.
The physician survey revealed that respondents provided care for a mean of 7 patients with HAE and a mean of 4.8 patients were currently treated with androgens. In addition, approximately 84% of physicians reported relying on C1-INH levels and function to establish the diagnosis of HAE while only 63.8% relied on C4 levels for screening and diagnosis of patients with symptoms frequently associated with HAE, such as angioedema or abdominal pain. Less than two-thirds of physicians retested their patients with only 12.2% conducting annual retests of their patients to verify and monitor the status and progression of HAE.
Among 313 patients who completed the online survey, 43% reported that they had waited more than 1 year after their first HAE episode before seeking medical care. Strikingly, the mean time between onset of first symptom(s) of HAE and diagnosis was 8.3 years and patients sought care from an average of 4.4 physicians before receiving a diagnosis of HAE. Sixty-five percent of patients indicated they had received a misdiagnosis of their condition and 19% of U.S. respondents and 24% of those in Europe reported having unnecessary surgical procedures attributed to a misdiagnosis. The patients reported an average of 2 immediate and 2 extended blood relatives who also were diagnosed with HAE, although only 48% of first-degree and 26% of extended family members had been tested for HAE. Only 32% of patients reported that all of their family members had been tested for the condition.
These findings emphasize the imperative for physician education about screening, diagnosis, and treatment of patients with suspected HAE including family members of patients with HAE. Screening of C4 levels was identified as an important strategy to establish a differential diagnosis of HAE, which would result in more rapid and accurate diagnosis as well as initiation of appropriate follow-up and treatment. Such educational efforts also should include patients. Patient and physician education, combined with guidelines for the diagnosis of HAE, were suggested by the investigators to have the potential to improve clinical outcomes for patients.
THE BERINERT® EXPERT NETWORK
The Berinert® Expert Network (B.E.N.™) program provides ongoing information and support to patients every day, 24 hours per day via a toll-free number (CSL Behring 2010). The program offers patients assistance with identification of physicians with expertise in the treatment of HAE and initiation of treatment with Berinert® by helping providers develop treatment plans that are appropriate for the individual patient. In addition, patients and physicians can contact the B.E.N.™ to identify specialty pharmacies that have been approved to provide Berinert®. The program also can be contacted to identify hospitals near patient locations that have access and the ability to administer Berinert® according to approved indications (CSL Behring 2010). This can facilitate access by patients and family members to programs, resources, and services developed by and for individuals with HAE. The B.E.N.™ program also provides assistance with insurance issues and questions by addressing prior treatment authorizations, coverage appeals, and letters of medical necessity. In addition, the provision of the Assurance and Assistance Program helps patients gain access to Berinert® if they experience a lapse in third-party, private insurance coverage as well as assistance in obtaining Berinert® for patients who are uninsured, under-insured, or cannot afford to pay for Berinert®.