Abstract
Increasing numbers of women are seeking evaluation of screen-detected breast abnormalities, and more women with breast cancer are living with the consequences of treatment. Improved technologies have helped to individualize diagnostic evaluation and treatment, improve efficacy and minimize morbidity. This article highlights some of these technologies. Superior imaging techniques have improved breast cancer screening and show promise for intraoperative surgical guidance and postoperative specimen evaluation. Digital mammography improves the sensitivity of mammography for women younger than 50 years with dense breasts, and tomosynthesis may improve specificity. Magnetic resonance imaging provides sensitive delineation of the extent of the disease and superior screening for women with a greater than 25% lifetime risk of breast cancer Minimally invasive techniques have been developed for the assessment of intraductal lesions, biopsy of imaging abnormalities, staging of the axilla and breast radiotherapy. Ductoscopy facilitates intraductal biopsy and localization of lesions for excision, sentinel lymph node biopsy is becoming standard for axillary staging, and intraoperative radiotherapy has the potential to reduce treatment time and morbidity. Three-dimensional imaging allows correlation of final histology with preoperative imaging for superior margin assessment. Related techniques show promise for translation to the intraoperative setting for surgical guidance. New classifications of breast cancers based on gene expression, rather than morphology, describe subtypes with different prognoses and treatment implications, and new targeted therapies are emerging. Genetic fingerprints that predict treatment response and outcomes are being developed to assign targeted treatments to individual patients likely to benefit. Surgeons play a vital role in the successful integration of new technologies into practice.
Abstract
De plus en plus de femmes demandent une évaluation d’anomalies du sein détectées au dépistage et plus de femmes qui ont un cancer du sein vivent avec les conséquences du traitement. L’amélioration des technologies a aidé à personnaliser l’évaluation diagnostique et le traitement, à améliorer l’efficacité et à réduire au minimum la morbidité. Cet article présente certaines de ces technologies. Des techniques d’imagerie supérieures ont amélioré le dépistage du cancer du sein et sont porteuses de promesses pour le guidage intraopératoire et l’évaluation des spécimens après l’intervention. La mammographie numérique améliore la sensibilité de l’examen chez les femmes de moins de 50 ans qui ont les seins denses et la tomosynthèse peut améliorer la spécificité. L’imagerie par résonance magnétique circonscrit de façon sensible l’étendue de la maladie et assure un meilleur dépistage chez les femmes qui présentent un risque total de cancer du sein de plus de 25 %. Des techniques à effraction minimale permettent d’évaluer les lésions intracanalaires, d’effectuer une biopsie d’anomalies révélées par l’imagerie, de déterminer le stade au niveau des aisselles et de traiter le sein par radiothérapie. La canaloscopie facilite les biopsies intracanalaires et la localisation des lésions à exciser. La biopsie du ganglion lymphatique sentinelle devient la norme pour la détermination du stade au niveau des aisselles et la radiothérapie intraopératoire pourrait réduire la durée du traitement et la morbidité. L’imagerie tridimensionnelle permet d’établir un lien entre l’histologie finale et l’imagerie préopératoire et de mieux évaluer les marges. Des techniques connexes sont porteuses de promesses pour application au contexte peropératoire aux fins de guidage chirurgical. De nouvelles classifications du cancer du sein basées sur l’expression génique plutôt que sur la morphologie décrivent des sous-types qui présentent des pronostics différents et nécessitent des traitements différents. De nouvelles thérapies ciblées font leur apparition. Les empreintes génétiques qui permettent de prédire la réaction au traitement et les résultats sont en développement et permettront d’affecter des traitements ciblés aux patientes qui sont susceptibles d’en bénéficier. Les chirurgiens jouent un rôle vital dans l’intégration réussie des nouvelles technologies dans la pratique.
Increased public awareness of breast cancer has been accompanied by a marked increase in the number of women seeking assessment of symptomatic or screen-detected breast abnormalities. In developed countries where breast cancer is most common, a large proportion of the female population is within the age group for which screening is recommended and breast abnormalities are prevalent. These demographic realities all increase the need for thorough and accurate breast assessment.
Over the last 2–3 decades, the incidence of breast cancer has risen, and this is predominantly related to the increased use of screening and the detection of early breast cancers, including ductal carcinoma in situ.1–3 More importantly, the mortality rate from breast cancer has decreased significantly, owing to both the detection of earlier disease and improved treatment modalities.4,5 Thus, there are more survivors of breast cancer than at any previous time, and, along with the expectation of longer life expectancy, it is essential that the modalities used to detect and treat breast cancer are accompanied by improvements that minimize long-term morbidity. The future of breast cancer treatment is maximum control of the disease with a minimum of complications and to tailor aggressive treatments to the individuals most likely to benefit.
Heightened public awareness of the societal burden of breast cancer has resulted in a substantial investment in breast cancer research. Such research has fostered the development of a number of important technological advances with the goal of improving diagnostic accuracy and individualizing treatment to maximize effectiveness while minimizing short- and long-term morbidity. In this article, we present several novel approaches to the diagnosis of breast abnormalities and treatment, which incorporate some of these new technologies. These new technologies were discussed at a joint symposium of the Canadian Association of General Surgeons and the Canadian Society of Surgical Oncology at the Canadian Surgery Forum in Toronto on Sept. 7, 2007. The information presented reflects the personal experience of the authors and is not intended to be a comprehensive review.
Ductoscopy
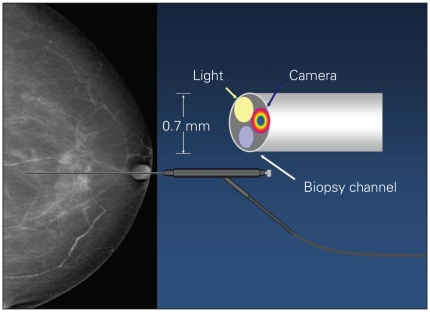
A tiny endoscope has been developed for direct visualization and assessment of intraluminal lesions, particularly within the proximal breast ducts. Despite the small diameter of the ductoscope, it contains several channels, including a light source, video camera and a biopsy channel, which allows saline to be flushed down the duct to aid in its passage and the aspiration of fluid for cytology (Fig. 1). Whereas the scope is fairly rigid, the central breast is quite malleable, and the camera can easily be guided down the ducts by moving the breast tissue as the scope is inserted. The scope is connected to a light, and a live, coloured video feed with reasonable resolution can be displayed on a computer screen. Normal ducts can be followed beyond several levels of bifurcations, and it is possible to differentiate between normal and abnormal ductal tissue. A biopsy catheter that fits over the ductoscope can biopsy most observed abnormalities.
Fig. 1.
Ductoscope used for the assessment of intraductal lesions. The ductoscope (Fibretech) contains several channels, including a light source, video camera and biopsy channel, which allows saline to be flushed down the duct to aid its passage and the aspiration of fluid for cytology.
Since 2005, we (A.E., W.L., D.M., M.R.) have performed over 50 ductoscopies in women with spontaneous uniductal nipple discharge. Under general anesthesia at the time of planned duct excision for suspected intraductal papilloma, the duct opening is gently dilated, and the ductoscope (Fibretech) is passed into the duct until the papilloma is visualized. Intraluminal localization of the papilloma directs the incision in the breast skin and dissection within the parenchyma to ensure that the papilloma is completely removed, thereby minimizing the removal of additional ductal tissue. Occasionally, no papilloma is found; this usually corresponds to a diagnosis of duct ectasia as a cause of the nipple discharge. In our initial series, we were able to cannulate the duct in 96% of patients and looked at the ducts for an average of 14 minutes per patient.6 Surgeons generally felt that the ductoscope was helpful in locating and defining the extent of duct excision. The learning curve was quite short.
Although our use has been limited to patients undergoing surgical excision of a breast or nipple duct, this technique has been used by others in the diagnostic workup of patients with nipple discharge. A ductoscope can be inserted in the outpatient or office setting, and there are even reports of papillomata being excised directly with the endoscope, thus avoiding the need for surgical duct excision.7 Although Sharma and colleagues8 reported that malignant lesions may be missed by intraluminal excision given the intraluminal origin of breast carcinoma, the potential application of ductoscopy to the management of malignant disease warrants further study.
Breast imaging
Mammography
Film mammography has been the gold standard for population-based screening for breast cancer and is the only breast imaging modality that has been proven to decrease mortality.9 However, mammographic breast density, a risk factor strongly associated with an increased risk of breast cancer, decreases the sensitivity of mammography.10,11 Consequently, there have been considerable efforts to improve and refine breast imaging technology.
Digital mammography acquires and records a digital image of the breast that is stored electronically rather than on film.12 The Digital Mammographic Imaging Screening Trial11 screened 49 528 asymptomatic women by both film and digital mammography. In the entire population, the diagnostic accuracy of film and digital mammography was the same; however, the accuracy of digital mammography was significantly higher among women younger than 50 years, those with heterogeneously or extremely dense breasts on mammography, and those who were pre- or perimenopausal. Other advantages of digital mammography include more efficient storage, transfer of images to readers distant from the site of image acquisition, lower doses of radiation and the capability to digitally manipulate images (e.g., change the contrast or magnification). A double read of a mammogram increases the rate of cancer detection by 10% but has not been widely adopted in North America.13 Digital mammography offers the possibility of computer-aided detection (CAD) of malignant disease, which may prove helpful in reducing variability among radiologists with different expertise.9 In a recent equivalence trial, CAD with a single reading of the mammogram was compared with a double reading or with double reading and CAD. The results suggested a single reading with CAD could be an alternative to double reading and could improve the rate of detection of cancer from screening mammograms read by a single reader.14 The main disadvantage of digital mammography is the increased capital investment required.15
Digital tomosynthesis
Tomosynthesis is a modified form of digital mammography in which multiple views of a stationary compressed breast are taken at different angles. These images are then reconstructed to generate 3-dimensional (3-D) radiographic images of the breast.16 It is hypothesized that this evaluation of the breast will decrease the number of false-positive and false-negative mammogram results from overlapping tissue and potentially minimize the number of recalls and additional views required to resolve these issues.15,17
Breast magnetic resonance imaging
Breast magnetic resonance imaging (MRI) generates images by recording the signals generated after radiofrequency excitation of nuclear particles in tissues exposed to a strong magnetic field. It typically involves use of an intravenous contrast agent, gadolinium diethylenetriamine penta-acetic acid (DPTA), which can locate tumours by highlighting areas containing dense vessel networks.18 Importantly, MRI is not influenced by breast density. There are important considerations when using MRI, such as its high sensitivity (94%–100%) but low specificity (37%–97%).17 Consequently, diagnostic imaging centres need to be prepared for the increased workload (higher recall rates and more benign biopsies) as a result of adding MRI capability17,19 by having the ability to perform MRI-guided biopsies of lesions imaged only with this highly sensitive but modestly specific diagnostic modality.
Indications for MRI screening
Among women who carry BRCA1 or BRCA2 mutations, MRI is more sensitive for the detection of breast cancer than mammography, ultrasound or clinical breast examination.20 In addition, there is evidence that untested first-degree relatives of known gene mutation carriers and women with a lifetime risk greater than 25% of breast cancer benefit from MRI breast screening.21 For other women with a high risk of breast cancer, including those with a history of chest radiation before age 30, lobular carcinoma in situ or atypical ductal hyperplasia, there is limited evidence for MRI screening, but there is expert panel support.21 Nonetheless, its high cost (10 times the cost of mammography), limited availability, low specificity (compared with mammography) and lack of standard machinery and methodology make it unlikely to become a population screening tool in its current form.9
Use of MRI in planning for surgery
A systematic review of diagnostic imaging, including MRI, in breast cancer was completed in 2006 by the Program in Evidence-Based Care at Cancer Care Ontario.19 This report suggested that MRI could provide useful information in a number of special clinical situations including in the setting of invasive lobular carcinoma, occult breast primary with axillary metastases, clinically palpable but mammographically occult breast cancer, preoperative assessment of the extent of disease especially in young patients with dense breasts, assessment of tumour response after neoadjuvant therapy for locally advanced breast cancer, assessment of the extent of disease in women with positive margins after initial breast-conserving surgery (BCS) and assessment of the extent of disease after local in-breast recurrence. In a more recent publication, MRI was shown to identify contralateral breast cancers that are occult to physical examination and mammography in 3% of women with proven breast cancer,22 suggesting that MRI will likely become part of the standard preoperative evaluation of patients with breast cancer in the future. Further study is required to determine if the identification of more extensive disease with MRI will lead to a corresponding reduction in breast cancer recurrence or mortality.17
Positron emission tomography
Positron emission tomography (PET) is unique among the diagnostic imaging techniques used in the management of breast cancer in that it identifies disease based on the detection of altered physiology rather than anatomy. Breast cancer cells frequently have increased glucose metabolism, which may be detected by increased uptake of a radiolabelled glucose compound (18-fluorodeoxyglucose). However, PET presently does not have a useful role in screening for breast cancer or in the assessment of patients with early breast cancer. Investigational use of PET for monitoring response to chemotherapy,15 distinguishing scars from recurrence12 and in the assessment of metastatic disease17 has been reported.
Image-guided surgery and specimen evaluation
Breast-conserving surgery, defined as complete tumour excision with microscopically clear resection margins and a good or excellent cosmetic result, is feasible in about 70% of women who undergo surgery for primary breast cancer. Women who undergo BCS for stage I or II breast cancer have comparable survival rates to those who receive a mastectomy;23 however, involvement of the resection margin with tumour has been associated with increased rates of local disease recurrence in numerous studies, even when radiotherapy is used.24–26 The cosmetic result after BCS is directly related to the volume of tissue excised, thus the current BCS practice is challenged to balance the needs of complete tumour excision with maximal preservation of normal breast tissue.
With the advent of screening, many breast cancers are not palpable at diagnosis and require localization to facilitate excision. Current localization techniques generally involve percutaneous wires placed preoperatively by a radiologist on the day of surgery. The wires are prone to being dislodged and therefore must be placed just before surgery. This creates important scheduling and other logistical issues. A titanium seed containing 125I developed for prostate cancer brachytherapy can be used to address this problem. The 60-day half-life of 125I allows the percutaneously placed seed to be positioned 1 day before surgery. The seed is detected intraoperatively using the gamma probes in routine use for sentinel lymph node biopsy. This approach is currently being evaluated by Peter Lovrics and colleagues, including the authors A.E., J.E., W.L., D.M. and M.R., in a clinical trial with the outcomes positive margin rate, procedural complication rate, localization and operative times, margin width and weight/volume of tissue excised.
Most localization techniques are effective at indicating the location of the tumour within the breast, and some techniques can often act as guides to disease extent along a single axis, leaving the surgeon to estimate the extent of disease in the other 2 dimensions. Even among women with palpable tumours, nonpalpable satellite lesions or in situ carcinoma that extends beyond the palpable invasive component are common. Optimal localization techniques will locate not only the position of the tumour in the breast but also the margin of the tumour in all dimensions. One approach has been to target tumour cells with antibodies labelled with tracers for intraoperative detection. We (C.H., F.W., M.L.Q.) have initiated a proof-of-principle study for this approach.
Three-dimensional imaging of many breast tumours can be achieved with a combination of mammography, ultrasound and MRI interpreted together by an experienced radiologist. Although such imaging may be highly accurate, it is not available to surgeons in a useable form at operation. Translation of preoperative 3-D imaging of breast tumours and their extent into the operative field is required for the benefits of such imaging to be fully used to improve treatment outcomes. Techniques for real-time intraoperative imaging of the extent of disease are under development. It is hoped that they will improve the accuracy of surgical excision, thereby reducing recurrence rates and extending the indications for BCS, although this remains to be proven.
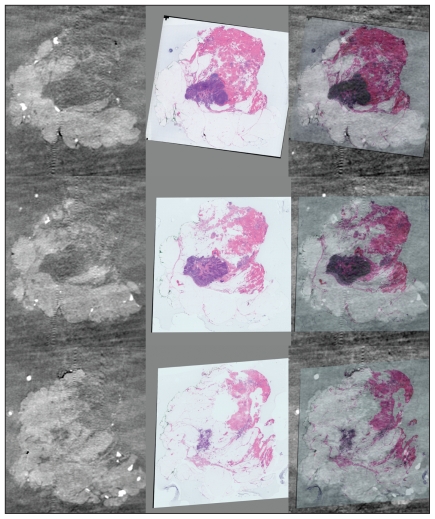
For the benefits of more accurate surgery to be realized, precise pathologic assessment of the margins of the excised specimen is required. Current methods of inking margins, formalin fixation and selection of histologic sections based on palpation or radiographic images of the sliced gross specimen are disadvantaged by alteration in specimen conformation on removal, the soft and fragile composition of the specimen and by the limitations of sampling. Techniques that maintain specimen conformation and orientation and combine 3-D specimen imaging and reconstruction with histologic examination have the potential to improve the accuracy of pathologic margin assessment (Fig. 2).
Fig. 2.
Correlation between imaging histology and 3-dimensional (3-D) reconstruction for comprehensive margin assessment. Whole-mount sections in series are digitally scanned. Each whole-mount section may be compared with slices imaged by micro-computed tomography (CT). The CT and digital images are then coregistered to allow 3-D reconstruction. Photograph courtesy of Dr. Gina Clarke.
Sentinel lymph node biopsy
The introduction of sentinel lymph node biopsy (SLNB) has been revolutionary in the surgical management of early breast cancer. The concept of the first node in the draining nodal basin reflecting the status of the entire basin was first described by Cabanas27 in penile cancer in 1977 and subsequently adopted for melanoma by Morton and colleagues28 in 1992. Sentinel lymph node biopsy for breast cancer was then reported using blue dye by Giuliano and colleagues29 and radioisotopes by Krag and colleagues30 in the mid 1990s. Since then, findings of numerous studies, including 2 randomized control trials demonstrating the accuracy of the procedure with no difference in survival, have been incorporated into guidelines by bodies such as the American Society of Clinical Oncology,31 supporting its use as the preferred method of evaluating the axilla in women with early breast cancer.32–37
There is no question that, unlike a lot of new surgical innovations, SLNB is a multidisciplinary procedure involving surgery, nuclear medicine and pathology. Close collaboration among these disciplines is required for optimal results. Appropriate training through residency or fellowship, formal courses or mentoring is needed before using SLNB as the sole staging procedure for the axilla.31 In addition, identification rates and longer-term results such as regional recurrences should be monitored.36 Whereas there have been various descriptions of the technical aspects of the procedure, mapping the sentinel node with both blue dye and radioisotopes yields consistent results. Radioisotopes can be injected subareolarly or subdermally over the tumour the day before or on the day of surgery.31 A hand-held intraoperative gamma probe is then used to remove, on average, 2 or 3 sentinel nodes using the “10% rule,” whereby nodes emitting a count greater than 10% of the highest count are considered to be sentinel nodes. In addition to those that stain blue, those that have a blue lymphatic vessel leading to it or are highly clinically suspicious on palpation during the procedure should also be removed as sentinel nodes. Whereas the use of intraoperative pathologic evaluation can help facilitate completion axillary dissection during the same procedure in cases of positive disease, this is not required and is not available in many centres.
One of the most important implications of SLNB has been the enhanced pathologic staging afforded by evaluation of a few nodes as opposed to the standard 10 or more nodes in a level I/II axillary dissection. Historically, axillary nodes were bivalved and evaluated using standard hematoxylin and eosin staining. Sentinel lymph node biopsy results in many fewer nodes, which can be sectioned serially, often at 2- to 3-mm intervals, allowing for more thorough analysis of each node. The most recent update of the American Joint Committee on Cancer staging system reflects this ability to detect increasingly small tumour deposits,38 although the clinical significance of such disease is less clear.
There is general agreement that completion axillary node dissection should be performed when macrometastases (> 2 mm) are present, given a rate of additional disease of up to 50%.31,39 However, controversy remains when micrometastases (> 0.2 mm to ≤ 2 mm) and isolated tumour cells (< 0.2 mm) are found. Micrometastatic disease has been associated with additional disease in up to 35% of patients,40 and retrospective data suggest that survival is poorer in those with micrometastatic disease compared with those who are truly node-negative.41–43 The finding of isolated tumour cells alone has been associated with additional nonsentinel node metastases in 0%–27% of patients undergoing a completion axillary lymph node dissection;44 however, this is difficult to interpret given the wide variation in SLNB protocols, methods of detection of additional metastases and the heterogeneous patient populations in these studies. This presents a challenge to clinicians in determining if completion dissection is warranted. In the absence of more definitive data, decisions about completion dissection in this subgroup should take into account the risk of additional disease and the impact on adjuvant treatment decisions on a case-by-case basis. Tools such as the Memorial Sloan Kettering nomogram, although not perfect, can help to quantify the risk of residual disease based on primary tumour characteristics and method of metastasis detection.45 We are awaiting long-term data on morbidity and outcomes from 2 North American randomized trials of SLNB: the National Surgical Adjuvant Breast and Bowel Project (protocol B-32; NSABP-B32) and the American College of Surgeons Oncology Group prognostic study of sentinel node and bone marrow micrometastases in women with clinical T1 or T2 N0 M0 breast cancer (ACOSOG Z0010).46 Other randomized trials of SLNB, including those evaluating recurrence and mortality in patients with micrometastases, are summarized in Table 1. In the interim, SLNB is rapidly becoming the preferred method of axillary staging for early-stage breast cancer, providing accurate assessment of nodal status with less morbidity.
Table 1.
Randomized trials of sentinel lymph node biopsy
| Study | Population | Comparison arms | Trial status |
|---|---|---|---|
| Milan47 | T1 tumours | SLNB; SLNB + ALND | Completed |
| GIVOM48 | T < 3 cm | ALND; SLNB | Completed |
| NSABP-B32 | T1–3 tumours | SLNB; SLNB + ALND | Closed, technical report published only |
| ACOSOG Z0010 | T1–2 tumours | SLNB and bone marrow aspiration (no comparison arm) | Closed |
| ALMANAC33 | Any T | SLNB; SLNB + ALND or 4 node sampling | Closed, quality of life data published |
| ACOSOG Z0011 | T1,2 with positive SLN | ALND; no further surgery | Closed early owing to poor accrual |
| IBCSG 23–01 | T1,2 tumours with micrometastases < 2 mm found in SLN | ALND; no further surgery | Open |
| AMAROS | T1,2 with positive SLN | ALND; RT to axilla level I/II | Open |
ACOSOG Z0010 = American College of Surgeons Oncology Group prognostic study of sentinel node and bone marrow micrometastases in women with clinical T1 or T2 N0 M0 breast cancer; ACOSOG Z0011 = American College of Surgeons Oncology Group randomized trial of axillary node dissection in women with clinical T1–2 N0 M0 breast cancer who have a positive sentinel node; ALND = axillary lymph node dissection; ALMANAC = Axillary Lymphatic Mapping Against Nodal Axillary Clearance; AMAROS = After Mapping of the Axilla: Radiotherapy Or Surgery?; GIVOM = Gruppo Interdisciplinare Veneto Oncologia Mammaria; IBCSG 23-01 = International Breast Cancer Study Group randomized trial of axillary dissection vs. no axillary dissection for patients with clinically node negative breast cancer and micrometastases in the sentinel node; NSABP B32 = National Surgical Adjuvant Breast and Bowel Project, Protocol B-32; RT = radiotherapy; SLN = sentinel lymph node; SLNB = sentinel lymph node biopsy.
Gene expression–based breast cancer subtype classification
Invasive breast cancer is a heterogeneous disease and has traditionally been classified histologically as either ductal or lobular. However, recent advances in our knowledge of the human genome and the availability of high-throughput, high-performance screening techniques of gene expression have permitted reclassification of breast cancer into luminal, normal-like, basal and Her2-positive subtypes.49 Thus, molecular profiling has allowed detailed definition of a number of breast cancer subtypes with different prognoses and treatment implications.
The luminal subtype makes up about 70% of breast cancers. Although luminal tumours are defined by a specific gene expression profile,50 an appropriate immunohistochemical surrogate for this subtype is the presence of estrogen receptor (ER) and/or progesterone receptor (PR). Compared with the other subtypes, luminal tumours have the most favourable overall and relapse-free survival times.49 This is partially because of the sensitivity of these breast cancers to therapies that target estrogen metabolism.
Cancers of the normal-like subtype resemble normal breast tissue in that basal epithelial cell and adipose cell genes are expressed at relatively high levels and luminal epithelial cell genes are expressed at relatively low levels. Patients with these tumours have intermediate survival times.
Her2-positive cancers (Her2-positive, basal keratins [5/6 and 17]–positive and ER-negative) make up 5%–10% of breast cancers. These tumours have traditionally been associated with a poorer prognosis; however, with the introduction of trastuzumab, a monoclonal antibody that targets Her2, the rate of disease-free survival has dramatically improved in patients with these lesions.51
Basal breast cancers (15%–20% of breast cancer) are associated with the lowest 5- to 10-year survival compared with the other subtypes. About 80% of basal breast cancers harbour mutations in the p53 tumour suppressor gene (v. 10%–15% for luminal), a known marker for poor prognosis and limited response to systemic treatment. Most basal breast cancers are ER-, PR- and Her2-negative (“triple negative”), making them refractory to the available targeted breast cancer therapies.
In contrast, basal breast cancers tend to overexpress growth factor receptors such as epithelial growth factor receptor and c-KIT; thus, agents that target these proteins may prove effective in this context. Furthermore, there is evidence that BRCA1-related breast cancer and basal breast cancer share common molecular defects.52 The observation that BRCA1-associated breast cancer may be sensitive to platinum-based chemotherapy has stimulated interest in using a similar chemotherapeutic approach to basal breast cancer.53 Interestingly, triple-negative patients who are alive and well 10 years after treatment have a relatively low rate of distant relapse and may form a stable “cured” population.54
At the same time that better classifications of breast cancer are being developed, new systemic and targeted therapies are emerging. To better match treatments to individual patients and tumours, genetic fingerprints that predict treatment response and outcomes are being developed.
Commercial gene panels
Current tools for predicting prognosis and treatment response are imprecise. Olson55 reported that patients who were considered “low risk” had a 23% mortality rate at 18.2 years, whereas 30% of “high-risk” patients remained disease-free with local–regional therapy alone. Consequently, oncologists tend to overprescribe chemotherapy because there is no accurate way to tell which patients’ disease has been treated adequately with surgery alone. Therefore, a better prognostic system has the potential to improve the risk-to-benefit ratio of potentially toxic and expensive treatments.
The development of DNA microarray technology has allowed the expression level of thousands of genes to be determined simultaneously. A process known as gene profiling measures the relative mRNA levels of individual genes, resulting in a genetic signature of the tumour that may better predict outcomes compared with current clinicopathologic parameters.
One of the first studies to demonstrate the potential utility of gene profiling in predicting outcomes was reported by the Netherland Cancer Institute.56,57 They retrospectively confirmed that a 70-gene panel could predict distant metastasis and overall survival better than any commonly used clinicopathologic systems. This 70-gene panel is now available commercially (MammaPrint; Agendia, about $ 1650 per test). The test is conducted on RNA extracted from fresh surgical specimens, and it must be completed quickly because RNA is not stable at room temperature. The test uses DNA microarray technology to determine the expression of 70 genes whose expression is correlated with clinical outcome. The initial study included women younger than 61 years with early-stage node-negative breast cancer and, therefore, may not be applicable to other patient populations. A Europe-wide multicentre prospective trial, MINDACT (Microarray In Node-negative disease and 1 to 3 positive lymph node Disease may Avoid ChemoTherapy), is now being conducted to evaluate MammaPrint for selection of chemotherapy in 5000 women with node-negative breast cancer.
Other gene profiling technologies, including quantitative real-time polymerase chain reaction, have also been developed. Oncotype DX (Genomic Health, about US$4200 per test) determines the expression level of 21 genes, 5 of which are controls. Oncotype DX has the advantage of using paraffin-embedded tissue, thereby eliminating the time restrictions for specimen processing. Thus, patients and their physicians may postpone the decision to order the test until after surgery. Oncotype DX was initially validated in patients with ER-positive node-negative early breast cancer treated in the NSABP trials.58 The test generates a recurrence score based on the 21-gene profile, and a few studies have shown that recurrence scores can predict recurrence in patients who received tamoxifen. Women with a high recurrence score (≥ 31) may benefit from the addition of chemotherapy to hormonal therapy, whereas those with low score (< 18) will not likely benefit. Currently, a North America–wide multicentre trial, the Trial for Assigning Individualized Options for Treatment (TAILORx), is being conducted to prospectively evaluate the utility of Oncotype DX in treatment planning for 10 000 women with ER-positive, Her2/neu-negative breast cancer.
Both Mammaprint and Oncotype DX represent the first generation of gene profiling tests that will facilitate better individualization of treatment plans. As our understanding of the molecular classification of breast cancer improves and new targeted therapies are developed, more refined gene profiling tests are expected.
Intraoperative radiotherapy
Standard breast-conserving therapy for invasive breast cancer mandates whole-breast radiation after wide local excision of the tumour. Radiation is generally given over a period of weeks and may result in altered texture, sensation and sensitivity of the breast skin and parenchyma. The evidence that breast-conserving therapy for early breast cancer yields comparable disease-free and overall survival to mastectomy has provided a rationale for the evaluation of more focused and less extensive radiotherapy after tumour excision, with the goal of reducing treatment time and morbidity. Whole-breast radiation after wide local excision is used primarily to reduce local recurrence. Because most in-breast recurrences occur at the site of the primary tumour,59–65 the necessity of whole-breast radiation has been questioned, and the concepts of partial breast radiation and delivery of radiation at the time of surgery have been developed.
There are several techniques for partial breast radiation, including interstitial brachytherapy with seeds66 or Mammosite67 conformal external breast radiation,68 intensity modulated radiotherapy69 and intraoperative breast radiation (IORT) either using the soft beam or the electron beam radiation.70–72
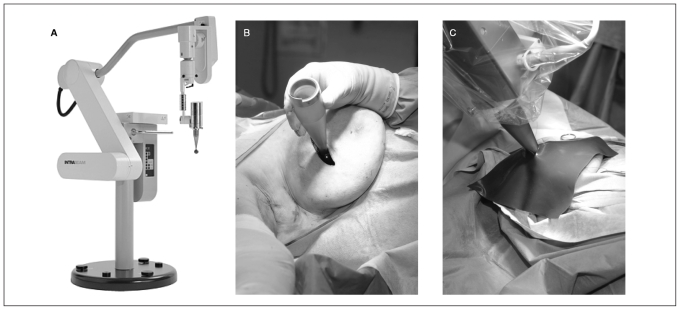
At the Princess Margaret Hospital, we (J.E., A.E., W.L., M.R., D.M.) are currently evaluating IORT using soft photon beam radiation as part of the targeted intraoperative radiotherapy (TARGIT) trial. This international study comparing IORT to standard external beam radiation had enrolled 779 patients at 16 institutions by 2006.73 Once the tumour has been excised, the duration of radiation, according to the size of the cavity, is calculated by the radiation physicist. The tumour bed receives 20 Gy using soft radiographs (50 kV) delivered with the Intrabeam Photon Radiosurgery System (Zeiss Inc.; Fig. 3). Following delivery of radiation, the source is removed, the cavity inspected for hemostasis and the skin closed in the usual way. The patient is followed at regular intervals to assess the condition of the skin over the operative site.
Fig. 3.
Intraoperative radiotherapy illustration of the Intrabeam unit (A) on its own, (B) in place with the applicator within the breast lumpectomy cavity and (C) in use with a tungsten shield over the breast.
Use of this technology has raised the question of optimal management in the event that surgical resection margins are found to be positive after tumour excision and IORT. Ideally, efforts should be made to avoid positive margins. By selecting patients according to age (> 50 yr), tumour size (< 3 cm), preoperative diagnosis with core needle biopsy and ultrasound-guided preoperative needle localization of nonpalpable tumours, multivariate analysis predicted a positive margin rate at first lumpectomy as low as 2.2%.74
Other groups have dealt with the issue of positive margins in different ways. Some have considered IORT as a boost and given postoperative whole-breast radiation;75 others have re-resected the margins before whole-breast radiation.76 An Australian group has opted for performing lumpectomy first and performing IORT during a second procedure after negative margins have been confirmed.77–81
The primary advantages of IORT are the precision in targeting the site of the primary tumour, thereby minimizing injury to normal breast tissue caused by whole-breast radiation, and the completion of surgery and radiation in 1 day. A possible limitation of IORT is the availability of operating room time. In our experience, IORT requires between 20 and 40 minutes for radiation time plus about 15 minutes to set up the radiation source.
Intraoperative breast radiation may represent an important technological advance that supports the paradigm shift from radical surgery and field radiation to targeted, minimally invasive surgery and partial breast irradiation.
Summary
Technological advances in many fields have been used in the diagnosis and treatment of breast cancer (Table 2). Whereas many of these advances have been clearly shown to improve outcomes for women with breast cancer, others require evaluation in prospective trials to determine their role and utility in clinical practice. Surgeons have an important role to play in the development, application and evaluation of new technologies aimed at improving patient outcomes.
Table 2.
Technological advances in the local evaluation and treatment of breast cancer
| Technique | Past | Present | Future |
|---|---|---|---|
| Definition of ductal anatomy | Contrast radiography | Direct endoscopic visualization | Intraductal excision of lesions |
| Breast cancer screening | Film mammography | Digital mammography | Possibly digital tomosynthesis |
| Preoperative evaluation of the extent of disease | Film mammography, ultrasonography | Digital mammography with computer-assisted detection, ultrasonography, MRI in selected circumstances | Possibly digital, tomosynthesis, ultrasonography, MRI |
| Breast-conserving surgery | Palpation | Wire localization | Molecular probes |
| Surgical specimen evaluation | Gross and microscopic evaluation | Orientation of specimen, inking of margins, specimen radiograph, directed sectioning | 3-D specimen imaging and imaging–pathology correlation |
| Axillary staging | Axillary node dissection | Sentinel lymph node biopsy | Preoperative imaging of axillary nodes +/− biopsy, sentinel lymph node biopsy |
| Tumour classification | Histologic type, size | Grade, lymphovascular invasion, ER, PR, Her2-neu oncogene expression | Genotyping |
| Gene expression | ER, PR | ER, PR, Her2-neu expression, Mammaprint,* Oncotype DX* | Gene array–based individualized treatment |
| Radiotherapy | Whole-breast radiation | Conformal whole-breast radiation | Partial-breast radiation |
3-D = 3-dimensional; ER = estrogen receptor; PR = progesterone receptor; MRI = magnetic resonance imaging.
Clinical trial.
Footnotes
This work was presented at the Annual Meeting of the Canadian Association of General Surgeons, Sept. 7, 2007, Toronto, Ont.
Competing interests: None declared.
Contributors: Drs. Leong, Escallon, Wright and Quan contributed to the conception and design of this article and the collection of data. Drs. Holloway and McCready contributed to the conception and design of the article and the interpretation of data. Dr. Easson contributed to the conception and design of the article. Drs. Wright, Quan and Reedjik contributed to the interpretation of data. All authors contributed to the writing of the article, which was revised by Drs. Holloway, McCready, Reedjik, Escallon and Liang. All authors approved the final version submitted for publication.
References
- 1.Ernster VL, Barclay J. Increases in ductal carcinoma in situ (DCIS) of the breast in relation to mammography: a dilemma. J Natl Cancer Inst Monogr. 1997;(22):151–6. doi: 10.1093/jncimono/1997.22.151. [DOI] [PubMed] [Google Scholar]
- 2.Duffy SW, Lynge E, Jonsson H, et al. Complexities in the estimation of overdiagnosis in breast cancer screening. Br J Cancer. 2008;99:1176–8. doi: 10.1038/sj.bjc.6604638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glass AG, Lacey JV, Jr, Carreon JD, et al. Breast cancer incidence, 1980–2006, combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst. 2007;99:1152–61. doi: 10.1093/jnci/djm059. [DOI] [PubMed] [Google Scholar]
- 4.Jatoi I, Miller AB. Why is breast-cancer mortality declining. Lancet Oncol. 2003;4:251–4. doi: 10.1016/s1470-2045(03)01037-4. [DOI] [PubMed] [Google Scholar]
- 5.Berry DA, Cronin KA, Plevritis SK, et al. Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–92. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 6.Simpson JS, Connolly EM, Leong WL, et al. Mammary ductoscopy in the evaluation of pathologic nipple discharge: a Canadian experience. Can J Surg. 2009;52:E245–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Hunerbein M, Raubach M, Gebauer B, et al. Ductoscopy and intraductal vacuum assisted biopsy in women with pathologic nipple discharge. Breast Cancer Res Treat. 2006;99:301–7. doi: 10.1007/s10549-006-9209-9. [DOI] [PubMed] [Google Scholar]
- 8.Sharma R, Dietz J, Wright H, et al. Comparative analysis of minimally invasive microductectomy versus major duct excision in patients with pathologic nipple discharge. Surgery. 2005;138:591–6. doi: 10.1016/j.surg.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Elmore JG, Armstrong K, Lehman CD, et al. Screening for breast cancer. JAMA. 2005;293:1245–56. doi: 10.1001/jama.293.10.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–36. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 11.Pisano ED, Gatsonis C, Hendrick E, et al. Digital Mammographic Imaging Screening Trial (DMIST) Investigators Group. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2005;353:1773–83. doi: 10.1056/NEJMoa052911. [DOI] [PubMed] [Google Scholar]
- 12.Smith JA, Andreopoulou E. An overview of the status of imaging screening technology for breast cancer. Ann Oncol. 2004;15(Suppl 1):I18–26. doi: 10.1093/annonc/mdh653. [DOI] [PubMed] [Google Scholar]
- 13.Taylor P, Potts HW. Computer aids and human second reading as interventions in screening mammography: two systematic reviews to compare effects on cancer detection and recall rate. Eur J Cancer. 2008;44:798–807. doi: 10.1016/j.ejca.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Gilbert FJ, Astley SM, Gillan MG, et al. Single reading with computer-aided detection for screening mammography. N Engl J Med. 2008;359:1675–84. doi: 10.1056/NEJMoa0803545. [DOI] [PubMed] [Google Scholar]
- 15.Bartella L, Smith CS, Dershaw DD, et al. Imaging breast cancer. Radiol Clin North Am. 2007;45:45–67. doi: 10.1016/j.rcl.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Smith AP, Hall PA, Marcello DM. Emerging technologies in breast cancer detection. Radiol Manage. 2004;26:16–24. [PubMed] [Google Scholar]
- 17.Reddy DH, Mendelson EB. Incorporating new imaging models in breast cancer management. Curr Treat Options Oncol. 2005;6:135–45. doi: 10.1007/s11864-005-0021-2. [DOI] [PubMed] [Google Scholar]
- 18.Smith RA. The evolving role of MRI in the detection and evaluation of breast cancer. N Engl J Med. 2007;356:1362–4. doi: 10.1056/NEJMe078006. [DOI] [PubMed] [Google Scholar]
- 19.Myers R, Minuk T, Johnston M Diagnostic Imaging Guidelines Panel. Diagnostic imaging in breast cancer: recommendations report. [(accessed 2009 Jan. 16)]. Available: www.cancercare.on.ca/pdf/pebcdibrf.pdf.
- 20.Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292:1317–25. doi: 10.1001/jama.292.11.1317. [DOI] [PubMed] [Google Scholar]
- 21.Warner E, Messersmith H, Causer P, et al. Magnetic resonance imaging screening of women at high risk for breast cancer: a clinical practice guideline. [(accessed 2008 Mar. 15)]. Available: www.cancercare.on.ca/pdf/pebcmris.pdf.
- 22.Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356:1295–303. doi: 10.1056/NEJMoa065447. [DOI] [PubMed] [Google Scholar]
- 23.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 24.Anscher MS, Jones P, Prosnitz LR, et al. Local failure and margin status in early-stage breast carcinoma treated with conservation surgery and radiation therapy [review] Ann Surg. 1993;218:22–8. doi: 10.1097/00000658-199307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heimann R, Powers C, Halpem HJ, et al. Breast preservation in stage I and II carcinoma of the breast. the university of chicago experience. Cancer. 1996;78:1722–30. doi: 10.1002/(sici)1097-0142(19961015)78:8<1722::aid-cncr12>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Horst KC, Smitt MC, Goffinet DR, et al. Predictors of local recurrence after breast-conservation therapy [review] Clin Breast Cancer. 2005;5:425–38. doi: 10.3816/cbc.2005.n.001. [DOI] [PubMed] [Google Scholar]
- 27.Cabanas RM. An approach for the treatment of penile carcinoma. Cancer. 1977;39:456–66. doi: 10.1002/1097-0142(197702)39:2<456::aid-cncr2820390214>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 28.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–9. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 29.Giuliano AE, Kirgan DM, Guenther JM, et al. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220:391–8. doi: 10.1097/00000658-199409000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krag D, Weaver D, Ashikaga T, et al. The sentinel node in breast cancer — a multicenter validation study. N Engl J Med. 1998;339:941–6. doi: 10.1056/NEJM199810013391401. [DOI] [PubMed] [Google Scholar]
- 31.Lyman GH, Giuliano AE, Somerfield MR, et al. American society of clinical oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–20. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Veronesi U, Paganelli G, Viale G, et al. Sentinel-lymph-node biopsy as a staging procedure in breast cancer: update of a randomised controlled study. Lancet Oncol. 2006;7:983–90. doi: 10.1016/S1470-2045(06)70947-0. [DOI] [PubMed] [Google Scholar]
- 33.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC trial [erratum in J Natl Cancer Inst 2006;98:876] J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 34.McMasters KM, Tuttle TM, Carlson DJ, et al. Sentinel lymph node biopsy for breast cancer: a suitable alternative to routine axillary dissection in multi-institutional practice when optimal technique is used. J Clin Oncol. 2000;18:2560–6. doi: 10.1200/JCO.2000.18.13.2560. [DOI] [PubMed] [Google Scholar]
- 35.Martin RC, II, Edwards MJ, Wong SL, et al. Practical guidelines for optimal gamma probe detection of sentinel lymph nodes in breast cancer: results of a multi-institutional study. for the university of louisville breast cancer study group. Surgery. 2000;128:139–44. doi: 10.1067/msy.2000.108064. [DOI] [PubMed] [Google Scholar]
- 36.American Society of Breast Surgeons. Consensus statement on guidelines for performing sentinel lymph node dissection in breast cancer. 4th revision. [(accessed 2008 March)]. Available: www.breastsurgeons.org/slnd.shtml.
- 37.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–8. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 38.Singletary SE, Allred C, Ashley P, et al. Staging system for breast cancer: revisions for the 6th edition of the AJCC cancer staging manual. Surg Clin North Am. 2003;83:803–19. doi: 10.1016/S0039-6109(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 39.Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer. 2006;106:4–16. doi: 10.1002/cncr.21568. [DOI] [PubMed] [Google Scholar]
- 40.McCready DR, Yong WS, Ng AK, et al. Influence of the new AJCC breast cancer staging system on sentinel lymph node positivity and false-negative rates. J Natl Cancer Inst. 2004;96:873–5. doi: 10.1093/jnci/djh142. [DOI] [PubMed] [Google Scholar]
- 41.Chen SL, Hoehne FM, Giuliano AE. The prognostic significance of micrometastases in breast cancer: a SEER population-based analysis. Ann Surg Oncol. 2007;14:3378–84. doi: 10.1245/s10434-007-9513-6. [DOI] [PubMed] [Google Scholar]
- 42.Tan LK, Giri D, Hummer AJ, et al. Occult axillary node metastases in breast cancer are prognostically significant: results in 368 node-negative patients with 20-year follow-up. J Clin Oncol. 2008;26:1803–9. doi: 10.1200/JCO.2007.12.6425. [DOI] [PubMed] [Google Scholar]
- 43.Cote RJ, Peterson HF, Chaiwun B, et al. Role of immunohistochemical detection of lymph-node metastases in management of breast cancer. International breast cancer study group. Lancet. 1999;354:896–900. doi: 10.1016/s0140-6736(98)11104-2. [DOI] [PubMed] [Google Scholar]
- 44.van Deurzen CH, de Boer M, Monninkhof EM, et al. Non-sentinel lymph node metastases associated with isolated breast cancer cells in the sentinel node. J Natl Cancer Inst. 2008;100:1574–80. doi: 10.1093/jnci/djn343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10:1140–51. doi: 10.1245/aso.2003.03.015. [DOI] [PubMed] [Google Scholar]
- 46.White RL, Jr, Wilke LG. Update on the NSABP and ACOSOG breast cancer sentinel node trials. Am Surg. 2004;70:420–4. [PubMed] [Google Scholar]
- 47.Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349:546–53. doi: 10.1056/NEJMoa012782. [DOI] [PubMed] [Google Scholar]
- 48.Zavagno G, De Salvo GL, Scalco G, et al. GIVOM Trialists. A randomized clinical trial on sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer: results of the Sentinella/GIVOM trial. Ann Surg. 2008;247:207–13. doi: 10.1097/SLA.0b013e31812e6a73. [DOI] [PubMed] [Google Scholar]
- 49.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 51.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–72. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 52.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer: therapeutic options. Lancet Oncol. 2007;8:235–44. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 53.Tutt AN, Lord CJ, McCabe N, et al. Exploiting the DNA repair defect in BRCA mutant cells in the design of new therapeutic strategies for cancer. Cold Spring Harb Symp Quant Biol. 2005;70:139–48. doi: 10.1101/sqb.2005.70.012. [DOI] [PubMed] [Google Scholar]
- 54.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 55.Olson JA., Jr Application of microarray profiling to clinical trials in cancer. Surgery. 2004;136:519–23. doi: 10.1016/j.surg.2004.05.041. [DOI] [PubMed] [Google Scholar]
- 56.van ‘t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 57.van de Vijver MJ, He YD, van’t Veer LJ, et al. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 58.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 59.Clark RM, Wilkinson RH, Mahoney LJ, et al. Breast cancer: a 21-year experience with conservative surgery and radiation. Int J Radiat Oncol Biol Phys. 1982;8:967–79. doi: 10.1016/0360-3016(82)90163-8. [DOI] [PubMed] [Google Scholar]
- 60.Boyages J, Recht A, Connolly JL, et al. Early breast cancer: predictors of breast recurrence for patients treated with conservative surgery and radiation therapy. Radiother Oncol. 1990;19:29–41. doi: 10.1016/0167-8140(90)90163-q. [DOI] [PubMed] [Google Scholar]
- 61.Kurtz JM, Spitalier JM. Local recurrence after breast-conserving surgery and radiotherapy: What have we learned. Int J Radiat Oncol Biol Phys. 1990;19:1087–9. doi: 10.1016/0360-3016(90)90038-l. [DOI] [PubMed] [Google Scholar]
- 62.Kurtz JM, Spitalier JM, Amalric R, et al. The prognostic significance of late local recurrence after breast-conserving therapy. Int J Radiat Oncol Biol Phys. 1990;18:87–93. doi: 10.1016/0360-3016(90)90271-k. [DOI] [PubMed] [Google Scholar]
- 63.Fisher ER, Anderson S, Redmond C, et al. Ipsilateral breast tumor recurrence and survival following lumpectomy and irradiation: pathological findings from NSABP protocol B-06. Semin Surg Oncol. 1992;8:161–6. [PubMed] [Google Scholar]
- 64.Fisher B, Wickerham DL, Deutsch M, et al. Breast tumor recurrence following lumpectomy with and without breast irradiation: an overview of recent NSABP findings. Semin Surg Oncol. 1992;8:153–60. [PubMed] [Google Scholar]
- 65.Veronesi U, Luini A, Del Vecchio M, et al. Radiotherapy after breast-preserving surgery in women with localized cancer of the breast. N Engl J Med. 1993;328:1587–91. doi: 10.1056/NEJM199306033282202. [DOI] [PubMed] [Google Scholar]
- 66.Van Zyl JA, Muller AG. Breast-conserving treatment for stage I and II cancer. tumour excision, axillary dissection, peri-operative interstitial irradiation, with or without peri-operative chemotherapy, followed by breast irradiation–the tygerberg hospital experience. S Afr Med J. 1989;75:519–23. [PubMed] [Google Scholar]
- 67.McCormick B. Partial-breast radiation for early staged breast cancers: hypothesis, existing data, and a planned phase III trial. J Natl Compr Canc Netw. 2005;3:301–7. doi: 10.6004/jnccn.2005.0017. [DOI] [PubMed] [Google Scholar]
- 68.Vicini FA, Remouchamps V, Wallace M, et al. Ongoing clinical experience utilizing 3D conformal external beam radiotherapy to deliver partial-breast irradiation in patients with early-stage breast cancer treated with breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2003;57:1247–53. doi: 10.1016/s0360-3016(03)01573-6. [DOI] [PubMed] [Google Scholar]
- 69.Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26:2085–92. doi: 10.1200/JCO.2007.15.2488. [DOI] [PubMed] [Google Scholar]
- 70.Haffty BG, Buchholz TA, McCormick B. Should intensity-modulated radiation therapy be the standard of care in the conservatively managed breast cancer patient. J Clin Oncol. 2008;26:2072–4. doi: 10.1200/JCO.2007.15.9442. [DOI] [PubMed] [Google Scholar]
- 71.Donovan EM, Yarnold JR, Adams EJ, et al. An investigation into methods of IMRT planning applied to breast radiotherapy. Br J Radiol. 2008;81:311–22. doi: 10.1259/bjr/28583675. [DOI] [PubMed] [Google Scholar]
- 72.Rusthoven KE, Carter DL, Howell K, et al. Accelerated partial-breast intensity-modulated radiotherapy results in improved dose distribution when compared with three-dimensional treatment-planning techniques. Int J Radiat Oncol Biol Phys. 2008;70:296–302. doi: 10.1016/j.ijrobp.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 73.Holmes DR, Baum M, Joseph D. The TARGIT trial: targeted intraoperative radiation therapy versus conventional postoperative whole-breast radiotherapy after breast-conserving surgery for the management of early-stage invasive breast cancer (a trial update) Am J Surg. 2007;194:507–10. doi: 10.1016/j.amjsurg.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 74.Schiller DE, Le LW, Cho BC, et al. Factors associated with negative margins of lumpectomy specimen: potential use in selecting patients for intraoperative radiotherapy. Ann Surg Oncol. 2008;15:833–42. doi: 10.1245/s10434-007-9711-2. [DOI] [PubMed] [Google Scholar]
- 75.Reitsamer R, Sedlmayer F, Kopp M, et al. Concepts and techniques of intraoperative radiotherapy (IORT) for breast cancer. Breast Cancer. 2008;15:40–6. doi: 10.1007/s12282-007-0001-4. [DOI] [PubMed] [Google Scholar]
- 76.Joseph DJ, Bydder S, Jackson LR, et al. Prospective trial of intraoperative radiation treatment for breast cancer. ANZ J Surg. 2004;74:1043–8. doi: 10.1111/j.1445-1433.2004.03264.x. [DOI] [PubMed] [Google Scholar]
- 77.Vaidya JS, Baum M, Tobias JS, et al. Targeted intra-operative radiotherapy (Targit): An innovative method of treatment for early breast cancer. Ann Oncol. 2001;12:1075–80. doi: 10.1023/a:1011609401132. [DOI] [PubMed] [Google Scholar]
- 78.Vaidya JS. Partial breast irradiation using targeted intraoperative radiotherapy (Targit) Nat Clin Pract Oncol. 2007;4:384–5. doi: 10.1038/ncponc0850. [DOI] [PubMed] [Google Scholar]
- 79.Vaidya JS, Baum M, Tobias JS, et al. Targeted intraoperative radiotherapy (TARGIT) yields very low recurrence rates when given as a boost. Int J Radiat Oncol Biol Phys. 2006;66:1335–8. doi: 10.1016/j.ijrobp.2006.07.1378. [DOI] [PubMed] [Google Scholar]
- 80.Vaidya JS, Tobias JS, Baum M, et al. TARGeted intraoperative radiotherapy (TARGIT): an innovative approach to partial-breast irradiation. Semin Radiat Oncol. 2005;15:84–91. doi: 10.1016/j.semradonc.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 81.Vaidya JS, Baum M, Tobias JS, et al. The novel technique of delivering targeted intraoperative radiotherapy (Targit) for early breast cancer. Eur J Surg Oncol. 2002;28:447–54. doi: 10.1053/ejso.2002.1275. [DOI] [PubMed] [Google Scholar]