INTRODUCTION
Epilepsy is a chronic medical disorder or condition, usually resulting in unpredictable, unprovoked recurrent seizures that affect a variety of mental and physical functions. It is one of the most common neurological diseases, affecting more than 3 million people in the U.S.1 and about 50 million people worldwide.2 Epilepsy was one of the first brain disorders to be described.3 It was mentioned in ancient Babylon more than 3,000 years ago.3 Through the ages, the strange behavior caused by some seizures has led to the creation of numerous superstitions and prejudices.
The term epilepsy is derived from the Greek word epilam-banein, meaning to attack or seize. People once thought that epileptic individuals were being visited by demons or gods. However, in 400 b.c., the early physician Hippocrates suggested that epilepsy was a disorder of the brain—and he was right.3
A person is considered to have epilepsy when two or more unprovoked seizures occur that can’t be explained by a medical condition such as fever or substance withdrawal. Seizures can be the result of a family tendency toward the disease, or they can occur after a brain injury, but the cause of epilepsy is largely unknown.4 Epileptic seizures are manifested by an abnormal, excessive, and hypersynchronous electrical discharge of neurons in the brain.4
Each distinct form of epilepsy has its own natural history and response to treatment.4 This diversity probably reflects the many different underlying causes of epilepsy and the variety of epilepsy syndromes in which the clinical and pathological characteristics are distinctive and suggest a specific underlying etiologic mechanism.5
There are many kinds of seizures, each with characteristic behavioral changes and electrophysiological disturbances that can usually be detected in scalp electroencephalographic (EEG) recordings.4 A seizure is a transient epileptic event, indicating a disturbance in brain function.4 Having a single seizure does not necessarily mean that a person has epilepsy.4,5 Ten percent of adults experience a seizure sometime during their lifetime.1 Seizures can last from a few seconds to a few minutes. Patients and health care professionals do not always recognize the signs or symptoms, which can include convulsions, a loss of consciousness, blank staring, lip smacking, or jerking movements of the arms and legs.1 A seizure has a clear beginning, middle, and end.
CLASSIFICATION
It is necessary to determine the type of seizure in order to focus the diagnostic approach on a particular etiologic factor, to select the appropriate drug therapy, to conduct scientific investigations that require delineation of clinical and EEG phenotypes, and to provide vital information regarding the prognosis.4,5
In 1981, the International League against Epilepsy (ILAE) published a modified version of the International Classification of Epileptic Seizures (ICES), which has continued to be a very useful system.5 This system is based on the clinical features of seizures and associated EEG findings. The etiology or cellular substrate is not considered.
There are three main types of seizures: partial, generalized, and unclassified.5 A modified version of the ICES is presented in Table 1.
Table 1.
Types of Epileptic Seizures
Partial (focal) seizures
|
Primarily generalized seizures
|
Unclassified seizures
|
Adapted from Harrison’s Principles of Internal Medicine, 17th ed., 2008,5 and Epilepsy.com, http://professionals.epilepsy.com/page/seizures_classified.html.
Partial. Partial seizures are confined to discrete areas of the cerebral cortex; only a certain area of the body is usually involved, at least at the start.5 By contrast, generalized seizures are noted in diffuse regions of the brain.
Simple partial seizures cause motor, sensory, autonomic, or psychic symptoms without an obvious alteration in consciousness. These seizures may also be manifested as changes in somatic sensation (e.g., paresthesias or tingling), vision, equilibrium, autonomic function olfactory changes, and hearing.
Complex partial seizures are characterized by focal seizure activity, accompanied by transient impairment of the patient’s ability to maintain normal contact with the environment. Partial seizures can spread to involve both cerebral hemispheres and may produce a generalized seizure, usually of tonic–clonic variety. Secondary generalization is often observed following simple partial seizures, especially those with a focus in the frontal lobe.5
Generalized. Generalized seizures arise from both cerebral hemispheres simultaneously.5 Absence seizures (petit mal) are characterized by sudden, brief lapses of consciousness without loss of postural control. The seizure typically lasts for only seconds; consciousness returns as suddenly as it was lost, and there is no postictal confusion.
Atypical absence seizures have features that deviate clinically and electrophysiologically from typical absence seizures. For example, the lapse of consciousness is usually of longer duration and less abrupt in onset and cessation.
A simple absence seizure is defined as a brief clouding of the sensorium, or loss of consciousness, accompanied by certain generalized epileptic discharges without other detectable clinical signs. A complex absence seizure indicates that other signs are also present.
Generalized, tonic–clonic seizures (formerly grand mal) are the main seizure type in approximately 20% of all persons with epilepsy. They are also the most common seizure type resulting from metabolic derangements and are therefore frequently encountered in many different clinical settings.5
Atonic seizures are characterized by sudden loss of postural muscle tone lasting 1 to 2 seconds. Consciousness is briefly impaired, but there is usually no postictal confusion.5
Myoclonus is a sudden and brief muscle contraction that may involve one part of the body or the entire body.
Unclassified. Not all seizure types are partial or generalized; this appears to be true of seizures that occur in neonates and infants.5 Some of the seizures that occur in neonates and infants likely result, in part, from differences in neuronal function and connectivity in the immature brain.5
EPILEPSY SYNDROMES
Epilepsy syndromes are disorders in which epilepsy is a predominant feature, with evidence indicating a common underlying mechanism. Important epilepsy syndromes include the following:
Juvenile myoclonic epilepsy is a generalized seizure disorder of unknown cause, appearing in early adolescence. It is usually characterized by bilateral myoclonic jerks that may be single or repetitive.5 Consciousness is not affected unless the myoclonus is particularly severe. In addition, many patients experience generalized tonic–clonic seizures, and up to one-third of patients have absence seizures.5
Lennox–Gastaut syndrome is associated with central nervous system (CNS) disease. It is a severe form of epilepsy in children when seizures usually begin before four years of age. There may be periods of frequent seizures mixed with brief, relatively seizure-free periods. It is defined by the following triad:5
multiple seizure types, usually including generalized tonic–clonic, atonic, and atypical absence seizures
EEG features showing slow (below 3 cycles per second [hertz, Hz]) spike-and-wave discharges and other abnormalities
impaired cognitive function in most cases
Seizure types include:
tonic (stiffening of the body, upward deviation of the eyes, dilation of the pupils, and altered respiratory patterns).
atonic (brief loss of muscle tone and consciousness, causing abrupt falls).
atypical absence (staring spells).
myoclonic (sudden muscle jerks).
Mesial temporal lobe syndrome is the most common epilepsy syndrome. It is associated with complex partial seizures and exemplifies a symptomatic, partial epilepsy with distinct clinical, EEG, and pathological features.5
INCIDENCE AND PREVALENCE
The annual incidence of epilepsy has been estimated at 50 per 100,000 with a prevalence of 5 to 10 per 1,000.6,7 According to the Centers for Disease Control and Prevention (CDC) in a 2010 report, epilepsy affects approximately 2.5 million people in the U.S. and each year accounts for $15.5 billion in direct costs (medical) and indirect costs (i.e., lost or reduced earnings and productivity).8 There is no central registry of cases of epilepsy or seizures in the U.S.9 Epidemiologists base their estimates on peer-reviewed studies of medical records at specific institutions or in defined local communities. Surveys of physicians and patients, self-reporting, and studies in matched populations or segments of populations overseas may also be used. From this mixture of sources, leading experts in the field have arrived at the following estimates of the incidence and prevalence of seizures and epilepsy in the U.S.9
A first epileptic seizure occurs in 300,000 people each year.9 Of these people, 120,000 are younger than 18 years of age, and between 75,000 and 100,000 of these are children younger than five years of age who are experiencing febrile seizures.9
Each year, 200,000 new cases of epilepsy are diagnosed, and 45,000 children younger than 15 years of age are affected. The incidence is highest in those younger than age two and those older than age 65.9 Males are slightly more at risk than females, and African-American and socially disadvantaged populations are affected at a higher rate than Caucasians.9 In 70% of new cases of epilepsy, no cause is apparent. Fifty percent of people with newly diagnosed epilepsy have generalized-onset seizures, which are more common in children younger than age 10. Afterward, more than 50% of all new patients with epilepsy have partial seizures.
Of all epileptic patients, 70% are expected to enter remission, defined as five or more years of remaining seizure-free with medication.9 Seventy-five percent of seizure-free people taking medication for two to five years can be successfully withdrawn from pharmacotherapy. Despite optimal medical management, 10% of new patients are not treated successfully.
The prevalence of active epilepsy in the U.S. tends to increase with age.1,9 More than 300,000 persons older than 65 years of age have epilepsy.9
ETIOLOGY
Seizures result from a shift in the normal balance of excitation and inhibition within the CNS as well as from abnormal brain function.5 Because various properties control neuronal excitability, it is not unusual that this normal balance can be disturbed in many different ways; thus, there are many causes of seizures and epilepsy.5 In about 70% of patients, no cause can be found.1 The normal brain is capable of experiencing a seizure under certain circumstances, and individuals vary in their susceptibility or threshold for seizures. Patients may have seizures intermittently, with periods of months to years between seizures. There are several possible causes of seizures in a given patient:5
Seizures may be induced by a high fever in children who are otherwise healthy, who have a structural defect, or who have genetic risk factors.
A severe, penetrating head trauma or injury is associated with almost a 50% risk of subsequent epilepsy.
In older patients, Alzheimer’s disease and stroke may precipitate epilepsy.
The pivotal role of synapses in mediating communication among neurons in the mammalian brain suggests that defective synaptic function might lead to seizures.10 That is, a decrease of inhibitory synaptic activity or enhancement of excitatory synaptic activity might be expected to trigger seizures, as corroborated by pharmacological studies.10
The neurotransmitters mediating the main part of synaptic transmission in mammalian brain are amino acids; gamma aminobutyric acid (GABA) and glutamate are the principal inhibitory and excitatory neurotransmitters, respectively.11 GABA is the major inhibitory amino acid neurotransmitter in the mammalian CNS.11 Its receptors have been divided into two main types: GABAA (the more prominent subtype) is a ligand-gated Cl− ion channel that is opened after the release of GABA from presynaptic neurons.11 The GABAA receptor protein has been well characterized by its high abundance and its role in almost every neuronal circuit.11 GABAB, a member of the G-protein coupled receptor family, is linked both to biochemical pathways and to regulation of the ion channels.11
Most primary epilepsies have a genetic basis. As in many other idiopathic diseases such as diabetes, the mode of inheritance is complex.12 Most cases of partial epilepsy appear to be acquired as a consequence of a focal lesion of the cortex, with a minority of cases identified by genetic determinants. In contrast to partial epilepsies, genetic determinants seem to underlie most cases of the most common generalized-onset epilepsies.10
That a genetic factor is involved in primary generalized tonic–clonic seizures is suggested by a familial incidence in 5% to 10% of such patients and, in particular families, by the inheritance of a generalized seizure disorder through specific genes or chromosomal regions.12 The most common form of generalized-onset epilepsy—juvenile myoclonic epilepsy—appears to be a polygenic disease. Expression of the phenotype requires the simultaneous inheritance of multiple genes.10
In practice, the etiologic mechanism is based on the patient’s age, which is one of the most important factors in determining both the incidence and the likely causes of seizures or epilepsy.5 Patients often have a family history of febrile seizures or epilepsy. Childhood marks the age at which many of the well-defined epilepsy syndromes are manifested.5
Head trauma is a common cause of epilepsy in adolescents and adults. The development of epilepsy is strongly correlated with the severity of the head injury.5 In adults older than 65 years of age, cerebrovascular disease causes about 50% of cases of epilepsy; trauma, CNS tumors, and degenerative diseases are also etiologic factors in this population.5 Metabolic disturbances such as electrolyte imbalances, hypoglycemia or hyperglycemia, endocrine disorders, hematological disorders, renal failure, and hepatic failure may cause seizures at any age.5
Conditions most likely to simulate a seizure are syncope and transient ischemic attacks; other possible conditions include unexplained falls (“drop attacks”), subarachnoid hemorrhage, sleep disorders (sleepwalking, rapid-eye-movement sleep behavior disorder), panic attacks, migraine, hypoglycemia, cataplexy, paroxysmal ataxia and choreoathetosis, recurrent transient global amnesia, and psychogenic pseudoseizures.12
The diagnosis of complex partial seizures is the most difficult.12 These attacks are variable and often induce disturbances of behavior and psychic function rather than overt interruptions or loss of consciousness. They may be mistaken for temper tantrums in children, psychogenic drug ingestion, hysteria, sociopathic behavior, or acute psychosis. The careful questioning of witnesses of an attack is critical. Emphasis on amnesia for the events of at least part of the seizure is a critical criterion for the diagnosis of complex partial epilepsy. In all obscure forms of epilepsy, prolonged EEG and video monitoring may prove diagnostic.
PATHOLOGY
Symptomatic epilepsy is associated with definable brain lesions.12 These lesions include zones of neuronal loss and gliosis (scars) or other signs of tissue loss. The frequency of occurrence of these lesions is not fully known. Epileptogenesis refers to the transformation of a normal neuronal network into one that is chronically hyperexcitable.5 There is a delay of months to years between an initial CNS injury such as trauma, stroke, or infection and the first seizure.5 The injury may lower the seizure threshold in the affected area until a spontaneous seizure takes place. In many genetic and idiopathic forms of epilepsy, epileptogenesis may be determined by developmentally regulated events.5
The most common histological finding in the brains of epileptic patients is a bilateral loss of neurons in the CA1 segment (Sommer sector) of the pyramidal cell layer of the hippo-campus, extending into the contiguous regions of both the pyramidal layer and the underlying dentate gyrus.12 In a specific group of epileptic disorders, idiopathic epilepsy may be caused by a disruption of ion channels by neurotransmitter receptors.12
A more complex genetic element is also identified in several childhood seizure disorders, for instance, (1) absence epilepsy with 3-per-second spike-and-wave discharges and (2) benign epilepsy of childhood with centrotemporal spikes. Both of these disorders are transmitted as autosomal dominant traits with incomplete penetrance, perhaps in a more complicated manner.12
Gene mutations observed in symptomatic epilepsy appear to be associated with pathways affecting CNS development or neuronal homeostasis.5 In patients with symptomatic epilepsy, other neurological abnormalities, such as cognitive impairment, coexist with seizures. The challenge is to identify the multiple susceptibility genes that underlie the more common forms of idiopathic epilepsies.
DIAGNOSIS
In the diagnosis of epilepsy, history is the key, because in most adults, the physical examination is relatively nondefinitive.12 The examination of infants and children is of greater value, because the presence of dysmorphic and cutaneous abnormalities allows for the diagnosis of a number of highly characteristic cerebral diseases that give rise to epilepsy. When a patient is seen shortly after a seizure, the first priorities are attention to vital signs, respiratory and cardiovascular support, and treatment of seizures if they resume.5
The physician’s main means of diagnosis is to take a careful medical history, gathering as much information as possible about what the seizures looked like and what happened just before they began. The physician should also perform a thorough physical examination, especially of the nervous system, as well as an analysis of blood and other body fluids.
Several laboratory studies are usually included in the initial diagnostic evaluation, such as a complete blood count, blood chemistry profiles, liver and thyroid function tests, an EEG, and a brain study, preferably with magnetic resonance imaging (MRI). Computed tomography (CT) scanning may be the only practical study in an emergency or for very young children.12 Some patients may later require video/EEG or prolonged EEG monitoring, either in the hospital or with portable equipment in the home.
Cardiac stress tests, Halter monitoring, tilt-table testing, long-term patient activated cardiac monitors, and sleep studies may also be performed. Any patient who has a possible seizure disorder should undergo EEG evaluation as soon as possible.5 Almost all patients with new-onset seizures should have a brain imaging study to detect any underlying structural abnormalities.5 MRI is superior to CT for detecting cerebral lesions associated with epilepsy.5
States of constitutional mental retardation and confusion associated with epilepsy present special problems in diagnosis.12 Seizures are more common in those with mental retardation, but recurrent seizures themselves rarely cause intellectual deterioration.13 When this situation does occur, an underlying degeneration or hereditary metabolic disease should be suspected.12 Migraine should not be mistaken for a seizure.
A second battery of diagnostic tools includes another EEG to record brain waves. Electrical signals from brain cells are recorded during or between seizures and may show special patterns to determine whether the person has epilepsy. CT or MRI scans may be used to search for any growths, scars, or other physical conditions in the brain that might be causing seizures. In a few research centers, positron emission tomography (PET) is used to identify areas of the brain that are producing seizures.
MANAGEMENT
When a neurologist or a physician has made the diagnosis of seizures or epilepsy, the next step is to select the best form of treatment. If epilepsy (a continuing tendency to have seizures) is diagnosed, the neurologist usually prescribes seizure-preventing medications. If drugs are not successful, surgery, a special diet, complementary therapy, or vagus nerve stimulation may be tried. The goal of treatment is to prevent further seizures, avoid adverse effects, and enable patients to lead active lives.5
Medical Treatment
Antiepileptic drug (AED) therapy, the mainstay of treatment for most patients, has four goals: to eliminate seizures or reduce their frequency to the maximum degree possible, to evade the adverse effects associated with long-term treatment, and to aid patients in maintaining or restoring their usual psychosocial and vocational activities, and in maintaining a normal lifestyle.4 The decision to start AED therapy should be based on an informed analysis of the likelihood of seizure recurrence, the consequences of continuing seizures for patients, and the beneficial and adverse effects of the pharmacological agent chosen.13
Whether to initiate therapy in a patient with a single seizure is controversial.5 A single seizure caused by an identified lesion such as a CNS tumor, an infection, or trauma, in which there is strong evidence that the lesion is epileptogenic, should be treated.5 The overall goal of AED therapy is to prevent seizures completely.5
The relative risk of recurrence of epilepsy can vary, depending on the seizure type or syndrome.14 Patients with epileptiform discharges on an EEG or with congenital neurological defects are at high risk of recurrence (approaching 90%).13 The patient’s and family’s views should also be considered when AED treatment is begun.15
To prevent further seizures, it may be best to introduce AEDs early. Future seizure activity may be distressing for those who must drive, continue to work, or care for other family members. The probability of seizure recurrence varies among patients, depending on the type of epilepsy and any associated neurological and medical problems.4 Pharmacotherapy, however, carries a risk of adverse effects, at a rate approaching 30% after initial treatment.4 Treating children presents additional problems, especially on brain development, learning, and behavior, when a drug is used chronically.
Selection of Antiepileptic Therapy
The ideal AED should suppress all seizures without causing any unwanted adverse effects.10 Unfortunately, currently available AEDs not only fail to control seizure activity in some patients but also frequently produce adverse effects that range in severity from minimal impairment of the CNS to death from aplastic anemia or hepatic failure.10 The treating physician or practitioner must choose the appropriate AED or combination of drugs that best controls seizures with a satisfactory degree of untoward effects. It is generally accepted that complete control of seizures can be achieved in up to 50% of patients and that another 25% of patients improve significantly.10 Successful therapy is greater in patients with newly diagnosed epilepsy, and the success rate depends on type of seizure, family history, and extent of associated neurological abnormalities.16
Initiating AED treatment can be based on the likelihood of seizure recurrence, the consequences of continuing seizures, and the beneficial and adverse effects of the agent in preventing recurrence.17 The relative risk of recurrence can vary, depending on the seizure type or syndrome.14 Patients with epileptiform discharges on an EEG or congenital neurological defects are at high risk (up to 90%) of recurrence.17 The risk of recurrence is also elevated in patients with previous symptomatic seizures, in those with cerebral lesions, and in patients with Todd’s paralysis (a brief, temporary paralysis following a seizure).18
Approved AEDs used in epilepsy treatment in the U.S. are presented in Table 2.5 Because of the large number of drugs currently approved for the treatment of epilepsy, only the most relevant aspects of each drug’s profile are presented here. For additional details on adverse events, contraindications, and warnings, readers may consult the references for each drug’s prescribing information and Appendix A on page 410.
Table 2.
Antiepileptic Drugs Approved for the Treatment of Seizures in the U.S.
| Primary Generalized Tonic–Clonic Seizures | Partial Seizures* | Absence Seizures | Atypical Absence Myoclonic, and Atonic Seizures |
|---|---|---|---|
| First-line agents | |||
| Valproic acid Lamotrigine Topiramate |
Carbamazepine Phenytoin Oxcarbazepine Valproic Acid |
Valproic acid Ethosuximide |
Valproic acid Lamotrigine Topiramate |
| Alternative agents | |||
| Zonisamide† Phenytoin Carbamazepine Oxcarbazepine Phenobarbital Primidone Felbamate |
Levetiracetam† Topiramate Tiagabine† Zonisamide† Gabapentin Phenobarbital Primidone Felbamate Eslicarbazepine Vigabatrin Lacosamide Pregabalin Rufinamide |
Lamotrigine Clonazepam |
Clonazepam Felbamate |
Includes simple partial, complex partial, and secondarily generalized seizures.
As adjunctive therapy.
Modified from Fauci AS, Kasper DL, Longo DL, eds. Harrison’s Principles of Internal Medicine, 17th ed. New York: McGraw-Hill; 2008;2498–2512.5
FIRST-LINE DRUGS FOR PRIMARY GENERALIZED TONIC–CLONIC SEIZURES
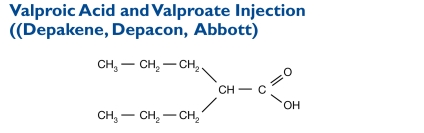
Valproic acid is a carboxylic acid. Its chemical name is di-N-propyl acetic acid, and the molecular weight is 144.
Indication and Mechanism of Action19
Valproic acid has been effective in partial and generalized seizures and is indicated as monotherapy and adjunctive therapy for complex partial seizures, which begin in a limited area of the brain. These seizures may occur either in isolation or in association with other types of seizures.5,10 The drug is also indicated for patients with simple and complex absence seizures and as an adjunctive therapy for patients with multiple seizure types, including absence seizures.20
Valproate is the name of valproic acid after it is converted to the form that works in the body. Valproic acid dissociates to the valproate ion in the gastrointestinal (GI) tract.
The mechanisms by which valproate exerts its antiepileptic effects have not been established, but its activity in epilepsy may be related to increased brain concentrations of GABA.10 To date, it has been difficult to correlate the increased GABA concentrations to the antiseizure activity of valproate.
Dosage and Administration19
Monotherapy. The initial dose is 10 to 15 mg/kg per day. The dose should be increased by 5 to 10 mg/kg per week to achieve optimal clinical response. Valproic acid capsules must be swallowed whole; if they are chewed, the mouth and throat may become irritated. Ordinarily, optimal clinical response is achieved at daily doses below 60 mg/kg per day. If the clinical response is not satisfactory, plasma concentrations should be measured to determine whether they are in the accepted therapeutic range of 50 to 100 mcg/mL. No recommendation can be made regarding its safety at doses above 60 mg/kg per day.
The probability of thrombocytopenia increases significantly at total trough valproate plasma concentrations above 110 mcg/mL in females and above 135 mcg/mL in males. The benefit of improved seizure control with higher doses should be weighed against the possibility of a greater incidence of adverse effects.
Adjunctive Therapy. Valproic acid may be added to the patient’s regimen at a dose of 10 to 15 mg/kg per day, which may be increased by 5 to 10 mg/kg per week to achieve an optimal response. The optimal response is ordinarily achieved at daily doses below 60 mg/kg per day. If the response is not satisfactory, plasma concentrations should be measured to determine whether they are in the accepted therapeutic range. No recommendation regarding the safety of valproate for use at doses above 60 mg/kg per day can be made. If the total daily dose exceeds 250 mg, the drug should be given in divided doses.
Simple and Complex Absence Seizures. The recommended initial dose is 15 mg/kg per day, increasing at one-week intervals by 5 to 10 mg/kg per day until seizures are controlled or until adverse effects preclude further increases. The maximum recommended dosage is 60 mg/kg per day. If the total daily dose exceeds 250 mg, the drug should be given in divided doses. A good correlation has not been established between daily dose, serum concentrations, and therapeutic effect. However, therapeutic valproate plasma concentrations for most patients with absence seizures range from 50 to 100 mcg/mL.
In some patients, seizures may be controlled with lower or higher serum concentrations. During adjunctive therapy, as the valproic acid dose is titrated upward, blood concentrations of concomitant phenobarbital, phenytoin, or both may be affected.
Contraindications, Warnings, and Adverse Effects19
A boxed warning mentions hepatotoxicity, teratogenicity, and pancreatitis. Contraindications, other warnings, and adverse effects are listed in Appendix A on page 410.
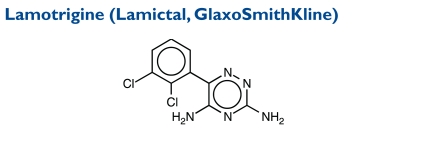
Lamotrigine, an AED of the phenyltriazine class, is chemically unrelated to existing AEDs. Its chemical name is 3,5-diamino-6-(2,3-dichlorophenyl)-as-triazine, and its molecular weight is 256.09.
Indication and Mechanism of Action21
Lamotrigine is indicated as an adjunctive therapy for partial seizures, primary generalized tonic–clonic seizures, and generalized seizures of Lennox–Gastaut syndrome in patients two years of age or older. This AED is also indicated for adults (older than age 16) with partial seizures who are switching to monotherapy and are receiving carbamazepine (Tegretol), phenytoin (Dilantin), phenobarbital (Luminal), primidone (Mysoline), or valproic acid (e.g., Depacon, Depakene) as the single AED.
In January 2010, the FDA approved lamotrigine extended-release tablets as once-daily, add-on therapy for epilepsy in patients 13 years of age and older with primary generalized tonic–clonic seizures22 and with partial-onset seizures with or without secondary generalization.
Lamotrigine’s action is related to inactivation of voltage-sensitive sodium channels, resulting in diminished neuronal activity.23 Lamotrigine may selectively influence neurons that synthesize glutamate and aspartate, because it diminishes the release of these excitatory neurotransmitters through its effect on sodium channels.
Dosage and Administration21
Dosing is based on concomitant medications, the indication, and the patient’s age. Lamotrigine should not be restarted in patients who discontinued therapy because of a rash unless the potential benefits clearly outweigh the risks. Patients starting or stopping estrogen-containing oral contraceptives usually require adjustments to maintenance doses of lamotrigine.
The escalation regimen for lamotrigine in patients older than age 12 who are not receiving other adjunctive therapies for epilepsy is as follows: During weeks 1 and 2, lamotrigine 25 mg is administered every day, then 50 mg/day is given during weeks 3 and 4. From week 5 onward to maintenance therapy, the dose is increased by 50 mg/day every one to two weeks. The usual maintenance dose is 225 to 375 mg/day in two divided doses.
For patients taking valproic acid in addition to a glucuron-idation-inducing antiseizure drug, the initial dose of lamotrigine should be 25 mg every other day for two weeks, followed by an increase to 25 mg/day for two weeks. The dose can then be increased by 25 to 50 mg every one to two weeks up to a maintenance dose of 100 to 400 mg/day in two divided doses. For patients taking valproic acid alone, the regimen for lamotrigine is the same, but the usual maintenance dose is 100 to 200 mg/day.
Contraindications, Warnings, and Adverse Effects21
A boxed warning mentions hepatotoxicity, teratogenicity, and pancreatitis. Contraindications, other warnings, and adverse effects are listed in Appendix A on page 410.
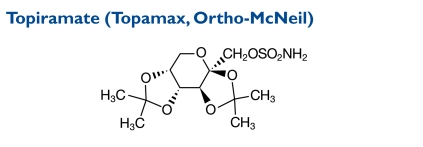
Topiramate is designated chemically as 2,3:4,5-Di-O-isopropylidene-β-d-fructopyranose sulfamate. Its molecular weight is 339.36.
Indication and Mechanism of Action24
Topiramate is an adjunctive therapy for adult and pediatric patients 2 to 16 years of age with partial-onset seizures or primary generalized tonic–clonic seizures in patients two years of age and older with seizures associated with Lennox–Gastaut syndrome. Topiramate tablets or sprinkle capsules are also indicated as initial monotherapy in patients 10 years of age and older with partial-onset or primary generalized tonic–clonic seizures.
The precise mechanism by which topiramate exerts its antiseizure effects is unknown. Electrophysiological and biochemical evidence suggests that topiramate, at pharmacologically relevant concentrations, blocks voltage-dependent sodium channels, augments the activity of the neurotransmitter GABA at some subtypes of the GABAA-receptor, antagonizes the AMPA/kainate subtype of the glutamate receptor, and inhibits the carbonic anhydrase enzyme, particularly isozymes II and IV.
Dosage and Administration24
Topiramate is available as 25-, 50-, 100-, and 200-mg round tablets for oral administration. Because of the bitter taste, tablets should not be broken. Capsules, available in strengths of 15 and 25 mg, may be taken whole or opened and sprinkled onto soft food.
Adjunctive Therapy. For adults 17 years of age and older with partial seizures, the recommended total daily dose of topiramate as adjunctive therapy is 200 to 400 mg/day in two divided doses. For adults with primary generalized tonic–clonic seizures, the recommended dose is 400 mg/day in two divided doses as adjunctive treatment. It is recommended that therapy be initiated at 25 to 50 mg/day, followed by titration to an effective dose in increments of 25 to 50 mg/week. Titrating the dose in increments of 25 mg/week may delay the time to reach an effective dose. Daily doses above 1,600 mg have not been studied. In studies of primary generalized tonic–clonic seizures, the initial titration rate was slower, and the assigned dose was reached at the end of eight weeks.
For pediatric patients with partial seizures, primary generalized tonic–clonic seizures, or seizures associated with Lennox–Gastaut syndrome, the recommended total daily dose of topiramate as adjunctive therapy is 5 to 9 mg/kg per day in two divided doses. Titration should begin at 25 mg (or less, based on a range of 1 to 3 mg/kg per day) nightly for the first week. The dose should then be increased at one- or two-week intervals by increments of 1 to 3 mg/kg per day (given in two divided doses) to achieve an optimal clinical response. Dose titration should be guided by the clinical outcome.
Monotherapy. The recommended dose for topiramate monotherapy in adults and children 10 years of age and older is 400 mg/day in two divided doses. The dose should be titrated according to the schedule in Table 3.
Table 3.
Topiramate Dosing Schedule for Epilepsy
| Initial Dose | Dose Titration | Recommended Dose | |
|---|---|---|---|
| Monotherapy: adults and pediatric patients ≥10 years | 50 mg/day in two divided doses | Titrate upward weekly by increments of 50 mg for the first 4 weeks, then 100 mg for weeks 5 and 6. | 400 mg/day in two divided doses |
| Adjunctive therapy: adults with partial-onset seizures or Lennox–Gastaut syndrome | 25–50 mg/day | Titrate upward weekly to an effective dose by increments of 25–50 mg. | 200–400 mg/day in two divided doses |
| Adjunctive therapy: adults with primary generalized tonic–clonic seizures | 25–50 mg/day | Titrate upward weekly to an effective dose by increments of 25–50 mg. | 400 mg/day in two divided doses |
| Adjunctive therapy: pediatric patients with partial-onset seizures, primary generalized tonic–clonic seizures or Lennox–Gastaut syndrome | 25 mg/day (or less, based on a range of 1–3 mg/kg per day nightly for the first week) | Titrate upward at 1- or 2-week intervals by increments of 1–3 mg/kg per day, given in two divided doses; dose titration should be guided by clinical outcome. | 5–9 mg/kg per day in two divided doses |
In dose–response studies of adults with partial-onset seizures, doses of topiramate above 400 mg/day (600, 800, or 1,000 mg/day) did not improve responses. It is not necessary to monitor topiramate plasma concentrations to optimize topiramate therapy. On occasion, adding topiramate to phenytoin may require an adjustment of the phenytoin dose to achieve an optimal outcome. Adding or withdrawing phenytoin and/or carbamazepine during adjunctive therapy with topiramate may require adjustment of the dose of topiramate.
Contraindications, Warnings, and Adverse Effects24
There are no contraindications. Warnings and adverse effects are presented in Appendix A on page 410.
FIRST-LINE DRUGS FOR PARTIAL SEIZURES
Carbamazepine is related to the tricyclic antidepressants (TCAs). The carbamyl moiety is essential for potent antiseizure activity. The chemical name is 5H-dibenz[b, f]azepine-5-carboxamide, and the molecular weight is 236.27. The drug is not related to other antiseizure agents.
Indication and Mechanism of Action25
Carbamazepine is indicated for use as an anticonvulsant drug. Evidence supporting its efficacy as an AED was derived from studies that enrolled patients with (1) partial seizures with complex symptomatology (psychomotor, temporal lobe); generalized tonic–clonic seizures (grand mal); and (3) mixed-seizure patterns, including the latter two types or other partial or generalized seizures.25 Absence (petit mal) seizures do not appear to be controlled by carbamazepine.
Carbamazepine appears to act by reducing polysynaptic responses and blocking post-tetanic potentiation.25 The mechanism of action remains unknown,25 but the drug’s anticonvulsant activity may result from use-dependent blockade of voltage-sensitive sodium channels.23 Carbamazepine has demonstrated anticonvulsant properties in animals with electrically and chemically induced seizures, and it reduces or abolishes pain induced by stimulation of the infraorbital nerve animals.25
Dosage and Administration25
For adults and children over 12 years of age, carbamazepine tablets are initiated as either 200 mg twice daily or as extended-release (XR) or as one teaspoon four times daily for suspension at a dose of 400 mg/day. The dosing should be increased at weekly intervals to total 200 mg/day with twice-daily carbamazepine XR or with other formulations given three or four times daily until an optimal response is obtained.
For children 12 to 15 years of age, generally the dosage should not exceed 1,000 mg daily. For patients older than 15 years of age, the dose should not exceed 1,200 mg daily. For maintenance therapy, the dose is adjusted to the minimum effective level, usually 800 to 1,200 mg daily.
For children 6 to 12 years of age, the initial dose for the tablets or the XR tablets is either 100 mg twice daily or a suspension of 200 mg/day using 0.5 teaspoon four times daily. The dose is increased at weekly intervals to a total of 100 mg/day in a twice-daily regimen of carbamazepine XR. Alternatively, other formulations are given three or four times daily until the optimal response is obtained. In general, the dose should not exceed 1,000 mg daily. For maintenance therapy, the dose should be adjusted to the minimum effective level, usually 400 to 800 mg daily.
For children younger than six years of age, the initial dose is 10 to 20 mg/kg per day twice or three times daily as tablets or four times daily as the suspension. To achieve optimal clinical response, the physician can increase the number of administrations to three or four times daily. If a satisfactory response is not achieved, plasma concentrations should be measured to determine whether they are in the therapeutic range.
Contraindications, Warnings, and Adverse Effects25
A boxed warning is included for carbamazepine. Further details are presented in Appendix A on page 410.
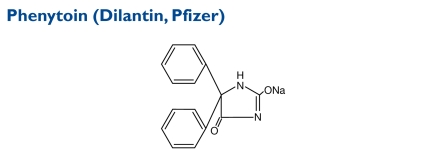
The chemical structure of phenytoin sodium is similar to that of the barbiturates, but it has a five-member ring. The chemical name is sodium 5,5-diphenyl-2, 4-imidazolidinedione. The molecular weight is 274.3.
Indication and Mechanism of Action26
Phenytoin is indicated for controlling generalized tonic–clonic (grand mal) and complex partial (psychomotor, temporal lobe) seizures and for preventing and treating seizures occurring during or following neurosurgery. The primary site of action appears to be the motor cortex, where the drug inhibits the spread of seizure activity. Possibly by promoting sodium efflux from neurons, phenytoin tends to stabilize the threshold against hyperexcitability caused by excessive stimulation or environmental changes capable of reducing membrane sodium gradient; this activity includes reducing post-tetanic potentiation at synapses. Loss of post-tetanic potentiation prevents cortical seizure foci from detonating adjacent cortical areas. Phenytoin reduces the maximal activity of brainstem centers responsible for the tonic phase of tonic–clonic seizures.
Dosage and Administration26
Patients who have not received previous treatment may begin with one 100-mg extended phenytoin capsule three times daily. The dose is then adjusted to suit individual requirements. For most adults, the satisfactory maintenance dose is one capsule three to four times daily. If necessary, the dose can be increased up to two capsules three times per day.
In adults, if seizure control is established with divided doses of three 100-mg phenytoin capsules daily, a once-daily dose of 300 mg of extended phenytoin capsules may be considered. Studies comparing divided doses of 300 mg with a single daily dose of this quantity indicated that absorption, peak plasma levels, biologic half-life, difference between peak and minimum values and urinary recovery were equivalent. Only extended phenytoin sodium capsules are recommended for once-daily dosing.
Some authors have advocated the use of an oral loading dose of phenytoin in adults who require rapid steady-state serum levels and when intravenous (IV) administration is not desirable. This dosing regimen should be reserved for patients in a clinic or hospital where phenytoin serum concentrations can be closely monitored. Patients with a history of renal or liver disease should not receive the oral loading regimen.
Initially, phenytoin capsules 1 g (1,000 mg) are divided into three doses (400, 300, and 300 mg) and are administered at two-hour intervals. The normal maintenance dose is then instituted 24 hours after the loading dose, with frequent serum concentration determinations.
In pediatric patients, the recommended initial administration is 5 mg/kg per day in two or three equally divided doses, with subsequent doses adjusted for each patient to a maximum of 300 mg daily. A recommended daily maintenance dosage is usually 4 to 8 mg/kg. Adolescents and children older than six years of age may require the minimum adult dose, 300 mg/day.
Contraindications, Warnings, and Adverse Effects26
There are no contraindications. Further details are presented in Appendix A on page 410.
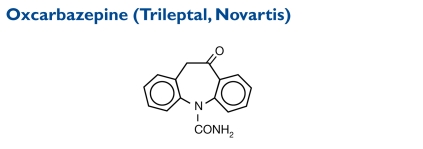
Oxcarbazepine (10,11-dihydro-10-oxo-5H-dibenz[b,f]aze-pine-5-carboxamide) has a molecular weight of 252.27.
Indication and Mechanism of Action27
Oxcarbazepine is used as monotherapy for adults and in children 4 to 16 years of age with partial seizures and as adjunctive therapy for children 2 years of age and older and for adults and children 2 to 16 years of age with partial seizures.
The drug’s activity is exerted primarily through its 10-mono-hydroxy metabolite (MHD). The precise mechanism by which oxcarbazepine and MHD exert their antiseizure effects is unknown. Preclinical evidence had indicated that they produce blockade of voltage-sensitive sodium channels, thereby stabilizing hyperexcited neural membranes, inhibiting repetitive neuronal firing, and diminishing the propagation of synaptic impulses.
These actions are thought to be important in preventing the spread of seizures in the intact brain. Increased potassium conductance and modulation of high-voltage activated calcium channels may also contribute to the agent’s antiseizure effects.
Dosage and Administration27
Oxcarbazepine is available as 150-, 300-, and 600-mg film-coated tablets for oral administration. It is also available as a 300-mg/5 mL (60-mg/mL) oral suspension.
Adults
Adjunctive Therapy. The initial dose is 600 mg/day twice daily. If indicated, the dose may be increased by a maximum of 600 mg/day at weekly intervals; the recommended daily dose is 1,200 mg/day. In controlled trials, doses above 1,200 mg/day were somewhat more effective; however, most patients were not able to tolerate the 2,400-mg/day dose, primarily because of CNS effects.
Switching to Monotherapy. Patients receiving concomitant AEDs may be switched to monotherapy by starting with oxcarbazepine at 600 mg/day twice daily (1,200 mg/day) while simultaneously initiating a reduced dose of a concomitant AED. The concomitant AED should be completely withdrawn over three to six weeks, and the maximum oxcarbazepine dose should be reached in about two to four weeks.
The oxcarbazepine dose may be increased, if necessary, by a maximum increment of 600 mg/day at approximately weekly intervals to achieve the recommended dose of 2,400 mg/day. In one study, a dose of 1,200 mg/day was effective when oxcarbazepine monotherapy was initiated.
Initiating Monotherapy. Patients who are not currently using AEDs may begin monotherapy with oxcarbazepine; the initial dose is 600 mg/day twice daily. The dose should be increased by 300 mg/day every third day to a total of 1,200 mg/day. A dose of 2,400 mg/day has been effective in patients who switched from other AEDs to oxcarbazepine monotherapy.
Pediatric Patients
Adjunctive Therapy. For children 4 to16 years of age, the initial daily dose of oxcarbazepine is 8 to 10 mg/kg twice daily, generally not to exceed 600 mg/day. The target maintenance dose should be achieved over a period of two weeks and is dependent on the patient’s weight.
For patients between 2 and 4 years of age, the initial daily dose is also 8 to 10 mg/kg twice daily. Generally, this dose should not exceed 600 mg/day. For patients weighing less than 20 kg, a starting dose of 16 to 20 mg/kg may be considered. The maximum maintenance dose should be achieved over a period of two to four weeks and should not exceed 60 mg/kg twice daily.
Switching to Monotherapy. Patients receiving concomitant AEDs may be switched to monotherapy with an initial oxcarbazepine dose of 8 to 10 mg/kg per day twice daily while simultaneously beginning a reduced dose of the concomitant AED. This AED may be completely withdrawn over a period of three to six weeks, whereas the oxcarbazepine dose may be increased.
Initiating Monotherapy. Patients who are not currently receiving another AED may try monotherapy. The initial oxcarbazepine dose is limited to 8 to 10 mg/kg per day in a twice-daily regimen.
Contraindications, Warnings, and Adverse Effects27
For further details, please see Appendix A on page 410.
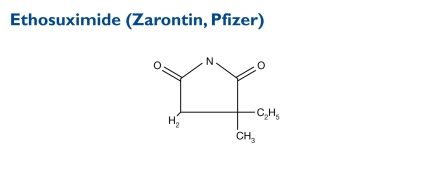
Ethosuximide is an antiseizure succinimide, chemically designated as alpha-ethyl-alpha methyl-succinimide. Its molecular weight is 141.17.
Indication and Mechanism of Action28,29
Ethosuximide is indicated for controlling absence (petit mal) epilepsy. It suppresses the paroxysmal three-cycles-per-second (3-Hz) spike-and-wave activity associated with lapses of consciousness, which are common in absence seizures. The frequency of epileptiform attacks is reduced, apparently by depression of the motor cortex and elevation of the threshold of the CNS to convulsive stimuli.28 The drug’s activity is accomplished via modulation of thalamic T-type calcium currents, thereby blocking synchronized firing of neurons associated with spike-and-wave discharges.29
Dosage and Administration28
Ethosuximide is given orally. The initial dose for patients three to six years of age is one 250-mg capsule per day; for patients six years of age and older, the dose is two 500-mg capsules per day. Thereafter, the dose must be individualized according to the patient’s response.
The dose should be augmented in small increments. One useful method is to increase the daily dose by 250 mg every four to seven days until seizure control is achieved with minimal side effects.
Dosages exceeding 1.5 g daily should be given in divided doses only under the strict supervision of a physician.
For most pediatric patients, the optimal dose is 20 mg/kg per day. This dose yields average plasma concentrations within the accepted therapeutic range of 40 to 100 mcg/mL. Subsequent dose schedules can be based on effectiveness and plasma concentration determinations.
Ethosuximide may be given in combination with other AEDs when other forms of epilepsy coexist with absence epilepsy.
Contraindications, Warnings, and Adverse Effects28
Additional details are listed in Appendix A on page 410.
ALTERNATIVE AGENTS
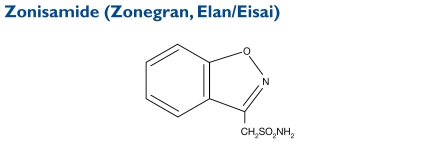
Zonisamide (1,2-benzisoxazole-3-methanesulfonamide) is an antiseizure sulfonamide, but it is not related to other AEDs. Its molecular weight is 212.23.
Indication and Mechanism of Action30
Zonisamide is indicated as adjunctive therapy for treating partial seizures in adults. Its precise mechanism of action is unknown, but the agent may produce its effects through action at sodium and calcium channels. It does not appear to potentiate the synaptic activity of GABA.
Dosage and Administration30
The initial dose should be 100 mg daily. After two weeks, the dose may be increased to 200 mg/day for at least two weeks. It can be increased to 300 mg/day and 400 mg/day, with the dose kept stable for at least two weeks to achieve steady state at each level. Evidence from controlled trials suggests that zonisamide doses of 100 to 600 mg/day are effective, but an increased response above 400 mg/day has not been reported.
Contraindications, Warnings, and Adverse Effects.30
Additional details are presented in Appendix A on page 410.
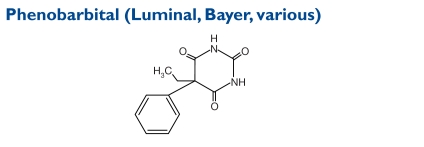
Phenobarbital tablets and elixir are given orally and are classified as Schedule IV controlled substances. Barbiturates are substituted pyrimidine derivatives in which the basic structure common to these drugs is barbituric acid, which has no CNS activity. The chemical name is 5-ethyl-5-phenylpyrim-idine-2,4,6(1H,3H,5H)-trione. The molecular weight is 232.24.
Indication and Mechanism of Action31–33
Phenobarbital is often used to treat seizures that occur in neonates and in the first year of life.31 It is effective for both generalized tonic–clonic seizures and partial seizures in patients of all ages.32 Phenobarbital is used to treat status epilepticus (continuous seizures with impaired consciousness between episodes).
The drug potentiates inhibitory neurotransmission by increasing the duration of time that GABA-mediated chloride channels remain open and reduces neurotransmitter release from nerve terminals, probably through its effect on calcium channels.33 Phenobarbital also diminishes excitatory neurotransmission by reducing the effects of glutamate.29
Dosage and Administration31,34
The therapeutic antiseizure concentration of phenobarbital in plasma is 10 to 25 mcg/mL.34 To achieve the blood levels considered therapeutic in children, higher per-kilogram dosages are generally necessary for this agent and for most other AEDs. In children and infants, phenobarbital at a loading dose of 15 to 20 mg/kg produces blood concentrations of about 20 mcg/mL shortly after administration.
In status epilepticus, it is imperative to achieve therapeutic phenobarbital blood levels as rapidly as possible, because a barbiturate-induced depression may occur along with postictal depression after the seizures are controlled. It is therefore important to use the minimal amount of drug required and to wait for the antiseizure effect to develop before a second dose is given.
For adults, as a daytime sedative, 30 to 120 mg is given orally daily in two or three divided doses. As a bedtime hypnotic agent, the dose is 100 to 320 mg. As an AED, the dose is 50 to 100 mg two to three times daily.
Contraindications, Warnings, and Adverse Effects31
More information is provided in Appendix A on page 410.
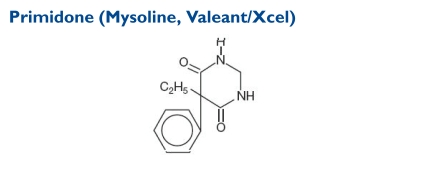
Primidone (5-ethyldihydro-5-phenyl-4,6[1H, 5H]-pyrim-idinedione) is used alone or together with other AEDs. Its molecular weight is 218.25.
Indication and Mechanism of Action35
Primidone is indicated for controlling psychomotor, focal epileptic, and grand mal seizures as well as grand mal seizures that have not responded to another AED. It raises electroshock or chemoshock seizure thresholds and may alter seizure patterns in experimental animals. The antiseizure mechanism is unknown.
Dosage and Administration35
Patients eight years of age and older who have received no previous treatment may start with either 50-mg or scored 250-mg primidone tablets: from days 1 to 3, 100 to 125 mg at bedtime; from days 4 to 6, 100 to 125 mg twice daily; from days 7 to 9, 100 to 125 mg three times daily; and from day 10 to the maintenance dose, 250 mg three times daily.
For most adults and children eight years of age and older, the usual maintenance dosage is three or four 250-mg primidone tablets daily in divided doses, taken as 250 mg three or four times daily. If necessary, the dose may be increased to five or six 250-mg tablets daily, but daily doses should not exceed 500 mg four times daily.
For patients already receiving another AED, primidone should be started at 100 to 125 mg at bedtime and gradually increased to the maintenance concentration as the dose of the other drug is gradually decreased. This regimen should be continued until a satisfactory dosage level is achieved for the combination or until the other medication is completely withdrawn. When therapy with primidone alone is the objective, the transition from concomitant therapy should not be completed in less than two weeks.
Children younger than eight years of age may receive 50 mg at bedtime from days 1 to 3; 50 mg twice daily from days 4 to 6; 100 mg twice daily from days 7 to 9; and 125 mg to 250 mg three times daily on day 10 to the maintenance dose. The usual maintenance dose for these patients is 125 to 250 mg three times daily or 10 to 25 mg/kg per day in divided doses.
Contraindications, Warnings, and Adverse Effects35
For further details, please see Appendix A on page 410.
Indication and Mechanism of Action36
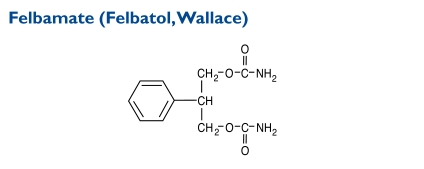
Felbamate (2-phenyl-1,3-propanediol dicarbamate) is not indicated as a first-line AED; it is recommended only for patients who respond inadequately to alternative treatments and whose epilepsy is so severe that a substantial risk of aplastic anemia and/or liver failure is deemed acceptable in light of the benefits conferred by its use.Its molecular weight is 238.24.
The mechanism by which felbamate exerts its antiseizure activity is unknown. Protection against maximal electroshock-induced seizures suggests that felbamate may reduce the spread of seizures, an effect possibly predictive of efficacy in generalized tonic–clonic or partial seizures. Felbamate may increase the seizure threshold, and it has weak inhibitory effects on GABA-receptor binding.
Dosage and Administration36
Felbamate has been studied as monotherapy and as adjunctive therapy in adults and as adjunctive therapy in children with seizures associated with Lennox–Gastaut syndrome. Because felbamate is added to or substituted for existing AEDs, it is strongly recommended that the dose be reduced to that of AEDs in the range of 20% to 33% to minimize adverse effects.
In clinical trials, most patients (adults 14 years of age and older) received 3,600 mg/day. Felbamate has not been systematically evaluated as initial monotherapy. The initial dose of felbamate is 1,200 mg/day in divided doses three or four times daily. The dose should be titrated in treatment-naive patients under close clinical supervision in 600-mg increments every two weeks to 2,400 mg/day, depending on the clinical response, and thereafter to 3,600 mg/day if indicated.
For patients switching to monotherapy, the starting dose is 1,200 mg/day in three or four divided doses. The dose of the concomitant AED is reduced by one-third when felbamate therapy is initiated. At week 2, the felbamate dose is increased to 2,400 mg/day; the dose of any other AED is decreased up to an additional one-third of its original dosage. At week 3, the felbamate dose is increased up to 3,600 mg/day; the dose of any other AED is reduced as indicated.
As adjunctive therapy, felbamate is added at 1,200 mg/day in three or four divided doses; any present AEDs are reduced by 20% in order to control plasma levels of concurrent phenytoin, valproic acid, phenobarbital, and carbamazepine and their metabolites. Further reductions of the concomitant AED dosage may be necessary in order to minimize adverse effects resulting from drug interactions. The felbamate dose is augmented in increments of 1,200 mg/day at weekly intervals to 3,600 mg/day.
Most adverse effects observed during felbamate adjunctive therapy resolve as the dose of any concomitant AEDs is decreased.
Contraindications, Warnings, and Adverse Effects36
For further details, please see Appendix A on page 410.
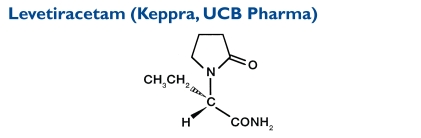
The chemical name of levetiracetam is (-)-(S)-α-ethyl-2-oxo-1-pyrrolidine acetamide. Levetiracetam, a single enantiomer, is not chemically related to existing AEDs.33 Its molecular weight is 170.21.
Indication and Mechanism of Action37
Levetiracetam is indicated as adjunctive therapy for (1) partial-onset seizures and epilepsy in adults and children four years of age and older; (2) myoclonic seizures in adults and adolescents 12 years of age and older with juvenile myoclonic epilepsy; and (3) primary generalized tonic–clonic seizures in adults and children six years of age and older with idiopathic generalized epilepsy.
Levetiracetam, at concentrations of up to 10 micromoles (μM), does not demonstrate binding affinity for a variety of known receptors, such as those associated with benzodiazepines, GABA, glycine, N-methyl-d-aspartate (NMDA), reuptake sites, and second messenger systems. Levetiracetam may selectively prevent hypersynchronization of epileptiform burst firing and propagation of seizure activity. This AED does not appear to facilitate GABAergic neurotransmission directly.
Dosage and Administration37
For partial-onset seizures in adults 16 years of age and older, the initial dose is 1,000 mg/day, given as 500 mg twice daily. Additional dosing increments may be given as 1,000 mg/day every two weeks to a maximum recommended daily dose of 3,000 mg. Doses higher than 3,000 mg/day have been used in open-label studies for periods of six months and longer. There is no evidence that doses greater than 3,000 mg/day confer additional benefit.
For pediatric patients between four and 16 years of age, the initial dose is 20 mg/kg daily in two divided doses (10 mg/kg twice daily). The daily dose should be augmented every two weeks in increments of 20 mg/kg to the recommended daily dose of 60 mg/kg (30 mg/kg twice daily). If the patient cannot tolerate a daily dose of 60 mg/kg, this dose may be reduced.
Patients weighing 20 kg or less should receive the oral solution. Patients weighing more than 20 kg can receive either the tablets or the oral solution.
Contraindications, Warnings, and Adverse Effects37
For more details, please see Appendix A on page 410.
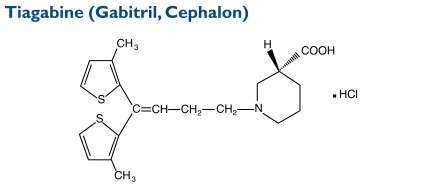
The chemical name of tiagabine HCl is (-)-(R)-1-[4,4-bis(3-methyl-2-thienyl)-3-butenyl]nipecotic acid HCl. Its molecular weight is 412.0.
Indication and Mechanism of Action38
Tiagabine is indicated as adjunctive therapy in adults and children 12 years of age and older with partial seizures. The precise mechanism by which tiagabine exerts its antiseizure effect is unknown, although it is believed to be related to its ability to enhance the activity of GABA, the major inhibitory neurotransmitter in the CNS. Tiagabine binds to recognition sites associated with the GABA uptake carrier, suggesting that this activity blocks GABA uptake into presynaptic neurons and thus permitting more GABA to be available for receptor binding on the surfaces of postsynaptic cells.
Dosage and Administration38
The drug is administered orally and is taken with food. A loading dose, a rapid escalation, and large dose increments of tiagabine should not be used. Dosage adjustments should be considered if a change in patient’s enzyme-inducing status occurs as a result of the addition, discontinuation, or dose change of the enzyme-inducing agent.
For adults and adolescents 12 years of age or older, certain recommendations apply if patients are already taking an enzyme-inducing AED (e.g., carbamazepine, phenytoin, primidone, or phenobarbital). When taking tiagabine, these are considered “induced patients.”
In adolescents 12 to 18 years of age, the initial tiagabine dose is 4 mg once daily. Modification of the concomitant AED dosage is not necessary unless clinically indicated. The total daily dose may be increased by 4 mg at the beginning of week 2. Thereafter, the total daily dose may be increased by 4 to 8 mg at weekly intervals until a clinical response is achieved or until the dose is increased to 32 mg/day. The total daily dose should be given in two to four divided doses. Doses above 32 mg/day have been tolerated in a small number of adolescents for a relatively short duration.
In adults, the initial tiagabine dose is 4 mg once daily. Dose adjustments of concomitant AEDs are not necessary unless clinically indicated. The total daily dose of tiagabine may be increased by 4 to 8 mg at weekly intervals until a clinical response is achieved or until the dose approaches 56 mg/day. The total daily dose should be given in two to four divided doses.
Doses above 56 mg/day have not been systematically evaluated in adequate, well-controlled clinical trials. Experience is limited in patients taking total daily doses above 32 mg/day using twice-daily dosing.
Contraindications, Warnings, and Adverse Effects38
For further details, please see Appendix A on page 410.
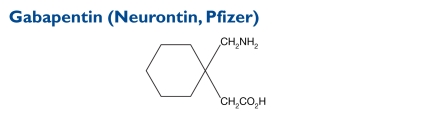
The chemical name for gabapentin is 1-(aminomethyl)cyclohexaneacetic acid. The molecular weight is 171.24.
Indication and Mechanism of Action39
Gabapentin is an adjunctive therapy for patients older than 12 years of age with partial seizures with and without secondary generalization and as an adjunctive therapy for pediatric patients three to 12 years of age with partial seizures.
Gabapentin is structurally related to the neurotransmitter GABA, but it does not modify GABAA or GABAB radioligand binding. It is not converted metabolically into GABA or a GABA agonist, and it does not inhibit GABA uptake or degradation. Gabapentin does not exhibit affinity for benzodiazepine, glutamate, NMDA, quisqualate, kainate, strychnine-insensitive or strychnine-sensitive glycine; alpha1, alpha2, or beta-adrenergic, adenosine A1 or A2, cholinergic muscarinic or nicotinic, dopamine D1 or D2; histamine H1; serotonin S1,or S2; or voltage-sensitive, calcium-channel receptor sites.
Dosage and Administration39
In patients older than 12 years of age, the effective dose of gabapentin is 900 to 1,800 mg/day, given in divided doses three times per day as 300-mg or 400-mg capsules or as 600-mg or 800-mg tablets. The starting dose is 300 mg three times per day. If necessary, the dose may be increased using 300-mg or 400-mg capsules, or 600-mg or 800-mg tablets three times a day, up to 1,800 mg/day.
Doses up to 2,400 mg/day have been well tolerated in long-term clinical studies. Doses of 3,600 mg/day, when administered to a small number of patients for a relatively short duration, have also been well tolerated. The maximum time between doses in the three-times-daily schedule should not exceed 12 hours.
In pediatric patients 3 to 12 years of age, the starting gabapentin dose ranges from 10 to 15 mg/kg per day in three divided doses. The effective dose is reached by upward titration over a period of approximately three days. The effective dose in patients five years of age and older is 25 to 35 mg/kg per day, given in three divided doses. The effective dose in pediatric patients three and four years of age is 40 mg/kg per day, given in three divided doses.
Contraindications, Warnings, and Adverse Effects39
Additional details are provided in Appendix A on page 410.
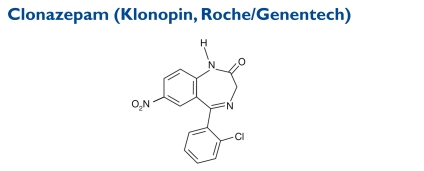
The formula for clonazepam is 5-(2-chlorophenyl)-1,3-dihydro-7-nitro-2H-1,4-benzodiazepin-2-one. Its molecular weight is 315.72.
Indication and Mechanism of Action40
A benzodiazepine derivative, clonazepam is useful alone or as an adjunctive therapy in Lennox–Gastaut syndrome (petit mal variant), akinetic seizures, and myoclonic seizures. In patients with absence (petit mal) seizures who have not responded to succinimides, clonazepam may be useful.
In some studies, up to 30% of patients have shown a loss of antiseizure activity, often within three months of administration. In some cases, a dosage adjustment may re-establish efficacy. The antiseizure actions of benzodiazepines result in large part from their ability to enhance GABA-induced increases in the conductance of Cl−.11
The precise mechanism by which clonazepam exerts its antiseizure and antipanic effects is unknown, although it is believed to be related to its ability to enhance the activity of GABA, the major inhibitory neurotransmitter in the CNS.
Dosage and Administration40,41
For adults, the initial clonazepam dose is divided into three doses. The total dose should not exceed 1.5 mg/day. The dose may be titrated upward in increments of 0.5 to 1 mg every three days until seizures are adequately controlled or until side effects preclude any further increase. The maintenance dose must be individualized for each patient, depending upon the response. The maximum recommended daily dose is 20 mg.41 The use of multiple anticonvulsants may result in an increase of depressant adverse effects. This consequence should be considered before clonazepam is added to an existing antiseizure regimen.
In children, clonazepam is administered orally. In order to minimize drowsiness, the initial dose for infants and children, including those up to 10 years of age or who weigh 30 kg, should be between 0.01 and 0.03 mg/kg per day but should not exceed 0.05 mg/kg per day, given in two or three divided doses. The dose should be increased by no more than 0.25 to 0.5 mg every third day until a daily maintenance dose of 0.1 to 0.2 mg/kg of body weight has been reached—unless seizures are controlled or side effects preclude further increases.
When possible, the daily dose should be divided into three equal doses. If doses are not equally divided, the largest dose should be given before the patient retires to bed.
Contraindications, Warnings, and Adverse Effects40
For further details, please see Appendix A on page 410.
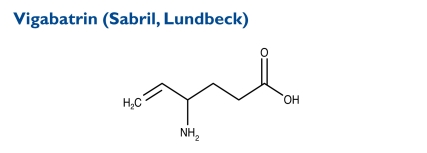
The chemical name of vigabatrin is (±) 4-amino-5-hexenoic acid. Its molecular weight is 129.16.
Indication and Mechanism of Action42
Vigabatrin is indicated as adjunctive therapy for adults with refractory complex partial seizures who have responded inadequately to alternative treatments and for whom the potential benefits outweigh the risk of vision loss.It is also approved as monotherapy for children one month to two years of age with infantile spasms when the potential benefits outweigh the potential risk of vision loss. The disorder can be difficult to treat because of the frequency of seizures.
The precise mechanism of the antiseizure effect is unknown, but it is believed to result from its action as an irreversible inhibitor of GABA transaminase (GABA-T), the enzyme responsible for the metabolism of the inhibitory neurotransmitter GABA. This action results in increased concentrations of GABA in the CNS.
No direct correlation between vigabatrin plasma concentration and efficacy has been established. The duration of drug effect is presumed to be dependent on the rate of enzyme resynthesis rather than on the rate of the drug’s elimination from the systemic circulation.
Dosage and Administration42
For refractory complex partial seizures in adults, vigabatrin oral 500-mg tablets are taken twice daily with or without food. Therapy should be initiated at 1 g/day (500 mg twice daily). The total daily dose may be increased in 500-mg increments at weekly intervals, depending on the patient’s response. The recommended dose in adults is 3 g/day (1.5 g twice daily). A 6-g/day dose has not been shown to confer additional benefit compared with the 3-g/day dose and is associated with an increased incidence of adverse events.
Vigabatrin is eliminated primarily via the kidneys. In patients with mild renal impairment (creatinine clearance [CrCl], 50–80 mL/minute), the dose should be decreased by 25%. With moderate renal impairment (CrCl, 30–50 mL/minute), the dose should be decreased by 50%. With severe renal impairment (CrCl, 10–30 mL/minute), the dose should be decreased by 75%.
Vigabatrin is not available in pharmacies because of the high risk of vision loss associated with the drug. It can be ordered directly only from Lundbeck Research in Deerfield, Illinois.
Contraindications, Warnings, and Adverse Effects42
There are no contraindications, but there is a boxed warning. Further details are given in Appendix A on page 410.
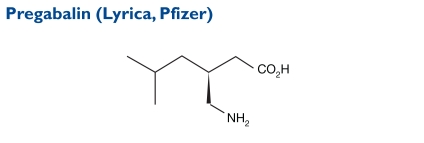
Pregabalin, (S)-3-(aminomethyl)-5-methylhexanoic acid), has a molecular weight of 159.23.
Indication and Mechanism of Action43
Pregabalin is indicated as an adjunctive therapy in the treatment of partial-onset epileptic seizures in adults. It binds with high affinity to the alpha2-delta site (an auxiliary subunit of voltage-gated calcium channels) in CNS tissues. Although pregabalin is a structural derivative of the inhibitory neurotransmitter GABA, it does not bind directly to GABAA, GABAB, or benzodiazepine receptors, nor does it augment GABAA responses in cultured neurons, alter rat brain GABA concentrations, or have acute effects on GABA uptake or degradation. However, in cultured neurons, the prolonged application of pregabalin increases the density of GABA transporter protein and increases the rate of functional GABA transports.
Dosage and Administration43
At doses of 150 to 600 mg/day, pregabalin has been effective as an adjunctive therapy for adults with partial-onset seizures. Both the efficacy and adverse-event profiles of pre-gabalin are dose-related. In general, it is recommended that patients begin with a total daily dose no greater than 150 mg (75 mg two times per day, or 50 mg three times per day). Based on individual patient response and tolerability, the dose may be increased to a maximum of 600 mg/day.
Because pregabalin is eliminated primarily by renal excretion, the dose should be adjusted in patients with reduced kidney function.
Contraindications, Warnings, and Adverse Effects43
Pregabalin is a controlled substance (Schedule V). For further details, please see Appendix A on page 410.
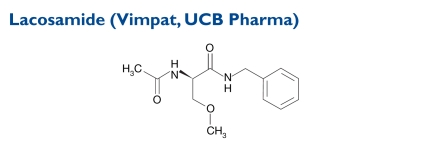
The chemical name of lacosamide, the single (R)-enantiomer, is (R)-2-acetamido-N-benzyl-3-methoxypropionamide (IUPAC). Its molecular weight is 250.3. Lacosamide is composed of functional amino acids.
Indication and Mechanism of Action44
Lacosamide tablets are indicated as adjunctive therapy for patients 17 years of age and older with partial-onset seizures. The precise mechanism by which the drug exerts antiepileptic effects in humans has not been fully elucidated. In vitro electrophysiological studies show that lacosamide selectively enhances slow inactivation of voltage-gated sodium channels, resulting in stabilization of hyperexcitable neuronal membranes and inhibition of repetitive neuronal firing.
Dosage and Administration44
Lacosamide can be initiated with either oral or IV administration. The initial dose is 50 mg twice daily (100 mg/day). The dose can be increased at weekly intervals by 100 mg/day, given as two divided doses up to the recommended maintenance dose of 200 to 400 mg/day, according to the patient’s response and tolerability. In clinical trials, 600 mg daily was not more effective than 400 mg daily and was associated with a substantially higher rate of adverse reactions.
When patients switch from the oral to the IV route, the initial total daily IV dose should be equivalent to the total daily dose and frequency of oral lacosamide and should be infused over a period of 30 to 60 minutes. Twice-daily IV infusion has been performed for up to five days.
At the end of the IV treatment period, patients may be switched from the IV to the oral route at the daily dose and frequency equivalent to the IV administration.
Contraindications, Warnings, and Adverse Effects44
Lacosamide is a Schedule V controlled substance. For further details, please see Appendix A on page 410.
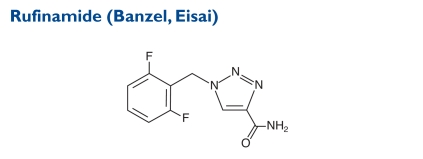
A triazole derivative, rufinamide is structurally unrelated to currently marketed AEDs. The chemical name is 1-[(2,6-difluorophenyl)methyl]-1H-1,2,3-triazole-4 carboxamide. The molecular weight is 238.19.
Indication and Mechanism of Action45
Rufinamide is indicated for the adjunctive treatment of seizures associated with Lennox–Gastaut syndrome in adults and children four years of age and older. The precise mechanism by which rufinamide exerts its antiepileptic effect is unknown. In vitro studies suggest that rufinamide modulates the activity of sodium channels and, in particular, prolongs the inactive state of the channel. At a concentration of 1M or greater, rufinamide significantly slowed sodium channel recovery from inactivation after a prolonged prepulse in cultured cortical neurons and limited sustained repetitive firing of sodium-dependent action potentials (EC50 of 3.8M).
Dosage and Administration45
For children four years of age and older who have Lennox–Gastaut syndrome, the initial daily dose is approximately 10 mg/kg per day, given in two equally divided doses. The dose should be titrated upward by approximately 10-mg/kg increments every other day to a target dose of 45 mg/kg per day or 3,200 mg/day, whichever is less, administered in two equally divided doses. It is not known whether doses lower than the target doses are effective.
For adults with Lennox–Gastaut syndrome, the starting daily dose is 400 to 800 mg/day, given in two equally divided doses. The dose should be increased by 400 to 800 mg/day every two days until a maximum daily dose of 3,200 mg, administered in two equally divided doses, is reached. It is not known whether doses lower than 3,200 mg/day are effective.
Contraindications, Warnings, and Adverse Effects45
For further details, please see Appendix A on page 410.
IN THE PIPELINE: AGENTS IN DEVELOPMENT
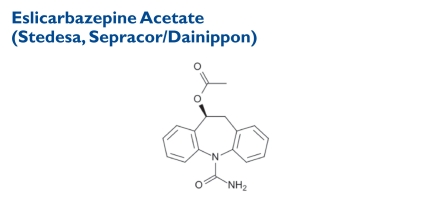
Indication and Mechanism of Action46,47
Known as ESL, this drug is a derivative of carbamazepine. The chemical formula is [(S)-(-)-10-acetoxy-10,11-dihydro-5H-dibenz[b,f]azepine-5-carboxamide. Its molecular weight is 296.32. ESL is a novel CNS-active drug with anticonvulsive activity. It is intended as adjunctive treatment in adults with refractory, partial-onset seizures.46 In clinical trials, ESL was associated with a significant reduction in seizure frequency compared with placebo in patients with partial-onset epilepsy.47
ESL acts as a voltage-gated sodium-channel blocker, inhibiting sodium channel-dependent release of neurotransmitter with a potency similar to that of carbamazepine and oxcarbazepine. The drug is an active metabolite of oxcarbazepine and has the same mechanism of action as carbamazepine.
In March 2009, ESL was submitted for FDA approval as an adjunctive therapy for adults with partial-onset seizures. The New Drug Application (NDA) was accepted for filing and has been under formal FDA review. In May 2010, however, the FDA decided not to approve Stedesa at that time. Dainippon declined to disclose the nature of the FDA’s concerns. Ultimately, results from ongoing studies will reveal the clinical utility of this agent as adjunctive therapy for partial-onset seizures.48
Dosage and Administration47
In a phase 3 clinical trial, once-daily ESL in doses of 400 mg, 800 mg, or 1,200 mg was administered to patients with partial seizures. The daily dose was titrated upward weekly by 400 mg to reach the maintenance dose. The frequency of seizures was significantly lower in patients receiving 1,200 and 800 mg than in the placebo group. Results were similar in patients receiving ESL with or without carbamazepine as a concomitant AED.
Contraindications, Warnings, and Adverse Effects47
Contraindications and warnings have not been published. Adverse effects include abnormal coordination, dizziness, somnolence, headache, nausea, diplopia, and vomiting.46,47
Indication and Mechanism of Action49
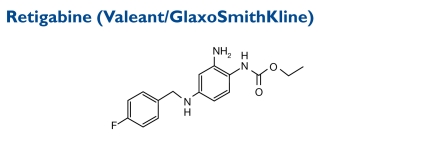
A first-in-its-class neuronal potassium-channel opener, retigabine is in late-stage development as an adjunctive therapy for partial-onset seizures. In phase 3 trials, retigabine was efficacious and reduced monthly seizure rates compared with placebo. In October 2009,Valeant and GSK submitted an NDA for retigabine to the FDA.
Contraindications, Warnings, and Adverse Effects49
Common adverse reactions in all completed trials to date (at an incidence greater than or equal to 5% and twice that of placebo) were dizziness, fatigue, confusion, vertigo, tremor, abnormal coordination, diplopia, attention disturbance, asthenia, and blurred vision. The drug is currently under review by the FDA for approval.
OTHER DRUGS UNDER INVESTIGATION
Clobazam (Lundbeck) is being studied for the treatment of Lennox–Gaustaut syndrome, and perampanel (E2007, Eisai) is being developed as a possible adjunctive therapy for patients with partial-onset seizures. Table 4 lists experimental agents in various stages of clinical development 50–54 and their structural relationship to FDA-approved AEDs.
Table 4.
Experimental Compounds In Phases 1 to 3 of Clinical Development
| Unapproved Antiepileptic Agents | Structural Relationship |
|---|---|
| Fluorofelbamate | Felbamate |
| Brivaracetam (Rikelta) | Levetiracetam |
| Seletracetam | Levetiracetam |
| Valrocemide | Valproic acid |
| Valnoctamide | Valproic acid |
| Propyl isopropyl acetamide | Valproic acid |
| Isovaleramide | Valproic acid |
| Carisbamate | Carbamate |
| Ganaxolone | Allopregnanolone |
| Talampanel | Benzodiazepine derivative |
| ICA-27243 | Retigabine |
| JZP-4 | Lamotrigine |
| Licarbazepine | Oxcarbazepine |
| ELB139 | Benzodiazepine receptor agonist |
| T2000 | Phenobarbital |
| XP13512 | Gabapentin |
| Tonabersat | Benzopyran |
| YKP-3089 | New chemical unrelated to others |
| NAX5055 | Galanin analogue |
| NPS1776 | Broad-spectrum antiseizure butanamide |
| ELB139 | Benzodiazepine agonist |
| ICA27243 | Selective KCNQ2/Q3 activators with anticonvulsant-like activity |
| Paxiline | Preclinical development only |
| NS1209 | Enhance GABAergic transmission |
| 2-deoxy-d-glucose | Antiseizure activity |
SURGICAL TREATMENT OF EPILEPSY
Surgery should be considered when seizures remain uncontrolled with optimal medical management and when they disrupt quality of life.4 Quantifying these problems, however, has been difficult, perhaps justly, because intractability is clearly more than continuing seizures. Some patients with refractory seizures experience little disability, whereas others find their lives severely compromised by infrequent attacks.4 Surgery has completely cured some patients, although they may remain disabled and may be unable to function productively.
Few patients benefit from further efforts at medical treatment if seizures have not been controlled after two trials of high-dose monotherapy with two appropriate AEDs and one trial of combination therapy.4 There are few blanket contraindications to epilepsy surgery today. If the seizure is caused by an underlying correctable brain condition, surgery may be a worthwhile option.
RECOMMENDATIONS FOR NEWLY DIAGNOSED EPILEPSY55,56
Both new and earlier AEDs are generally equally effective in new-onset epilepsy. Newer drugs tend to have fewer adverse effects. Guidelines of the American Academy of Neurology were based on a careful critique of the current clinical data and were designed to provide a strategy for decision making in patient care; they are not intended to exclude any other reasonable alternative treatments. Therefore, patients with newly diagnosed epilepsy can begin treatment with a standard AED (carbamazepine, phenytoin, valproic acid/divalproex, phenobarbital) or with a newer agent (gabapentin, lamotrigine, oxcarbazepine, topiramate). The choice depends on each patient’s characteristics. For children with newly diagnosed absence seizures, lamotrigine can be included in the options.
For children with refractory partial seizures, the summary of evidence-based guidelines for refractory epilepsy suggests gabapentin, lamotrigine, oxcarbazepine, and topiramate. In a previous guideline, neurology experts found that felbamate, another new AED, was also effective for partial seizures. However, felbamate has special risks that should be considered before practitioners prescribe it.
When considering which newer drugs were effective as monotherapy for partial seizures, the neurologists concluded that oxcarbazepine, topiramate, and possibly lamotrigine were effective in preventing refractory partial seizures. When the experts studied the data on generalized epilepsy that was refractory to other drug therapies, only topiramate was noted to be effective. Studies have not been conducted with the other AEDs. The group also recommended that lamotrigine and topiramate be used to treat drop attacks associated with the Lennox–Gastaut syndrome in adults and children.
AEDs can enhance quality of life and can help to reduce the number of seizures. Although all medications have some side effects, the choice of which drug and which side effects can be tolerated depends on each individual. AED therapy is the primary treatment for most patients with epilepsy. Early diagnosis and treatment of seizure disorders with a single appropriate therapeutic agent offer the best prospect of achieving prolonged seizure-free periods with the lowest risk of toxicity. The overall goals are to prevent seizures entirely with a minimum of adverse events and to provide a dosage schedule that is easy to follow.
An attempt should be made to determine the cause of the epilepsy with the intention of finding a correctable lesion, either structural or metabolic. Such a finding is more likely in very young patients or in patients whose first seizure appears during adulthood. After a decision is made to use drugs to control seizures—and this is often the case even if a specific etiologic mechanism is found—the goal of therapy is to keep the patient free of seizures without interfering with normal functioning.
In most cases, the regimen is started with a single drug. Classifying the seizure is an important element in treatment plan decisions, because some AEDs act differently against various seizure types. However, because of the considerable overlap between many AEDs, the agent can be chosen according to the patient’s specific needs, especially his or her assessment of adverse effects.
After the drug is selected, the initial dosage is usually expected to provide a plasma drug concentration during the plateau state, at least in the lower part of the range associated with clinical efficacy. However, to reduce the risk of dose-related adverse events, some drugs are initiated at a reduced dose and titrated upward to the lowest effective therapeutic dose. A loading dose is used only if the urgency to control seizures surpasses the risk of adverse events during initial therapy.
Health care professionals should assess the initial results by recognizing the time needed to reach the plateau state and the usual variability of incidence of seizures, and they should expect that some tolerance ordinarily develops to the sedative and minor adverse events associated with these AEDs. It is customary for the dose to be raised at suitable intervals in order to control seizures or as restricted by toxicity, and such change is preferably aided by monitoring plasma drug concentrations.
If a single drug does not sufficiently control seizures with the maximal tolerated dose and if patient compliance has been confirmed, another drug should be substituted. Unless serious adverse events direct otherwise, the dose should always be reduced slowly when a drug is being withdrawn to minimize the risk of precipitating status epilepticus.
If a second drug proves to be inadequate in eliminating seizures, many physicians substitute another agent before instituting a combined-drug regimen.
If two or more drugs do not control seizures, it is possible that the patient is experiencing more than one type of seizure.
CONCLUSION
Approximately 20% to 30% of patients have epilepsy that is resistant to medical therapy despite efforts to find an effective combination of AEDs.4 Surgery may be used to reduce the occurrence of seizures.
Newer AEDs are more expensive than the older drugs; this is clearly a problem for some individuals who must pay for their own medications. Common older drugs include valproic acid, phenytoin, carbamazepine, primidone, ethosuximide, clonazepam, and phenobarbital. Newer agents are gabapentin, lamotrigine, topiramate, tiagabine, levetiracetam, zonisamide, oxcarbazepine, pregabalin, eslicarbazepine, vigabatrin, lacosamide, and rufinamide. Felbamate, approved in 1993, is now rarely used because of its potential for serious adverse effects.
Because more monitoring of blood tests is required with earlier AEDs, this tends to puts newer drugs at an advantage. Older drugs are also associated with the potential to cause birth defects. Unfortunately, there is no certainty that new drugs are better in this regard, although there is promise. The newer drugs have not been clearly associated with birth defects, but data are insufficient to confirm that they are safe in pregnancy.
Patients are encouraged not to settle for unsatisfactory outcomes; instead, they should work with an informed physician to find a suitable treatment. Individuals starting on medication for the first time should discuss the relative advantages and disadvantages of the different choices.
Appendix A.
Warnings, Adverse Effects, and Contraindications for Selected Antiepileptic Agents
| 1 = FDA Black Box Warning | 2 = Warning | 3 = Adverse Effect | 4 = Contraindication | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbamazepine Tegretol | Clonazepam Klonopin | Eslicarbazepine Acetate Stedesa | Ethosuximide Zarontin | Felbamate Felbatol | Gabapentin Neurontin | Lacosadomide Vimpat | Lamotrigine Lamictal | Levetiracetam Keppra | Oxcarbazepine Trileptal | Phenobarbital Luminal | Phenytoin Sodium Dilantin | Pregabalin Lyrica | Primidone Mysoline | Rufinamide Banzel | Tiagabine Gabatril | Topiramate Topamax | Valproic Acid, Valproate, Depakene, Depacon | Vigabatrin Sabril | Zonisamide Zonegran | |
| Adenopathy | 3 | |||||||||||||||||||
| Adjustment of Dose in Renal Failure | 2 | |||||||||||||||||||
| Aggressivness | 3 | |||||||||||||||||||
| Agitation/Irritability | 3 | 3 | 3 | 3 | 3 | 3 | ||||||||||||||
| Agranulocytosis | 3 | 3 | 3 | 3 | ||||||||||||||||
| Alcohol Use, Effects on Phenytoin Serum Levels | 2 | |||||||||||||||||||
| Alcohol Use, No Consumption Allowed | 2 | |||||||||||||||||||
| Allergic Reaction | 3 | |||||||||||||||||||
| Alopecia | 3 | 3 | 3 | |||||||||||||||||
| Amnesia | 3 | |||||||||||||||||||
| Anaphylactic Reactions | 2 | |||||||||||||||||||
| Anemia | 3 | 3 | 1 | 2/3 | ||||||||||||||||
| Angioedema | 2 | |||||||||||||||||||
| Ankle and Facial Edema | 3 | |||||||||||||||||||
| Anorexia | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |||||||||||||
| Anticonvulsant Hypersensitivity Syndrome | 2 | |||||||||||||||||||
| Anxiety | 3 | 3 | 3 | |||||||||||||||||
| Aphonia | 3 | |||||||||||||||||||
| Aplastic Anemia and Agranulocytosis | 1/4 | 1 | 3 | |||||||||||||||||
| Apnea | 3 | |||||||||||||||||||
| Appearance, “Glassy-Eyed” | 3 | |||||||||||||||||||
| Appetite, Increased | 3 | |||||||||||||||||||
| Appetite, Loss of | 3 | |||||||||||||||||||
| Arrhythmias | 3 | |||||||||||||||||||
| Arthralgias | 3 | |||||||||||||||||||
| Asterixis | 3 | |||||||||||||||||||
| Asthenia | 3 | 3 | 3 | 3 | ||||||||||||||||
| Ataxia | 3 | 3 | 2/3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |||||||
| Atrial Fibrillation, Atrial Flutter | 2 | |||||||||||||||||||
| Behavorial Abonormalities | 3 | |||||||||||||||||||
| Binding in Eye and Other Melanin-Containing Tissues | 2 | |||||||||||||||||||
| Bipolar Disease, Use in Patients with | 2 | |||||||||||||||||||
| Bleeding, Vaginal | 3 | |||||||||||||||||||
| Blood Dyscrasias | 2 | 4 | 2 | |||||||||||||||||
| Bone Marrow Depression | 2/3 | |||||||||||||||||||
| Bradycardia | 3 | |||||||||||||||||||
| Bronchitis | 3 | |||||||||||||||||||
| Cardiac arrest | 3 | |||||||||||||||||||
| Central Nervous System Reactions/Depression | 3 | 2 | 2 | |||||||||||||||||
| Chest Congestion | 3 | |||||||||||||||||||
| Children, Use in | 2 | |||||||||||||||||||
| Chills | 3 | |||||||||||||||||||
| Chorea/Choreiform Movements | 3 | 3 | ||||||||||||||||||
| Chronic Idiopathic Constipation | 3 | |||||||||||||||||||
| Circulatory Depression | 3 | |||||||||||||||||||
| Coarsening of Facial Features | 3 | |||||||||||||||||||
| Cognition, Abnormal | 3 | 3 | 3 | |||||||||||||||||
| Cognitive/Neuropsychiatric Adverse Effects/Reactions | 2 | 2 | ||||||||||||||||||
| Coma | 3 | |||||||||||||||||||
| Concomitant Use with Oral Contraceptives | 2 | |||||||||||||||||||
| Confusion | 3 | 3 | 3 | 3 | ||||||||||||||||
| Constipation | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |||||||||||||
| Contusion | 3 | 3 | ||||||||||||||||||
| Convulsion | 3 | 3 | ||||||||||||||||||
| Coordination Problems | 3 | 3 | 3 | |||||||||||||||||
| Coordination, Abnormal | 3 | 3 | ||||||||||||||||||
| Coronary Artery Disease, Aggravation of | 3 | |||||||||||||||||||
| Cramps | 3 | 3 | ||||||||||||||||||
| Creatine Kinase Elevations | 2 | |||||||||||||||||||
| Death, Sudden, Unexplained | 2 | 2 | 2 | 2 | ||||||||||||||||
| Depression | 3 | 3 | 3 | 3 | 3 | |||||||||||||||
| Dermatological Reactions/Rash/Photosensitivity/Serious (Stevens– Johnson Syndrome, Toxic Epidermal Necrolysis) | 1 | 3 | 1/3 | 2/3/4 | 2,3 | 3 | 2 | |||||||||||||
| Diarrhea | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | ||||||||||||
| Difficulty with Concentration/Attention | 3 | 3 | 3 | |||||||||||||||||
| Digestive System Disorder | 3 | |||||||||||||||||||
| Diplopia | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | ||||||||||||
| Discontinuation, Abrupt or Rapid | 2 | |||||||||||||||||||
| Distribution Program, Restricted | 1/2 | |||||||||||||||||||
| Dizziness | 3 | 3 | 3 | 3 | 3 | 2/3 | 3 | 3 | 3 | 3 | 2/3 | 3 | 3 | 3 | 3 | 3 | 3 | |||
| Drowsiness | 3 | 3 | 3 | 3 | 3 | 3 | ||||||||||||||
| Dry Mouth | 3 | 3 | ||||||||||||||||||
| Dysarthria | 3 | |||||||||||||||||||
| Dysdiadochokinesis | 3 | |||||||||||||||||||
| Dysgeusia | 3 | |||||||||||||||||||
| Dyskinesias | 3 | |||||||||||||||||||
| Dyspepsia | 3 | 3 | 2 | 3 | ||||||||||||||||
| Dystonia | 3 | |||||||||||||||||||
| Dysuria | 3 | |||||||||||||||||||
| Edema | 3 | 2/3 | ||||||||||||||||||
| Emotional Disturbances | 3 | |||||||||||||||||||
| Encephalopathy, Hyperammonemic (w/wo Concomitant Valproic Acid Use) | 2 | 2 | ||||||||||||||||||
| Eosinophilia | 3 | 3 | 3 | |||||||||||||||||
| Exfoliative or Purpuric Dermatitis | 3 | |||||||||||||||||||
| Eye Movement, Abnormal | 3 | |||||||||||||||||||
| Familial Short QT Syndrome | 4 | |||||||||||||||||||
| Fatigue | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | ||||||||||
| Fever and/or Chills | 3 | 3 | 3 | 3 | 3 | 3 | ||||||||||||||
| Gait, Abnormal | 3 | 3 | 3 | 3 | 3 | |||||||||||||||
| Gastric Upset, Vague | 3 | |||||||||||||||||||
| Gastrointestinal System | 3 | |||||||||||||||||||
| Genitourinary System | 3 | |||||||||||||||||||
| Gingival Hyperplasia | 3 | |||||||||||||||||||
| Gingivitis | 3 | |||||||||||||||||||
| Glaucoma, Acute Narrow-Angle | 4 | |||||||||||||||||||
| Glaucoma, Secondary Angle-Closure | 2 | |||||||||||||||||||
| Glossitis | 3 | |||||||||||||||||||
| Granulocytopenia | 3 | |||||||||||||||||||
| Habit-forming | 2 | |||||||||||||||||||
| Hallucinations | 3 | 3 | ||||||||||||||||||
| Headache Disorder | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |||||||
| Hematological Events, Serious | 3 | 2 | ||||||||||||||||||
| Hemiparesis | 3 | |||||||||||||||||||
| Hepatic Failure/Dysfunction/Damage | 4 | 1/4 | 3 | 3 | 4 | |||||||||||||||
| Hepatic Function, Decreased | 2 | |||||||||||||||||||
| Hepatitis | 3 | |||||||||||||||||||
| Hepatotoxicity | 3 | 1 | ||||||||||||||||||
| Hiccups | 3 | |||||||||||||||||||
| Hyperkinesia | 3 | |||||||||||||||||||
| Hypersecretion in Upper Respiratory Tract | 3 | |||||||||||||||||||
| Hypersensitivity Reactions | 2/4 | 3 | 2 | 4 | ||||||||||||||||
| Hypertension | 3 | |||||||||||||||||||
| Hypertrophy and Swelling of Tongue | 3 | |||||||||||||||||||
| Hyponatremia | 2 | |||||||||||||||||||
| Hypotension | 3 | 3 | ||||||||||||||||||
| Hypotonia | 3 | |||||||||||||||||||
| Hypoventilation | 3 | |||||||||||||||||||
| Hysteria | 3 | |||||||||||||||||||
| Imbalance Disorder | 3 | |||||||||||||||||||
| Immunoglobulin Abnormalities | 3 | |||||||||||||||||||
| Impotence | 3 | 3 | ||||||||||||||||||
| Indigestion | 3 | |||||||||||||||||||
| Infection | 3 | |||||||||||||||||||
| Influenza | 3 | |||||||||||||||||||
| Injection-Site Reactions | 3 | |||||||||||||||||||
| Injury, Accidental | 3 | |||||||||||||||||||
| Insomnia | 3 | 3 | 3 | 3 | 3 | 3 | ||||||||||||||
| Integumentary | 3 | 3 | ||||||||||||||||||
| Interference with Cognitive or Motor Performance | 2 | |||||||||||||||||||
| Irritable Bowel Syndrome with Constipation | 3 | |||||||||||||||||||
| Jaundice, Cholestatic and Hepatocellular | 3 | |||||||||||||||||||
| Joint Pain | 3 | |||||||||||||||||||
| Kidney Changes | 3 | |||||||||||||||||||
| Kidney Stones | 2 | |||||||||||||||||||
| Kidney, Effects on | 2 | |||||||||||||||||||
| Laboratory Tests | 2 | 2 | ||||||||||||||||||
| Laceration | 3 | |||||||||||||||||||
| Language or Speech Problems | 2 | 3 | ||||||||||||||||||
| Lethargy | 3 | |||||||||||||||||||
| Leukocytosis | 3 | |||||||||||||||||||
| Leukopenia | 3 | 3 | 3 | |||||||||||||||||
| Libido, Increased | 3 | 2 | ||||||||||||||||||
| Liver Disease (see also Hepatic) | 4 | |||||||||||||||||||
| Liver, Effects on | 2 | |||||||||||||||||||
| Lymphadenopathy | 2/3 | |||||||||||||||||||
| Lymphoma | 3 | |||||||||||||||||||
| Magnetic Resonance Imaging Abnormalities | 2 | |||||||||||||||||||
| Medication Errors, Potential | 2 | |||||||||||||||||||
| Megaloblastic Anemia | 3 | 3 | ||||||||||||||||||
| Memory Loss | 3 | 3 | 3 | |||||||||||||||||
| Mental and Physical Slowing | 3 | 3 | ||||||||||||||||||
| Mental Confusion | 3 | |||||||||||||||||||
| Metabolic Acidosis | 2 | |||||||||||||||||||
| Monoamine Oxidase Inhibitors | 3 | |||||||||||||||||||
| Mood Problems/Changes | 3 | 3 | ||||||||||||||||||
| Morbilliform Skin Eruptions | 3 | |||||||||||||||||||
| Motor Twitching | 3 | |||||||||||||||||||
| Multiorgan Hypersensitivity Reactions | 2 | 2 | ||||||||||||||||||
| Multiorgan Failure, Acute | 2 | |||||||||||||||||||
| Muscle Weakness | 3 | |||||||||||||||||||
| Myoclonic Seizures | 3 | |||||||||||||||||||
| Myopia | 3 | |||||||||||||||||||
| Myopia, Acute | 2 | |||||||||||||||||||
| Nasopharyngitis | 3 | |||||||||||||||||||
| Nausea | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |||
| Nervousness | 3 | 3 | 3 | 3 | ||||||||||||||||
| Neural Tube Defects | 2 | |||||||||||||||||||
| Neuropsychiatric Events in Pediatric Patients | 3 | |||||||||||||||||||
| Neurotoxicity | 2/3 | |||||||||||||||||||
| Night Terrors/Nightmares | 3 | 3 | ||||||||||||||||||
| Nystagmus | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |||||||||||||
| Oligohidrosis and Hyperthermia in Pediatric Patients | 2 | |||||||||||||||||||
| Oligohidrosis/Hyperthermia | 2 | 2 | ||||||||||||||||||
| Pain, Abdominal | 3 | 3 | 3 | 3 | 3 | 3 | ||||||||||||||
| Pain, Acute or Chronic | 2 | |||||||||||||||||||
| Pain, Back | 3 | |||||||||||||||||||
| Pain, Chest | 3 | |||||||||||||||||||
| Palpitations | 3 | 3 | ||||||||||||||||||
| Pancreatitis | 1 | |||||||||||||||||||
| Pancytopenia | 3 | 3 | ||||||||||||||||||
| Paranoid Psychosis | 3 | |||||||||||||||||||
| Paresthesias | 1/3 | |||||||||||||||||||
| Periartertis Nodosa | 3 | |||||||||||||||||||
| Peripheral Edema | 2/3 | |||||||||||||||||||
| Peripheral Neuropathy | 2 | |||||||||||||||||||
| Pharyngitis | 3 | |||||||||||||||||||
| Platelet Count, Decreased | 1 | 2 | ||||||||||||||||||
| Porphyria | 3 | 2 | 4 | |||||||||||||||||
| Porphyria, Acute Intermittent | 3 | |||||||||||||||||||
| Porphyria, Exacerbation of | 2 | |||||||||||||||||||
| PR Interval Prolongation | 2 | 2 | 2 | |||||||||||||||||
| Pregnancy and Birth Defects | 2 | 3 | 2 | 2 | 2 | |||||||||||||||
| Pregnancy and Blood Clotting | 2 | |||||||||||||||||||
| Pregnancy and Withdrawal | 2 | 2 | ||||||||||||||||||
| Pregnancy, Use in | 2 | 2 | ||||||||||||||||||
| Pregnancy, Use in (Fetal Damage) | 2 | |||||||||||||||||||
| Pressure, High Intraocular | 3 | |||||||||||||||||||
| Primary Generalized Tonic–Clonic Seizures | 3 | |||||||||||||||||||
| Psychiatric Disorder | 3 | 2 | ||||||||||||||||||
| Psychotic Disorder | 3 | 3 | ||||||||||||||||||
| Purpura | 3 | |||||||||||||||||||
| Pyrexia | 3 | |||||||||||||||||||
| Rash, Serious/Pruritic | 3 | 1 | ||||||||||||||||||
| Red Blood Cell Hypoplasia | 3 | |||||||||||||||||||
| Renal Failure, Adustment of Dose in | 2 | |||||||||||||||||||
| Respiratory Arrest | 3 | 3 | ||||||||||||||||||
| Respiratory Depression | 3 | |||||||||||||||||||
| Respiratory Tract Infection, Upper | 3 | 3 | ||||||||||||||||||
| Rhinitis | 3 | |||||||||||||||||||
| Rhinorrhea | 3 | |||||||||||||||||||
| Seizure, Increased Frequency of | 2 | |||||||||||||||||||
| Seizures and Status Epilepticus in Patients without Epilepsy | 2 | 2 | ||||||||||||||||||
| Seizures on Withdrawal (see Withdrawal Symptoms, Seizure) | 2 | |||||||||||||||||||
| Sense of Touch, Decreased | 3 | |||||||||||||||||||
| Sensitivity to Tricyclic Compounds | 3 | |||||||||||||||||||
| Sensitivity, History of | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | |||||||||
| Serious Dermatological Reactions (Stevens– Johnson Syndrome, Toxic Epidermal Necrolysis) | 1 | 2 | 2/3 | |||||||||||||||||
| Sinus Infection or Irritation | 3 | |||||||||||||||||||
| Sleep Disturbances | 3 | |||||||||||||||||||
| Somnolence and Fatigue | 3 | 3 | 3 | 3 | 3 | 3 | 2/3 | 3 | 3 | 3 | 3/4 | 3 | ||||||||
| Sore Gums | 3 | |||||||||||||||||||
| Speech, Slurred | 3 | 3 | ||||||||||||||||||
| Stevens–Johnson Syndrome | 1 | 3 | 3 | 2 | 3 | 2 | ||||||||||||||
| Stomatitis | ||||||||||||||||||||
| Suicidal Behavior and Ideation | 1 | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | |||
| Sulfonamides, Fatal Reactions to | 2 | |||||||||||||||||||
| Syncope | 2 | 3 | 3 | |||||||||||||||||
| Synergistic Effects (Alcohol, Central Nervous System Depressants) | 2 | |||||||||||||||||||
| Systemic Lupus Erythematosus | 2 | |||||||||||||||||||
| Tachycardia | 3 | |||||||||||||||||||
| Taste Changes | 3 | |||||||||||||||||||
| Teratogenicity | 1 | 2 | ||||||||||||||||||
| Throat, Sore | 3 | |||||||||||||||||||
| Thrombocytopenia | 3 | |||||||||||||||||||
| Thromboembolism | 3 | |||||||||||||||||||
| Thrombophlebitis | 3 | |||||||||||||||||||
| Tongue, Coated | 3 | |||||||||||||||||||
| Tonic–Clonic Seizures, Primary Generalized | 3 | |||||||||||||||||||
| Tremor | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |||||||||||
| Tumorigenic Potential | 2 | 2 | 2 | |||||||||||||||||
| Unsteadiness | 3 | |||||||||||||||||||
| Urea Cycle Disorders | 2/4 | |||||||||||||||||||
| Urinary Retention | 3 | |||||||||||||||||||
| Urticaria | 3 | 3 | ||||||||||||||||||
| Vertigo | 3 | 3 | 3 | 3 | 3 | |||||||||||||||
| Vision, Abnormal | 3 | 3 | ||||||||||||||||||
| Vision, Blurred | 3 | |||||||||||||||||||
| Vision, Effects on | 2 | |||||||||||||||||||
| Vision, Loss of | 1/2 | |||||||||||||||||||
| Vision, Monitoring of (required) | 1/2 | |||||||||||||||||||
| Visual Field Defect | 3 | |||||||||||||||||||
| Vomiting | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |||||||
| Weakness | 3 | |||||||||||||||||||
| Weight Gain | 3 | 3 | 3 | 2/3 | 3 | 3 | 2/3 | |||||||||||||
| Weight Loss | ||||||||||||||||||||
| Weight Gain or Loss | 3 | |||||||||||||||||||
| White Blood Cell Count, Decreased | 1 | |||||||||||||||||||
| Withdrawal Seizures Leading to Status Epilepticus | 2 | 2 | 2 | |||||||||||||||||
| Withdrawal Symptoms/Seizure (see Seizures on Withdrawal) | 2/3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | ||||||||
Footnotes
Disclosure: The author reports no financial or commercial relationships in regard to this article.
REFERENCES
- 1.Epilepsy Foundation About epilepsy and seizures. Available at: www.epilepsyfoundation.org/about/statistics.cfm. Accessed January 10, 2010.
- 2.World Health Organization Epilepsy: Key facts. Available at: www.who.int/mediacentre/factsheets/fs999/en Accessed June 7, 2010. [Google Scholar]
- 3.National Institute of Neurological Disorders and Stroke . Seizures and epilepsy: Hope through research. Available at: www.ninds.nih.gov/disorders/epilepsy/detail_epilepsy.htm. Accessed January 19, 2010. [Google Scholar]
- 4.Bazil CW, Morrell MJ, Pedley TA. Epilepsy. In: Rowland LP, editor. Merritt’s Neurology. 11th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 990–1008. [Google Scholar]
- 5.Lowenstein DH. Seizures and epilepsy. In: Fauci AS, Kasper DL, Longo DL, editors. Harrison’s Principles of Internal Medicine. 17th ed. New York: McGraw-Hill; 2008. pp. 2498–2512. Section 2: Diseases of the Central Nervous System. [Google Scholar]
- 6.Tatum WO, Benbadis SR, Vale FL. The neurosurgical treatment of epilepsy. Arch Fam Med. 2000;9:1142–1146. doi: 10.1001/archfami.9.10.1142. [DOI] [PubMed] [Google Scholar]
- 7.Middleton DB. Seizure disorders. In: Taylor RB, editor. Family Medicine: Principles and Practice. 6th ed. New York: Springer-Verlag; 2003. [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Available at: www.cdc.gov/chronicdisease/resources/publications/AAG/epilepsy.htm. Accessed March 14, 2010.
- 9.Epilepsy and seizure statistics. Epilepsy Foundation. Available at: www.epilepsyfoundation.org/about/statistics.cfm. Accessed January 11, 2010.
- 10.McNamara JO. Drugs effective in the therapy of the epilepsies. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 9th ed. New York: McGraw-Hill; 1999. pp. 461–486. [Google Scholar]
- 11.Bloom FE. Neurotransmission and the central nervous system. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 9th ed. New York: McGraw-Hill; 1999. pp. 267–293. [Google Scholar]
- 12.Ropper AH, Samuels MA. Adams and Victor’s Principles of Neurology. 9th ed. New York: McGraw-Hill Medical; 2009. Epilepsy and other seizure disorders; pp. 304–338. [Google Scholar]
- 13.Ellenberg JG, Hirtz DG, Nelson KB. Do seizures in children cause intellectual deterioration? N Engl J Med. 1986;314:1085–1088. doi: 10.1056/NEJM198604243141705. [DOI] [PubMed] [Google Scholar]
- 14.Scottish Intercollegiate Guidelines Network (SIGN)Guideline No 70. Diagnosis and Management of Epilepsy in Adults Edinburgh, U.K.SIGN; 2003Available at: www.sign.ac.uk. Accessed April 2009 [Google Scholar]
- 15.Stephen LJ, Brodie MJ. The management of a first seizure: Still a major debate. Special problems—adults and elderly. Epilepsia. 2008;49(Suppl 1):45–49. doi: 10.1111/j.1528-1167.2008.01450.x. [DOI] [PubMed] [Google Scholar]
- 16.Elwes RDC, Johnson AL, Shorvon SD, Reynolds EH. The prognosis for seizure control in newly diagnosed epilepsy. N Engl J Med. 1984;311:944–947. doi: 10.1056/NEJM198410113111503. [DOI] [PubMed] [Google Scholar]
- 17.Stephen LJ, Brodie MJ. Selection of antiepileptic drugs in adults. Neurol Clin. 2009;27:967–992. doi: 10.1016/j.ncl.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Dulac O, Leppik IE, Chadwick DW, et al. Starting and stopping treatment. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. 2nd ed. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 1301–1309. [Google Scholar]
- 19.Valproic acid, package insert. Available at: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=12886; Accessed January 21,2010; Depakene, www.rxabbott.com/pdf/depakene.pdf Accessed June 23, 2010.
- 20.Schachter SC. Epilepsy therapy project, July 2008. Available at: www.epilepsy.com/pdfs_med/epilepsy_valproicacid.pdf. Accessed January 21, 2010.
- 21.Lamotrigine, package insert. Available at: http://dailymed.nlm.nihgov/dailymed/drugInfo.cfm?id=11858#section-13 Accessed January 22, 2010.
- 22.Lamictal XL. Update1: GlaxoSmithKline gets U.S. Lamictal XR approval. Available at: www.reuters.com/article/idUSL167633120090601. Accessed March 16, 2010.
- 23.Lang DG, Wang CM, Cooper BR. Lamotrigine, phenytoin, and carbamazepine interactions on the sodium current present in N4TG1 mouse neuroblastoma cells. J Pharmacol Exp Ther. 1993;266:829–835. [PubMed] [Google Scholar]
- 24.Topiramate, package insert. Available at: https://www.topamax.com/topamax/assets/topamax.pdf Accessed June 22, 2010.
- 25.Carbamazepine, package insert. Available at: www.pharma.us.novartis.com/product/pi/pdf/tegretol.pdf Accessed January 24, 2010.
- 26.Phenytoin, package insert. Available at: http://media.pfizer.com/files/products/uspi_dilantin.pdf Accessed January 25, 2020.
- 27.Oxcarbazepine, prescribing information. Available at: www.pharma.us.novartis.com/product/pi/pdf/trileptal.pdf Accessed January 25, 2010.
- 28.Ethosuximide, prescribing information: Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2009/012380s031lbl.pdf Accessed January 26, 2010.
- 29.White SH. Comparative anticonvulsant and mechanistic profile of the established and new antiepileptic drugs. Epilepsia. 1999;40(Suppl 5):2–10. doi: 10.1111/j.1528-1157.1999.tb00913.x. [DOI] [PubMed] [Google Scholar]
- 30.Zonisamide, prescribing information. Available at: www.eisai.com/pdf_files/ZonegranPIrev-1ver-1May2004.pdf Accessed January 29, 2010.
- 31.Holland KD. Efficacy, pharmacology and adverse effects of antiepileptic drugs. Neurol Clin. 2001;19:313–345. doi: 10.1016/s0733-8619(05)70021-9. [DOI] [PubMed] [Google Scholar]
- 32.Mattson RH, Cramer JA, Collins JF, et al. A comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondary generalized tonic–clonic seizures in adults. N Engl J Med. 1985;313:145–151. doi: 10.1056/NEJM198507183130303. [DOI] [PubMed] [Google Scholar]
- 33.Twyman RE, Rogers CJ, MacDonald RL. Differential regulation of gamma-aminobutyric acid receptor channels by diazepam and phenobarbital. Ann Neurol. 1989;25:213–220. doi: 10.1002/ana.410250302. [DOI] [PubMed] [Google Scholar]
- 34.Phenobarbital, prescribing information. Available at: www.rxlist.com/phenobarbital-drug.htm#ad Accessed January 26, 2010.
- 35.Primidone, prescribing information. Available at: www.healthyplace.com/other-info/psychiatric-medications/primidone-full-prescribing-information/menu-id-122 Accessed January 25, 2010.
- 36.Felbamate, prescribing information: Available at: www.felbatol.com/pi.pdf Accessed January 27, 2010.
- 37.Levetiracetam, prescribing information. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2009/021035s078s080,021505s021s024lbl.pdf Accessed January 28, 2010.
- 38.Tiagabine, prescribing information. Available at: http://daily-med.nlm.nih.gov/dailymed/drugInfo.cfm?id=14960 Accessed January 28, 2010.
- 39.Neurontin, prescribing information, Available at: http://media.pfizer.com/files/products/uspi_neurontin.pdf Accessed January 29, 2010.
- 40.Clonazepam, prescribing information. Available at: www.healthyplace.com/other-info/psychiatric-medications/clonazepam-klonopin-full-prescribing-information/menu-id-72/#dosage Accessed January 29, 2010.
- 41.Sato S, Malow BA. Benzodiazepines: Clonazepam. In: Levy RH, Mattson RH, Meldrum BS, editors. Antiepileptic Drugs. 4th ed. New York: Raven Press; 2005. [Google Scholar]
- 42.Vigabatrin, prescribing information. Available at: www.lundbeckinc.com/USA/products/CNS/Sabril/sabril_PI_CPS.pdf Accessed January 31, 2010.
- 43.Pregabalin, prescribing information. Available at: www.pfizer.com/files/products/uspi_lyrica.pdf Accessed February 1, 2010.
- 44.Lacosamide, prescribing information. Available at: www.vimpat.com/hcp/pdfs/vimpat%20PI.pdf Accessed February 1, 2010.
- 45.Rufinamide, prescribing information. Available at: www.eisai.com/package_inserts/Banzel%20PI%200109.PDF Accessed February 1, 2010.
- 46.Almeida L, Soares-da-Silva P. Eslicarbazepine acetate (BIA-2-093) Neurotherapeutics. 2007;4:88–96. doi: 10.1016/j.nurt.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talati R, White CM, Coleman CI. Eslicarbazepine: A novel antiepileptic agent designed for improved efficacy and safety. Formulary. 2009;44:357–361. [Google Scholar]
- 48.Larkin C.Bloomberg News, May 3, 2010. Available at: www.bloomberg.com/news/2010-05-03/dainippon-fails-to-win-approval-for-epilepsy-drug-update1-.html Accessed June 23, 2010.
- 49.Retigabine. Available at: www.dancewithshadows.com/pillscribe/new-epilepsy-drug-retigabine-under-fda-review-in-us Accessed January 31, 2010.
- 50.Advances in anti-epileptic drug therapy. Available at: www.epilepsyfoundation.org/epilepsyusa/magazine/Issue2-2009/AntiepilepticDrugTherapy.cfm Accessed February 2, 2010.
- 51.Bialer M, Johannessen SI, Kupferberg HJ, et al. Progress report on new antiepileptic drugs: A summary of the Seventh Eilat Conference (EILAT VII) Epilepsy Res. 2004;61(1–3):1–48. doi: 10.1016/j.eplepsyres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 52.Pitkänen A, Mathiesen C, Rønn CL, et al. Effect of novel AMPA antagonist, NS1209, on status epilepticus: An experimental study in rat. Epilepsy Res. 2009;74:45–54. doi: 10.1016/j.eplepsyres.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Wickenden AD, Krajewski JL, London B, et al. N-(6-chloro-pyridin-3-yl)-3,4-difluoro-benzamide (ICA-27243): A novel, selective KCNQ2/Q3 potassium channel activator. Mol Pharmacol. 2008;73:977–986. doi: 10.1124/mol.107.043216. [DOI] [PubMed] [Google Scholar]
- 54.Stafstrom CE, Ockuly JC, Murphree L, et al. Anticonvulsant and antiepileptic actions of 2-deoxy-d-glucose in epilepsy models. Ann Neurol. 2009;65:435–448. doi: 10.1002/ana.21603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.American Academy of Neurology Guideline summary for clinicians: Efficacy and tolerability of the new antiepileptic drugs, part 1: Treatment of new onset epilepsy. Available at: http://aan.com/professionals/practice/pdfs/clinician_ep_onset_e.pdf. Accessed February 2, 2010.
- 56.American Academy of Neurology Guideline summary for patients and their families: Efficacy and tolerability of the new antiepileptic drugs for the treatment of refractory epilepsy. Available at: http://aan.com/professionals/practice/pdfs/patient_ep_refract_c.pdf. Accessed February 2, 2010.