Abstract
Transforming growth factor-β (TGF-β) has been shown to play an essential role in the suppression of inflammation, yet recent studies have revealed the positive roles of TGF-β in inflammatory responses. For example, TGF-β induces Foxp3-positive regulatory T cells (iTregs) in the presence of interleukin-2 (IL-2), while in the presence of IL-6, it induces pathogenic IL-17 producing Th17 cells. TGF-β inhibits the proliferation of immune cells as well as cytokine production via Foxp3-dependent and -independent mechanisms. Little is known about molecular mechanisms involved in immune suppression via TGF-β; however, Smad2/3 have been shown to play essential roles in Foxp3 induction as well as in IL-2 and IFN-γ suppression, whereas Th17 differentiation is promoted via the Smad-independent pathway. Interaction between TGF-β and other cytokine signaling is important in establishing the balance of immunity and tolerance.
Keywords: Immunity, tolerance, signal transduction, smad, T cell
Autoimmunity and inflammatory diseases can be caused by both excess immune reactions and decreased immune suppression. Among immune cells, helper T (Th) cells are known to function as central regulators of immune responses. After activation by antigenic stimulation, naïve Th cells differentiate into either effector T cells responsible for positive immune reactions or regulatory T cells (Tregs) responsible for the negative regulation of immunity. The balance between effector T cells and Tregs has been shown to play an important role in the establishment of immunity or tolerance (1).
Active immune suppression is mediated primarily through anti-inflammatory cytokines and specialized cells. The pleiotropic cytokines, transforming growth factor-β (TGF-β), and interleukin-10 (IL-10) play critical roles in suppressing the immune response (2–5). Recently, a direct connection between Treg and TGF-β has been discovered; TGF-β has been shown to induce Foxp3, a master regulator of Tregs in naïve T cells (6, 7). However, TGF-β has also been identified as an inducer of effector T cells, such as Th17 cells (8, 9). It has been shown that Tregs and Th17 cells are interchangeable at least in in vitro systems (10). Thus, T cell development, tolerance, homeostasis and differentiation are highly dependent on a regulatory network that is modulated by TGF-β. In this review, we will focus on the regulation of both Th cells functions and differentiation via TGF-β and its signals.
TGF-β and signal transduction
TGF-β1, -β2 and -β3 are the three isoforms that have been identified in mammals. Among these three isoforms, TGF-β1 is predominantly expressed in the immune system and is believed to be an important pleiotropic cytokine with potent immunoregulatory properties (11, 12). Mice deficient in TGF-β1 develop a multiorgan autoimmune inflammatory disease and die a few weeks after birth (13, 14). T cells have been shown to play important roles in this severe inflammtion, since such neonatal death and inflammation were eliminated by depleting mature T cells (15, 16). Various transgenic mice whose T cells are unable to respond specifically to TGF-β have also been shown to develop autoimmune diseases, indicating that TGF-β signaling is essential for T cell homeostasis (17–19). Thus, in this review, TGF-β1 will be representative of all TGF-βs unless otherwise specified.
TGF-β is synthesized in an inactive form, the pre-pro-TGF-β precursor. The dimeric proprotein is called the latency-associated peptide (LAP). The LAP/TGF-β complex binds to the latent TGF-β-binding protein (LTBP), a 125- to 160-kDa protein, and the LTBP/LAP/TGF-β complex is then secreted from cells and bound to collagen and other tissue matrix proteins (20, 21). It has also been shown that the LAP/TGF-β complex is highly expressed in Tregs. Additional stimuli, such as low pH, proteolysis, and binding to the cell surface proteins are required to liberate active TGF-β (22, 23).
The major signaling pathways of the TGF-β receptors (TGF-βR) are relatively simple (24). TGF-β first binds to the TGF-βR, which then primarily activates Smad transcription factors, including three structurally similar proteins: two receptor-associated Smads, Smad2 and Smad3 and one common Smad, Smad4 (25). Smad2 or Smad3 is directly phosphorylated and activated by TGF-βR and heterodimerizes with Smad4 or TIF1γ (7, 26). The activated Smad-complex translocates into the nucleus, and, in a cooperative manner with other nuclear cofactors, regulates the transcription of target genes. Apparently, however, there exist Smad-independent pathways (27, 28). Through mechanisms yet to be determined, TGF-β induces rapid activation of Ras-extracellular signal-regulated kinase (Erk), TGF-β-activated kinase-mitogen-activated protein kinase (MAPK) kinase 4-c-Jun N-terminal kinase (TAK1-MKK4-JNK), TAK1-MKK3/6-p38, Rho-Rac-cdc42 MAPK and phosphatidylinositol 3-kinase (PI3K)-Akt pathways. Therefore, TGF-β exerts its regulation of target cell function via a range of mechanisms.
How TGF-β inhibits immune responses
Multiple types of immune cells can be regulated by TGF-β. The following mechanisms are proposed: (i) Suppression of effector Th cell differentation; (ii) conversion of naïve T cells into regulatory T cells; (iii) inhibition of the proliferation of T cells and B cells; (iv) inhibition of effector cytokine production, such as IL-2, IFN-γ and IL-4; (v) suppression of macrophages, dendritic cells (DCs) and natural killer (NK) cells.
One of the most important effects of TGF-β on T cells is the suppression of IL-2 production (29), which leads to the anti-proliferative effect on activated T cells. This is supported by the fact that addition of exogenous IL-2 partially relieved TGF-β-mediated suppression (30). However, TGF-β still inhibits several actions of IL-2, indicating that TGF-β inhibits both the production and intracellular signaling of IL-2.
TGF-β also regulates cell proliferation through controlling the expression of cell cycle regulators, including cyclin-dependent kinase inhibitors (CKIs), such as p15, p21 and p27 (up-regulation) and cell cycle promoters, such as c-myc, cyclin D2, CDK2 and cyclin E (down-regulation) (31–33). TGF-β inhibits naïve T-cell proliferation more profoundly than that of activated T cells, which may be due to reduced TGF-β receptor II expression on activated T cells (34).
In addition to T cells, TGF-β modulates the development and functions of various immune cells. DCs are potent antigen-presenting cells (APCs) that activate naïve T cells and induce their proliferation and differentiation. TGF-β is necessary for the development of Langerhans cells (LCs), which are resident DCs present within keratinocytes in the epidermis (35, 36). TGF-β also regulates the maturation of differentiated DCs and DC-mediated T cell responses (37, 38). Additionally, it regulates the antigen-presentation function of differentiated DCs in vitro (39). Autocrine TGF-β has been shown to be necessary for tolerogenic future of DCs by inducing indoleamine 2,3-dioxygenase (IDO), which is an enzyme that inhibits T-cell proliferation (40). TGF-β inhibits macrophage activation, such as induction of inducible nitric-oxide synthase (iNOS) and matrix metalloproteinase (MMP)-12 via the Smad3 pathway (41) and also inhibits MyD88-dependent TLR signaling pathways (42). Macrophages are also an important producer of TGF-β, which is activated by the phagocytosis of apoptotic cells. Usually, uptake of apoptotic cells elicits anti-inflammatory effect. Thus, induction of TGF-β is a mechanism involving the anti-inflammatory effect of apoptotic cells (43).
TGF-β also suppresses NK cells, mast cells, granulocytes and also controls CD8+ T-cell proliferation and effector functions (2, 44). Recent studies have shown that TGF-β is important for Treg-induced inhibition of the exocytosis of granules and the cytolytic function of CD8+ T cells (45).
Although these immune cells are negatively regulated by TGF-β, Th cells play most essential roles in the immunosuppresive effect of TGF-β, because the neonatal lethality of TGF-β1-deficient mice was eliminated by depletion of CD4+ T cells (46), and the crossing of TGF-β1-deficient mice onto a major histocompatibility complex (MHC) class II null background prevented this inflammation (47). We will therefore focus on the effect of TGF-β on Th cells in the following sections.
Overview of helper T cell differentiation
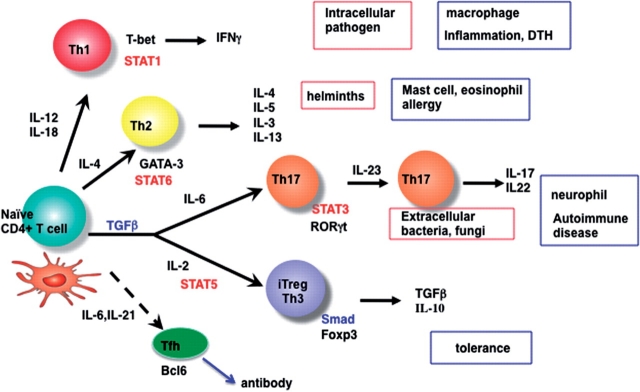
After emigrating from the bone marrow, thymocyte progenitors enter the thymus, and following positive selection, CD4+ or CD8+ single positive (SP) cells migrate to the periphery as naïve T cells. Naturally occurring CD4+CD25+ Foxp3+regulatory T cells (nTregs) also develop in the thymus from immature CD4+ T cells, but the mechanism of their development remains unclear (1). After exiting the thymus, naïve T cells are activated by APCs and differentiate into effector or memory T cells (Fig. 1).
Fig. 1.
Schematic overview of Th cell differentiation. See detail in the text.
Upon antigen stimulation, CD4+ Th cells follow distinct developmental pathways, attaining specialized properties and effector functions. Th cells are traditionally thought to differentiate into Th1 and Th2 cell subsets. Cells of the Th1 lineage, which are evolved to enhance eradication of intracellular pathogens (e.g. intracellular bacteria, viruses and some protozoa), are characterized by their production of interferon-γ (IFN-γ), a potent activator of cell-mediated immunity; cells of the Th2 lineage, which evolved to enhance elimination of parasitic infections (e.g. helminths), are characterized by production of IL-4, IL-5 and IL-13, which are potent activators of B-cell immunoglobulin (Ig)E production, eosinophil recruitment and mucosal expulsion mechanisms (mucous production and hypermotility), respectively. Immune pathogenesis that results from dysregulated Th1 responses to self or commensal floral antigens can promote tissue destruction and chronic inflammation, whereas dysregulated Th2 responses can cause allergy and asthma (Fig. 1).
Recently, a novel Th cell subset has been described that produces IL-17 (Th17) and has been identified as a subset distinct from Th1 or Th2 cells (48–52). Th17 cells secrete a distinctive set of immunoregulatory cytokines, including IL-17A, IL-17F, IL-22 and IL-21. These cytokines collectively play roles in inflammation and autoimmunity and in elimination of extracellular bacterial and fungal pathogens. Murine autoimmue models, such as experimental autoimmune encephalitis (EAE) and collagen-induced arthritis (CIA), have been shown to be dependent on Th17 cells.
Th1 polarization is primarily driven by IL-12 and IFN-γ, while Th2 polarization is primarily driven by IL-4. These respective cytokines signal via STAT4, STAT1 and STAT6 to directly control the transcription factors T-bet and GATA3, which, in turn, determine Th1 and Th2 differentiation, respectively (53). Th1 cells produce IFN-γ, which facilitates their differentiation while inhibiting IL-4-mediated Th2 differentiation. Reciprocally, Th2 cells produce IL-4 and IL-10, which strongly inhibit IL-12/IFN-γ-driven Th1 differentiation.
The Th17 differentiation of naïve T cells is initiated by IL-6 and TGF-β (54–56). In addition, IL-23, as well as IL-21, is thought to be a key cytokine for the maturation and/or maintenance of Th17 cells (49, 50, 57, 58). IL-6, IL-21 and IL-23 all activate STAT3, which is shown to be essential for Th17 differentiation (59–61). It has also been reported that STAT3 plays a critical role in the induction of the orphan nuclear receptor, RORγt, which directs Th17 cell differentiation by inducing the IL-23 receptor (62). The critical role of STAT3 in Th17 differentiation was also confrimed in human patients lacking functional STAT3 (63–65).
TGF-β also induces differentiation of naïve T cells into Foxp3+ Tregs (iTregs) in the peripheral immune compartment (6; Fig. 1). The role of TGF-β in Th17 and iTreg differentiation will be discussed later.
Regulation of effector Th-differentiation by TGF-β
Local TGF-β activation through Treg/DC interaction seems to be necessary for both immune suppression and Th17 generation. T cell specific TGF-β1 knockout (KO) revealed that T cell-produced TGF-β1 promoted Th17 cell differentiation and was essential for the induction of the EAE model (66). Local, but not systemic, administration of anti-TGF-β antibody inhibited EAE development (67). Since the TGF-β/LAP complex is highly expressed on Tregs, these studies suggest that TGF-β1 originating from Tregs is responsible for Th17 differentiation.
TGF-β inhibits Th1 and Th2 differentiation from naïve T cells in vitro (2). TGF-β blockade of Th1 cell differentiation is associated with reduced IL-12 receptor β2 (IL-12Rβ2) and T-bet expression (68). T-bet is required for the induction of IL-12Rβ2 (69). Therefore, reduced IL-12Rβ2 levels upon TGF-β treatment is probably due to its inhibition of T-bet expression, which is dependent on the IFN-γ/Stat1 pathway (69). TGF-β also inhibits Th2 differentiation by suppressing GATA-3 expression and IL-4 mediated STAT6 activity (70, 71). It has been suggested that the role of TGF-β in Th17 differentiation is the suppression of Th1 and Th2 differentiation (i.e. suppression of the production of IFN-γ and IL-4), since these cyokines strongly inhibit Th17 differentiation. This is supported by a report showing that IL-6 alone was sufficient in inducing robust differentiation of Th17 cells in STAT6−/−T-bet−/− mice, which are unable to generate Th1 and Th2 cells (72). TGF-β,however, may play a specific role in Th17 differentiation, other than Th1 and Th2 suppression, because antibodies against IFN-γ and IL-4 could not completely replace TGF-β (55), and RORγt, the master regulator of Th17, was induced by TGF-β alone even in the absence of IL-6 (73).
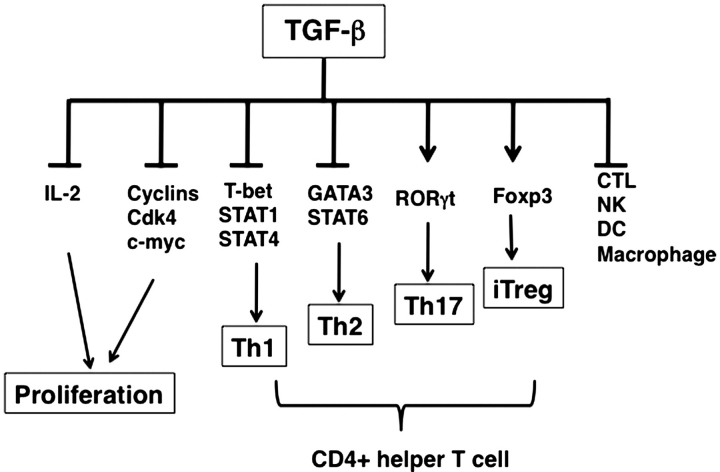
Interestingly, TGF-β partially inhibits IFN-γ production and IL-12 mediated STAT4 phosphorylation in fully-differentiated Th1 cells, while IL-4 production and IL-4 mediated STAT6 activation in fully-differentiated Th2 cells were unaffected by TGF-β (74). Recently, it has been shown that TGF-β induces robust IL-9 production in the presence of IL-4, which are now called Th9 cells (75). Regulation of Th cell differentiation by TGF-β is summarized in Fig. 2.
Fig. 2.
Effect of TGF-β on immune cells. TGF-β inhibits proliferation of various immune cells, inhibits Th1 and Th2 differentiation, induces Th17 and iTregs and inhibits maturation of other cells such as CD8+ CTL, NK cell, DC and macrophages.
Regulation of Treg-differentiation by TGF-β
TGF-β has been shown to induce Foxp3 (6), a master transcriptional factor of Treg cells (1). Foxp3 in CD4+ T cells is responsible for the suppression activity of Tregs. Foxp3 inhibits secretion of proinflammatory cytokines, including IL-2, IFN-γ, IL-4 and IL-17, enhances expression of anti-inflammatory cytokines, such as IL-10 and TGF-β, and up-regulates an inhibitor for co-stimulation, CTLA4 (76–78). TGF-β plays an important role in generating induced Tregs (iTregs) from naïve T cells. TGF-β has also been implicated in the maintenance of Foxp3 in thymus-derived nTregs (1). TGF-β1 deficient mice showed normal nTreg developement in the thymus but the peripheral Tregs were significantly reduced in number (79). Recently, however, TGF-β has been implicated in the development of nTregs during the neonatal stage in the thymus (80). The role of TGF-β in nTreg generation is still unclear.
When naïve T cells were stimulated with DCs in the presence of TGF-β, antigen-specific Foxp3+ iTregs were generated (81). These in vitro-generated iTregs can prevent experimental autoimmune diseases (81). DCs in the presence of TGF-β or specific DC subsets (CD8+ CD205+ DCs) also promote nTreg expansion by selectively suppressing effector T-cell expansion (82).
Endogenous TGF-β during T/DC interaction participates in maintaining the balance between effector T cells and Tregs. We have shown that SOCS3-deficient DCs, in which STAT3 was constitutively activated, selectively enhance expansion of nTregs (83). This effect was canceled by anti-TGF-β antibody and SOCS3-deficient DCs produced higher levels of TGF-β1 than did WT DCs (83). TGF-β promoter analysis revealed that STAT3 binds to the region of the TGF-β promoter, which may explain high levels of TGF-β in SOCS3-deficeint DCs (84). Adoptive transfer of SOCS3-deficient DCs suppresses EAE. Thus, TGF-β during T/DC interaction is important for the determination of immunity or tolerance.
Molecular mechanism of Foxp3 induction by TGF-β
Foxp3 expression is tightly regulated by various factors. The Foxp3 promoter/enhancer region contains three evolutionary conserved non-coding sequence (CNS) elements where several essential transcription factors bind. Rudensky’s group described the function of three Foxp3 CNS elements (CNS1-3) in Treg cell fate determination in mice using a KO strategy (85). CNS1, which contains a TGF-β-NFAT response element, is superfluous in nTreg cell differentiation, but plays a prominent role in iTreg cell generation in gut-associated lymphoid tissues.
We, and others, have found Smad-binding elements in the CNS1 region of the Foxp3 promoter (86, 87). This region contains two consecutive Smad-binding elements and one NFAT binding site. Previously, Smad3, but not Smad2, was implicated in the induction of Foxp3 (87) because Smad2 has a low DNA-binding activity compared to that of Smad3. However, using Smad2-deficient T cells, we demonstrated that both Smad2 and Smad3 are essential for TGF-β-mediated induction and maintenance of Foxp3 expression (88). Like TGF-β1 KO mice, T-cell specific Smad2- and Smad3-deficient mice possess normal nTreg cells in the thymus, but their number was decreased at the periphery (88).
TGF-β mediated Foxp3 expression is regulated by various factors. The IL-2/STAT5 signal is an essential factor for iTreg generation (89–91), whereas inflammatory cytokines IL-6 and IL-4 suppress iTreg (55, 86). STAT6 activated by IL-4 may bind to the Foxp3 promoter, thereby inducing chromatin remodeling (86). Recently, retinoic acid (RA), has been discovered as a potent inducer and preserver of Foxp3 in iTregs (92). The RA receptor directly interacts with the Foxp3 promoter (86). A reporter assay using a series of deletion mutants revealed that RA-responsive element was present between +2114 and +2350 and interacts with the RA receptor complex, RAR-α/RXR-α. This region was 300 bp upstream of a putative STAT6-binding site (86). The mechanisms by which IL-6/STAT3 inhibits Foxp3 expression are still unknown.
STAT1 seems to have different effects on TGF-β-mediated Foxp3 gene expression in humans and mice. The STAT1-activating cytokines IL-27 and IFN-γ amplify TGF-β-induced FOXP3 expression in human T cells (93). This study showed that the STAT1 binding element was present within the proximal region of the human FOXP3 promoter. While IFN-γ-activated STAT1 has been shown to inhibit Foxp3 induction in murine T cells (94, 95), the reason for this difference between human and mouse has not been clarified.
The Notch and TGF-β signaling pathways cooperatively regulate Foxp3 expression and regulatory T-cell maintenance (96). Pharmacologic inhibition of Notch signaling using γ-secretase inhibitor (GSI) treatment blocks TGF-β1-induced Foxp3 expression (96). Since Smads interact with various transcription factors, additional factors involving the regulation of iTreg generation will undoubtedly be discovered.
Treg is a major source of TGF-β, and TGF-β is one of the effector molecules of Tregs
As described above, Tregs express LAP on their membrane surface at high levels (22, 23). The CD25+ CD4+ LAP+ T cells (i.e. LAP+ Tregs) are more potent in their regulatory activity than are CD25+CD4+ LAP− T cells and the LAP+ cells are considered to be a major source of active TGF-β. To be expressed on the cell surface as LAP, the TGF-β precursor must be cleaved by the endopeptidase furin in the Golgi. Consistent with this hypothesis, conditional deletion of furin in T cells allows for normal T-cell development but impairs the function of regulatory and effector T cells, which, in turn, produce less TGF-β. Furin-deficient Tregs are less functional in a T-cell transfer colitis model and fail to induce Foxp3 in T cells (97). The LAP-activating receptors, such as CD36/TSP-1 and integrin αVβ6, are expressed on monocytes, endothelial cells, and DC, but not on T cells (20). Thus, LAP/TGF-β on Tregs will be activated via the interaction between Tregs and APCs. This is consistent with reports showing that conditional deletion of integrin αVβ6 or αVβ8 on DCs resulted in autoimmune diseases (98, 99). TGF-β produced by Foxp3-expressing regulatory T cells was required to inhibit Th1-cell differentiation and inflammatory-bowel disease in a transfer model (18). As mentioned, TGF-β on Tregs is required for Th17 development (66). These data suggest that the major source of TGF-β in the immune system is regulatory T cells, which are activated by Treg/DC interaction.
Smad-dependent and -independent regulation of Th differentiation by TGF-β
The downstream mechanism for the regulation of T cells by TGF-β remains unclear. It has been reported that Smad2 or Smad3 regulates a distinctive sets of genes in fibroblasts and tumor cells (24). Smad2-KO mice are embryonic-lethal (100), and Smad3-KO mice exhibit inflammatory diseases (101), suggesting that Smad2 is involved in mediating signals during development, while Smad3 is important for anti-inflammation. Moreover, the disruption of Smad4, specifically in T cells, results in colitis and an increased susceptibility to spontaneous colo-rectal tumorigenesis (102). These reports suggest that the Smad3/4 pathway is an important mediator of TGF-β signaling in immune regulation. However, the phenotypes of Smad3- or Smad4-single deficient mice were much milder than those of T-cell-specific TGF-βRII KO mice (19), suggesting that Smad2 may also play a role in immune regulation.
T-cell-specific Smad2 conditional KO mice revealed unexpected overlapping functions of Smad2 and Smad3 in TGF-β-induced Foxp3 induction as well as in Th functions (88). Smad2/Smad3-double KO mice, but not single KO mice, developed fatal inflammatory diseases, with higher IFN-γ production and reduced Foxp3 expression in CD4+ T cells at the periphery (88). TGF-β mediated induction of Foxp3, as well as suppression of IFN-γ and IL-2 was partialy impaired in Smad2- and Smad3-deficient T cells, and was completely eliminated in Smad2/3-double KO T cells (88). Thus, Smad2 and Smad3 are redundantly essential for iTreg induction and Th suppression.
Recent studies have demonstrated that TGF-β-induced Foxp3 antagonizes RORγt, which is also induced by TGF-β, to inhibit Th17 cell differentiation (73, 78). It has not yet been clarified, however, how TGF-β induces both the transcription factor Foxp3 and RORγt which have diametrically opposed physiological functions: one interacts with anti-inflammatory Tregs and the other induces inflammatory Th17 cells. It has been suggested that RORγt induction by TGF-β is independent of Smad4 (103). Takimoto, T et al. also demonstarted that both Smad2 and Smad3 were dispensable for the induction of RORγt (88). Interestingly, however, Th17 development was indirectly regulated by Smad2/3 signaling. Th17 cell development was reduced in Smad-deficient CD4+ T cells because of the higher production of Th17-inhibitory cytokines, such as IL-2 and IFN-γ, from these T cells. Therefore, Smad signaling indirectly promotes the inducing of Th17 cell differentiation by suppressing Th17 inhibitory cytokine production.
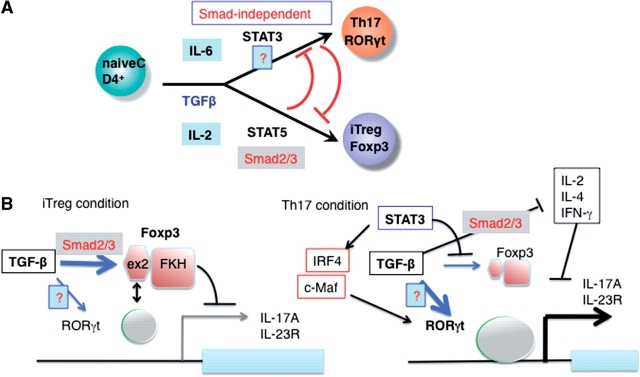
It is important to understand the role of IL-6/STAT3 in the generation of Th17 differentiation in the presence of TGF-β. IL-6 is apparently necessary for the suppression of Foxp3 and for maintaining high levels of RORγt (62, 78). STAT3 may suppress Foxp3 expression via a direct binding (104). In addition, IRF4 (105) and c-Maf (106, 107), which are upregulated by STAT3, have been shown to be necessary for RORγt expression. Since Foxp3 inhibits the transcriptional activity of RORγt, in the absence of IL-6/STAT3 signals, Foxp3 will overwhelm the activity of RORγt. Regulation of Th17 and iTregs through Smad-dependent and independent mechanisms are illustrated in Fig. 3.
Fig. 3.
Role of TGF-β in Th17 and iTreg differentiation. (A) RORγt, a master transcription factor for Th17 is induced by TGF-β+ IL-6, which requires STAT3 but not Smad2/3/4. Smad-independent mechanism is shown as ‘?'. Foxp3, a master transcription factor for Treg is induced by TGF-β, and Foxp3 levels become higher by the IL-2/STAT5 signaling. This step is Smad2/3 dependent. STAT3 and STAT5 inhibit Foxp3 and RORγt induction, respectively. (B) Regulation of iTreg and Th17 by IL-6. In iTreg condition, Foxp3 binds to RORγt, thereby suppressing transcriptional activity of RORγt and Th17 differentiation. STAT3 induces IRF4 and c-Maf, which supports expression of RORγt expression. STAT3 also inhibits Foxp3 expression. Suppression of IL-2, IFN-γ and IL-4 by TGF-β, which is Smad2/3-dependent also promotes Th17 differentiation.
Smad-mediated suppression of the cytokine production
TGF-β mediated suppression of IFN-γ, IL-2 and IL-4 production was partially impaired in Smad2-KO T cells and Smad3-KO T cells (88, 108), and completely eliminated in Smad2/3-double KO T cells (88). Therefore, suppression of cytokine production by TGF-β is Smad2/3-dependent (Fig. 3B). However, molecualr mechanism of this suppression has not been clarified yet.
TGF-β suppresses IL-2 production in T cells potentially through direct inhibition of IL-2 promoter activity. A cis-acting enhancer DNA element was identified as critical in suppressing IL-2 production via TGF-β (109). Tob, a member of an anti-proliferative gene family, was shown to bind to Smad2, thereby inhibiting IL-2 production (110). The interaction between Tob and Smad3, however, was not observed. Runx1/3 also play essential roles in cytokine production from CD4+ T cells, and may be potential interaction partners of Smad2 and/or Smad3 (111). NFAT could be a common target of Smad2 and Smad3, because NFAT is an essential transcription factor for IL-2 mRNA induction. However, the interactions between NFAT and Smad2/3 have not been identified.
TGF-β inhibits IFN-γ production by suppressing T-bet, which is a transcription factor critical for IFN-γ production and Th1 differentiation of CD4+ T cells (68). T-bet expression is induced by STAT1 and STAT4, thus Smads may inhibit IFN-γ production by suppressing STAT1 and STAT4. Similarly, TGF-β inhibits IL-4 production probably by suppressing IL-4-mediated STAT6 activation. The molecular mechanism by which Smads inhibit STAT have not been well understood. One paper has suggested that TGF-β1 suppresses IFN-γ-induced T-bet expression through the hemopoietic protein tyrosine phosphatase Src homology region 2 domain-containing phosphatase-1 (Shp-1) (112). Shp-1 was shown to play a vital role in TGF-β1's suppressive effects, because the suppression activity of TGF-β was completely eliminated in Shp-1 deficient CD4+ T cells. The way in which Smads are involved in the induction of Shp-1, however, still remains unclear.
Reciprocal regulation of TGF-β signaling and IFN-γ signaling
There is extensive crosstalk between the TGF-β1/Smad signaling and the JAK-STAT pathway (113, 114). For example, IFN-γ suppresses TGF-β1 signaling through upregulation of the inhibitory Smad7. IFN-γ also inhibits TGF-β1 responses via STAT1-mediated sequestration of the nuclear coactivator p300/CREB-binding protein, preventing its association with Smads and blocking Smad transcriptional activity (115). In contrast, little is known about the suppression mechanisms of the JAK-STAT pathway via TGF-β1. TGF-β1 suppresses NO production from macrophages stimulated with LPS and IFN-γ, and TGF-β1 functions as a negative autocrine feedback regulator to prevent tissue injury caused by excessive NO (116, 117). Previous reports have suggested that TGF-β1 reduces IFN-γ-induced iNOS mRNA and protein levels (116, 118) . We have also found that TGF-β1 not only accelerated proteosomal degradation of iNOS but also inhibited iNOS mRNA transcription by suppressing STAT1 activation (119). Additional analyses showed that TGF-βRI interacted with and phosphorylated IFN-γ receptor1 (IFNGR1), which is a novel mechanism of STAT1 repression by TGF-β1 (119). Another study suggested that TGF-β inhibits IFN-γ mediated STAT1 activation via the induction of STAT1-PIAS1 (a protein inhibitor of activated STAT1) interaction (120).
SOCS1 is a potent inhibitor of signaling events stimulated by IFN-γ, and in the absence of the SOCS1 protein, STAT1 is highly activated, and, subsequently, T cells are unconditionally hyperactivated (121, 122). SOCS1-deficient mice die within 3 weeks after birth due to very severe inflammtion, just as do TGF-β1-deficient mice. We therefore hypothesized that TGF-β signaling was impaired in SOCS1-deficient T cells. SOCS1-deficient T cells were resistant to all effects of TGF-β. TGF-β could not suppress IFN-γ production very efficiently in SOCS1-deficient CD4+ T cells (123). Moreover, TGF-β mediated induction of Foxp3 and RORγt was impaired in SOCS1-deficient T cells (123, 124). Such TGF-β resistance was IFN-γ-dependent, because TGF-β functioned normally in SOCS1/IFN-γ-double KO T cells. In other words, SOCS1 is necessary for proper TGF-β signaling by protecting cells from the strong antagonistic effect of IFN-γ.
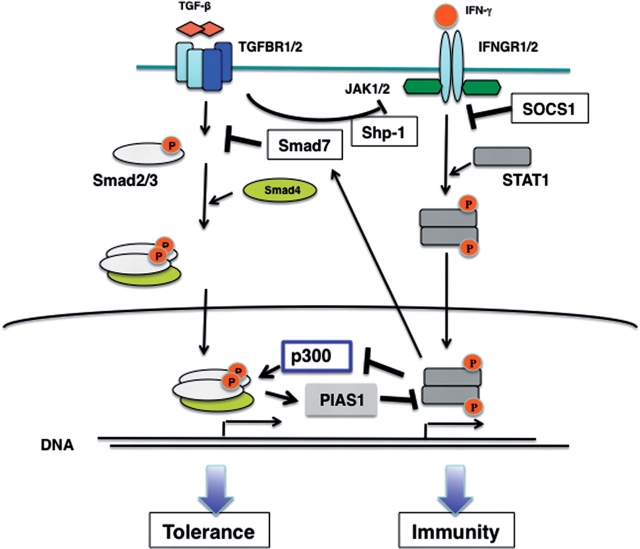
The molecular mechanism of IFN-γ-mediated TGF-β signal suppression in T cells has not been clearly identified. We could observe neither Smad7 induction nor suppression of Smad2 phosphorylation in SOCS1-deficient cells. In STAT1−/− cells, IFN-γ-mediated suppression was eliminated (Ichiyama et al., unpublished data). Therefore, our data suggest that STAT1 suppresses TGF-β signaling, while SOCS1 enhances TGF-β signaling by repressing STAT1. The precise molecular mechanism for STAT1-mediated Smad suppression is still unknown. However, it is apparent that the reciprocal suppression of IFN-γ and TGF-β is significant in the detemination of immunity or tolerance. Current model is illustrated in Fig. 4.
Fig. 4.
Reciprocal inhibition mechanism by TGF-β and IFN-γ. IFN-γ receptor activates STAT1, then STAT1-target genes such as Smad7 and unidentified molecules inhibit TGF-β signaling. TGF-β and Smads also inhibits the IFN-γ/STAT1 pathway by several ways. See detail in the text.
Conclusion
The importance of active immune suppression is widely acknowledged. Studies on TGF-β and Tregs have shed light on immune suppression applications. Advances in these areas have been and are currently being translated into clinical benefits. Further investigations are warranted to clarify the mechanism through which TGF-β and Tregs control immune responses. In addition, as TGF-β function in non-lymphoid systems, further studies on both the roles of TGF-β and Foxp3 in non-lymphoid systems and on the interaction between lymphoid and non-lymphoid systems are essential for achieving a more comprehensive view of our immune system.
Funding
Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO) and CREST Program by Japan Science and Technology Agency (JST).
Conflict of interest
None declared.
Acknowledgements
We thank F. Kotaki and N. Soma for article preparation.
Glossary
Abbreviations
- APC
antigen-presenting cell
- CD
cluster of differentiation or cluster of designation
- CIA
collagen-induced arthritis
- CREB
cAMP response element binding protein
- CTLA4
Cytotoxic T-Lymphocyte Antigen 4
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- IFN
interferon
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- IRF
Interferon regulatory factor
- iTreg
induced Treg
- KO
knockout
- LAP
Latency associated protein
- MHC
major histocompatibility complex
- NFAT
Nuclear factor of activated T-cells
- NK
natural killer
- NO
nitric oxide
- PIAS1
protein inhibitor of activated STAT1
- RAR
retinoic acid receptor
- ROR
retinoic-acid-related orphan receptor
- Runx
Runt-related transcription factor
- RXR
retinoid X receptor
- Shp-1
Src homology region 2 domain-containing phosphatase-1
- Smad
Sma- and Mad-related
- SOCS
suppressor of cytokine signaling
- STAT
Signal Transducers and Activator of Transcription
- TGF
Transforming growth factor
- Th
helper T
- Tob
Transducer of ErbB-2
- Treg
regulatory T
- TSP-1
Thrombospondin 1
- WT
wild type
References
- 1.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 2.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 3.Wan YY, Flavell RA. ‘Yin-Yang' functions of transforming growth factor-beta and T regulatory cells in immune regulation. Immunol. Rev. 2007;220:199–213. doi: 10.1111/j.1600-065X.2007.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 5.Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28:468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J. Exp. Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li MO, Flavell RA. TGF-beta: a master of all T cell trades. Cell. 2008;134:392–404. doi: 10.1016/j.cell.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: effector T cells with inflammatory properties. Semin. Immunol. 2007;19:362–371. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubtsov YP, Rudensky AY. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat. Rev. Immunol. 2007;7:443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 10.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 11.Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr. Rev. 2002;23:787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- 12.Govinden R, Bhoola KD. Genealogy, expression, and cellular function of transforming growth factor-beta. Pharmacol. Ther. 2003;98:257–265. doi: 10.1016/s0163-7258(03)00035-4. [DOI] [PubMed] [Google Scholar]
- 13.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc. Natl Acad. Sci. USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diebold RJ, Eis MJ, Yin M, Ormsby I, Boivin GP, Darrow BJ, Saffitz JE, Doetschman T. Early-onset multifocal inflammation in the transforming growth factor beta 1-null mouse is lymphocyte mediated. Proc. Natl Acad. Sci. USA. 1995;92:12215–12219. doi: 10.1073/pnas.92.26.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bommireddy R, Engle SJ, Ormsby I, Boivin GP, Babcock GF, Doetschman T. Elimination of both CD4+ and CD8+ T cells but not B cells eliminates inflammation and prolongs the survival of TGFbeta1-deficient mice. Cell Immunol. 2004;232:96–104. doi: 10.1016/j.cellimm.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorelik L, Flavell RA. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 18.Marie JC, Liggitt D, Rudensky AY. Cellular mechanisms of fatal early-onset autoimmunity in mice with the T cell-specific targeting of transforming growth factor-beta receptor. Immunity. 2006;25:441–454. doi: 10.1016/j.immuni.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-β controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Taylor AW. Society for Leukocyte Biology Review of the activation of TGF-β in immunity. J. Leukocyte Biol. 2009;85:29–33. doi: 10.1189/jlb.0708415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGF β activation. J. Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J. Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 23.Chen ML, Yan BS, Bando Y, Kuchroo VK, Weiner HL. Latency-associated peptide identifies a novel CD4+CD25+ regulatory T cell subset with TGFβ-mediated function and enhanced suppression of experimental autoimmune encephalomyelitis. J. Immunol. 2008;180:7327–7337. doi: 10.4049/jimmunol.180.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massague J. TGF-beta signal transduction. Annu. Rev. Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 25.Huse M, Muir TW, Xu L, Chen YG, Kuriyan J, Massague J. The TGF β receptor activation process: an inhibitor- to substrate-binding switch. Mol. Cell. 2001;8:671–682. doi: 10.1016/s1097-2765(01)00332-x. [DOI] [PubMed] [Google Scholar]
- 26.He W, Dorn DC, Erdjument-Bromage H, Tempst P, Moore MA, Massague J. Hematopoiesis controlled by distinct TIF1gamma and Smad4 branches of the TGFbeta pathway. Cell. 2006;125:929–941. doi: 10.1016/j.cell.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 27.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 28.Yu L, Hebert MC, Zhang YE. TGF-β receptor-activated p38 MAP kinase mediates Smad-independent TGF-β responses. EMBO J. 2002;21:3749–3759. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brabletz T, Pfeuffer I, Schorr E, Siebelt F, Wirth T, Serfling E. Transforming growth factor β and cyclosporin A inhibit the inducible activity of the interleukin-2 gene in T cells through a noncanonical octamer-binding site. Mol. Cell. Biol. 1993;13:1155–1162. doi: 10.1128/mcb.13.2.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruegemer JJ, Ho SN, Augustine JA, Schlager JW, Bell MP, McKean DJ, Abraham RT. Regulatory effects of transforming growth factor-beta on IL-2- and IL-4-dependent T cell-cycle progression. J. Immunol. 1990;144:1767–1776. [PubMed] [Google Scholar]
- 31.Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 32.Wolfraim LA, Walz TM, James Z, Fernandez T, Letterio JJ. p21Cip1 and p27Kip1 act in synergy to alter the sensitivity of naive T cells to TGF-beta-mediated G1 arrest through modulation of IL-2 responsiveness. J. Immunol. 2004;173:3093–3102. doi: 10.4049/jimmunol.173.5.3093. [DOI] [PubMed] [Google Scholar]
- 33.Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 34.Cottrez F, Groux H. Regulation of TGF-beta response during T cell activation is modulated by IL-10. J. Immunol. 2001;167:773–778. doi: 10.4049/jimmunol.167.2.773. [DOI] [PubMed] [Google Scholar]
- 35.Jaksits S, Kriehuber E, Charbonnier AS, Rappersberger K, Stingl G, Maurer D. CD34+ cell-derived CD14+ precursor cells develop into Langerhans cells in a TGF-β1-dependent manner. J. Immunol. 1999;163:4869–4877. [PubMed] [Google Scholar]
- 36.Zhang Y, Zhang YY, Ogata M, Chen P, Harada A, Hashimoto S, Matsushima K. Transforming growth factor-β1 polarizes murine hematopoietic progenitor cells to generate Langerhans cell-like dendritic cells through a monocyte/macrophage differentiation pathway. Blood. 1999;93:1208–1220. [PubMed] [Google Scholar]
- 37.Strobl H, Knapp W. TGF-β1 regulation of dendritic cells. Microbes Infect. 1999;1:1283–1289. doi: 10.1016/s1286-4579(99)00256-7. [DOI] [PubMed] [Google Scholar]
- 38.Strobl H, Riedl E, Scheinecker C, Bello-Fernandez C, Pickl WF, Rappersberger K, Majdic O, Knapp W. TGF-β1 promotes in vitro development of dendritic cells from CD34+ hemopoietic progenitors. J. Immunol. 1996;157:1499–1507. [PubMed] [Google Scholar]
- 39.Geissmann F, Revy P, Regnault A, Lepelletier Y, Dy M, Brousse N, Amigorena S, Hermine O, Durandy A. TGF-β1 prevents the noncognate maturation of human dendritic Langerhans cells. J. Immunol. 1999;162:4567–4575. [PubMed] [Google Scholar]
- 40.Belladonna ML, Volpi C, Bianchi R, Vacca C, Orabona C, Pallotta MT, Boon L, Gizzi S, Fioretti MC, Grohmann U, Puccetti P. Cutting edge: autocrine TGF-beta sustains default tolerogenesis by IDO-competent dendritic cells. J. Immunol. 2008;181:5194–5198. doi: 10.4049/jimmunol.181.8.5194. [DOI] [PubMed] [Google Scholar]
- 41.Werner F, Jain MK, Feinberg MW, Sibinga NE, Pellacani A, Wiesel P, Chin MT, Topper JN, Perrella MA, Lee ME. Transforming growth factor-β1 inhibition of macrophage activation is mediated via Smad3. J. Biol. Chem. 2000;275:36653–36658. doi: 10.1074/jbc.M004536200. [DOI] [PubMed] [Google Scholar]
- 42.Naiki Y, Michelsen KS, Zhang W, Chen S, Doherty TM, Arditi M. Transforming growth factor-β differentially inhibits MyD88-dependent, but not TRAM- and TRIF-dependent, lipopolysaccharide-induced TLR4 signaling. J. Biol. Chem. 2005;280:5491–5495. doi: 10.1074/jbc.C400503200. [DOI] [PubMed] [Google Scholar]
- 43.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J. Clin. Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat. Immunol. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 45.Mempel TR, Pittet MJ, Khazaie K, Weninger W, Weissleder R, von Boehmer H, von Andrian UH. i Regulatory T cells reversibly suppress cytotoxic T cell function independent of effector differentiation. Immunity. 2006;25:129–141. doi: 10.1016/j.immuni.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 46.Rudner LA, Lin JT, Park I, Kates JM, Dyer DA, Franz DM, Fresnch MA, Duncan EM, White HD, Gorham JD. Necroinflammatory liver disease in BALB/c background, TGF-beta 1-deficient mice requires CD4+ T cells. J. Immunol. 2003;170:4785–4792. doi: 10.4049/jimmunol.170.9.4785. [DOI] [PubMed] [Google Scholar]
- 47.Letterio JJ, Geiser AG, Kulkarni AB, Dang H, Kong L, Nakabayashi T, Mackall CL, Gress RE, Roberts AB. Autoimmunity associated with TGF-beta1-deficiency in mice is dependent on MHC class II antigen expressions II antigen expression. J. Clin. Invest. 1996;98:2109–2119. doi: 10.1172/JCI119017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J. Immunol. 2000;165:6107–6115. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 49.Aggarwal S, Ghilardi N, Xie M.HJ, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 50.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing, C.D4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 52.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glimcher LH, Murphy KM. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 2000;14:1693–1711. [PubMed] [Google Scholar]
- 54.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 55.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 56.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 57.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 58.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho ML, Kang JW, Moon YM, Nam HJ, Jhun JY, Heo SB, Jin HT, Min SY, Ju JH, Park KS, Cho YG, Yoon CH, Park SH, Sung YC, Kim HY. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J. Immunol. 2006;176:5652–5661. doi: 10.4049/jimmunol.176.9.5652. [DOI] [PubMed] [Google Scholar]
- 60.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 61.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O'Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc. Natl Acad. Sci. USA. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 63.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O'Shea J, Holland SM, Paul WE, Douek DC. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma CS, Chew G.YJ, Simpson N, Priyadarshi A, Wong M, Grimbacher B, Fulcher DA, Tangye SG, Cook MC. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J. Exp. Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, Feinberg J, von Bernuth H, Samarina A, Janniere L, Fieschi C, Stephan J.-L, Boileau C, Lyonnet S, Jondeau G, Cormier-Daire V, Le Merrer M, Hoarau C, Lebranchu Y, Lortholary O, Chandesris M.-O, Tron F, Gambineri E, Bianchi L, Rodriguez-Gallego C, Zitnik SE, Vasconcelos J, Guedes M, Vitor AB, Marodi L, Chapel H, Reid B, Roifman C, Nadal D, Reichenbach J, Caragol I, Garty B.-Z, Dogu F, Camcioglu Y, Gulle S, Sanal O, Fischer A, Abel L, Stockinger B, Picard C, Casanova J.-L. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J. Exp. Med. 2008;205:1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-cell differentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 67.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 68.Gorelik L, Constant S, Flavell RA. Mechanism of transforming growth factor β-induced inhibition of T helper type 1 differentiation. J. Exp. Med. 2002;195:1499–1505. doi: 10.1084/jem.20012076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat. Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 70.Gorelik L, Fields PE, Flavell RA. Cutting edge: TGF-β inhibits Th type 2 development through inhibition of GATA-3 expression. J. Immunol. 2002;165:4773–4777. doi: 10.4049/jimmunol.165.9.4773. [DOI] [PubMed] [Google Scholar]
- 71.Heath VL, Murphy EE, Crain C, Tomlinson MG, O'Garra A. TGF-β1 down-regulates Th2 development and results in decreased IL-4-induced STAT6 activation and GATA-3 expression. Eur. J. Immunol. 2000;30:2639–2649. doi: 10.1002/1521-4141(200009)30:9<2639::AID-IMMU2639>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 72.Das J, Ren G, Zhang L, Roberts AI, Zhao X, Bothwell AL, Van Kaer L, Shi Y, Das G. Transforming growth factor beta is dispensable for the molecular orchestration of Th17 cell differentiation. J. Exp. Med. 2009;206:2407–2416. doi: 10.1084/jem.20082286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, Takaesu G, Hori S, Yoshimura A, Kobayashi T. Foxp3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J. Biol. Chem. 2008;283:17003–1708. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- 74.Ludviksson BR, Seegers D, Resnick AS, Strober W. The effect of TGF-beta1 on immune responses of naive versus memory CD4+ Th1/Th2 T cells. Eur. J. Immunol. 2000;30:2101–2111. doi: 10.1002/1521-4141(200007)30:7<2101::AID-IMMU2101>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 75.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-beta ‘reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 76.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 77.Bettelli E, Dastrange M, Oukka M. Foxp3 interacts with nuclear factor of activated T cells and NF-kappa B to repress cytokine gene expression and effector functions of T helper cells. Proc. Natl Acad. Sci. USA. 2005;102:5138–5143. doi: 10.1073/pnas.0501675102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-b-induced Foxp3 inhibits TH17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yongzhong L, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-b signaling in the development of natural CD4+CD25+Foxp3+regulatory T cells. Nat. Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 81.Yamazaki S, Patel M, Harper A, Bonito A, Fukuyama H, Pack M, Tarbell KV, Talmor M, Ravetch JV, Inaba K, Steinman RM. Effective expansion of alloantigen-specific Foxp3+ CD25+ CD4+ regulatory T cells by dendritic cells during the mixed leukocyte reaction. Proc. Natl Acad. Sci. USA. 2006;103:2758–2763. doi: 10.1073/pnas.0510606103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, Steinman RM. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J. Immunol. 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matsumura Y, Kobayashi T, Ichiyama K, Yoshida R, Hashimoto M, Takimoto T, Tanaka K, Chinen T, Shichita T, Wyss-Coray T, Sato K, Yoshimura A. Selective expansion of foxp3-positive regulatory T cells and immunosuppression by suppressors of cytokine signaling 3-deficient dendritic cells. J. Immunol. 2007;179:2170–2179. doi: 10.4049/jimmunol.179.4.2170. [DOI] [PubMed] [Google Scholar]
- 84.Kinjyo I, Inoue H, Hamano S, Fukuyama S, Yoshimura T, Koga K, Takaki H, Himeno K, Takaesu G, Kobayashi T, Yoshimura A. Loss of SOCS3 in T helper cells resulted in reduced immune responses and hyperproduction of interleukin 10 and transforming growth factor-beta 1. J. Exp. Med. 2006;203:1021–1031. doi: 10.1084/jem.20052333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takaki H, Ichiyama K, Koga K, Chinen T, Takaesu G, Sugiyama Y, Kato S, Yoshimura A, Kobayashi T. STAT6 Inhibits TGF-beta1-mediated Foxp3 induction through direct binding to the Foxp3 promoter, which is reverted by retinoic acid receptor. J. Biol. Chem. 2008;283:14955–14962. doi: 10.1074/jbc.M801123200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat. Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 88.Takimoto T, Wakabayashi Y, Sekiya T, Inoue N, Morita R, Takahashi R, Asakawa M, Muto G, Mori T, Hasegawa E, Shizuya S, Hara T, Nomura M, Yoshimura A. Smad2 and Smad3 are redundantly essential for the TGF-β-mediated regulation of Treg plasticity and Th1 cell development. J. Immunol. 2010 doi: 10.4049/jimmunol.0904100. In press. [DOI] [PubMed] [Google Scholar]
- 89.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J. Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 90.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, O'Shea JJ. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the develop- ment of Foxp3+ regulatory T cells. J. Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 92.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 93.Ouaked N, Mantel PY, Bassin C, Burgler S, Siegmund K, Akdis CA, Schmidt-Weber CB. Regulation of the foxp3 gene by the Th1 cytokines: the role of IL-27-induced STAT1. J. Immunol. 2009;182:1041–1049. doi: 10.4049/jimmunol.182.2.1041. [DOI] [PubMed] [Google Scholar]
- 94.Caretto D, Katzman SD, Villarino AV, Gallo E, Abbas AK. Cutting edge: the Th1 response inhibits the generation of peripheral regulatory T cells. J. Immunol. 2010;184:30–34. doi: 10.4049/jimmunol.0903412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang JH, Kim YJ, Han SH, Kang CY. IFNgamma-STAT1 signal regulates the differentiation of inducible Treg: potential role for ROS-mediated apoptosis. Eur. J. Immunol. 2009;39:1241–1251. doi: 10.1002/eji.200838913. [DOI] [PubMed] [Google Scholar]
- 96.Samon JB, Champhekar A, Minter LM, Telfer JC, Miele L, Fauq A, Das P, Golde TE, Osborne BA. Notch1 and TGFbeta1 cooperatively regulate Foxp3 expression and the maintenance of peripheral regulatory T cells. Blood. 2008;112:1813–1821. doi: 10.1182/blood-2008-03-144980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pesu M, Watford WT, Wei L, Xu L, Fuss I, Strober W, Andersson J, Shevach EM, Quezado M, Bouladoux N, Roebroek A, Belkaid Y, Creemers J, O'Shea JJ. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature. 2008;455:246–250. doi: 10.1038/nature07210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, Bluestone JA, Sheppard D. αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aluwihare P, Mu Z, Zhao Z, Yu D, Weinreb PH, Horan GS, Violette SM, Munger JS. Mice that lack activity of alphavbeta6- and alphavbeta8-integrins reproduce the abnormalities of Tgfb1- and Tgfb3-null mice. J. Cell Sci. 2009;122:227–232. doi: 10.1242/jcs.035246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nomura M, Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;25:737–739. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- 101.Yang XO, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, Roberts AB, Deng C. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-β. EMBO J. 1999;18:1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim BG, Li C, Qiao W, Mamura M, Kasprzak B, Anver M, Wolfraim L, Hong S, Mushinski E, Potter M, Kim SJ, Fu XY, Deng C, Letterio JJ. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441:1015–1019. doi: 10.1038/nature04846. [DOI] [PubMed] [Google Scholar]
- 103.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huber M, Brüstle A, Reinhard K, Guralnik A, Walter G, Mahiny A, von Löw E, Lohoff M. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc. Natl Acad. Sci. USA. 2008;105:20846–20851. doi: 10.1073/pnas.0809077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang Y, Ochando J, Yopp A, Bromberg JS, Ding Y. IL-6 plays a unique role in initiating c-Maf expression during early stage of CD4 T cell activation. J. Immunol. 2005;174:2720–2729. doi: 10.4049/jimmunol.174.5.2720. [DOI] [PubMed] [Google Scholar]
- 107.Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat. Immunol. 2009;10:167–75. doi: 10.1038/ni.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McKarns SC, Schwartz RH, Kaminski NE. Smad3 is essential for TGF-beta 1 to suppress IL-2 production and TCR-induced proliferation, but not IL-2-induced proliferation. J. Immunol. 2004;172:4275–4284. doi: 10.4049/jimmunol.172.7.4275. [DOI] [PubMed] [Google Scholar]
- 109.Brabletz T, Pfeuffer I, Schorr E, Siebelt F, Wirth T, Serfling E. Transforming growth factor beta and cyclosporin A inhibit the inducible activity of the interleukin-2 gene in T cells through a noncanonical octamer-binding site. Mol. Cell. Biol. 1993;13:1155–1162. doi: 10.1128/mcb.13.2.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tzachanis D, Freeman GJ, Hirano N, van Puijenbroek AA, Delfs MW, Berezovskaya A, Nadler LM, Boussiotis VA. Tob is a negative regulator of activation that is expressed in anergic and quiescent T cells. Nat. Immunol. 2001;2:1174–1182. doi: 10.1038/ni730. [DOI] [PubMed] [Google Scholar]
- 111.Miyazono K, Maeda S, Imamura T. Coordinate regulation of cell growth and differentiation by TGF-β superfamily and Runx proteins. Oncogene. 2004;23:4232–4237. doi: 10.1038/sj.onc.1207131. [DOI] [PubMed] [Google Scholar]
- 112.Park IK, Shultz LD, Letterio JJ, Gorham JD. TGF-beta1 inhibits T-bet induction by IFNgamma in murine CD4+ T cells through the protein tyrosine phosphatase Src homology region 2 domain-containing phosphatase-1. J. Immunol. 2005;175:5666–5674. doi: 10.4049/jimmunol.175.9.5666. [DOI] [PubMed] [Google Scholar]
- 113.Eickelberg O, Pansky A, Koehler E, Bihl M, Tamm M, Hildebrand P, Perruchoud AP, Kashgarian M, Roth M. Molecular mechanisms of TGF-(beta) antagonism by interferon (gamma) and cyclosporine A in lung fibroblasts. FASEB J. 2001;15:797–806. doi: 10.1096/fj.00-0233com. [DOI] [PubMed] [Google Scholar]
- 114.Ulloa L, Doody J, Massague J. Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature. 1999;397:710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- 115.Ghosh AK, Yuan W, Mori Y, Chen S, Varga J. Antagonistic regulation of type I collagen gene expression by interferon-gamma and transforming growth factor-beta. Integration at the level of p300/CBP transcriptional coactivators. J. Biol. Chem. 2001;276:11041–11048. doi: 10.1074/jbc.M004709200. [DOI] [PubMed] [Google Scholar]
- 116.Ding A, Nathan CF, Graycar J, Derynck R, Stuehr DJ, Srimal S. Macrophage deactivating factor and transforming growth factors-beta 1 -beta 2 and -beta 3 inhibit induction of macrophage nitrogen oxide synthesis by IFNgamma. J. Immunol. 1990;145:940–944. [PubMed] [Google Scholar]
- 117.Nelson BJ, Ralph P, Green SJ, Nacy CA. Differential susceptibility of activated macrophage cytotoxic effector reactions to the suppressive effects of transforming growth factor-beta 1. J. Immunol. 1991;146:1849–1857. [PubMed] [Google Scholar]
- 118.Mitani T, Terashima M, Yoshimura H, Nariai Y, Tanigawa Y. TGF-beta1 enhances degradation of IFNgamma-induced iNOS protein via proteasomes in RAW 264.7 cells. Nitric Oxide. 2005;13:78–87. doi: 10.1016/j.niox.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 119.Takaki H, Minoda Y, Koga K, Takaesu G, Yoshimura A, Kobayashi T. TGF-beta1 suppresses IFNgamma-induced NO production in macrophages by suppressing STAT1 activation and accelerating iNOS protein degradation. Genes Cells. 2006;11:871–882. doi: 10.1111/j.1365-2443.2006.00988.x. [DOI] [PubMed] [Google Scholar]
- 120.Reardon C, McKay DM. TGF-beta suppresses IFNgamma-STAT1-dependent gene transcription by enhancing STAT1-PIAS1 interactions in epithelia but not monocytes/macrophages. J. Immunol. 2007;178:4284–4295. doi: 10.4049/jimmunol.178.7.4284. [DOI] [PubMed] [Google Scholar]
- 121.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 122.Kubo M, Hanada T, Yoshimura A. Suppressors of cytokine signaling and immunity. Nat. Immunol. 2003;4:1169–1176. doi: 10.1038/ni1012. [DOI] [PubMed] [Google Scholar]
- 123.Tanaka K, Ichiyama K, Hashimoto M, Yoshida H, Takimoto T, Takaesu G, Torisu T, Hanada T, Yasukawa H, Fukuyama S, Inoue H, Nakanishi Y, Kobayashi T, Yoshimura A. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFNgamma on STAT3 and Smads. J. Immunol. 2008;180:3746–3756. doi: 10.4049/jimmunol.180.6.3746. [DOI] [PubMed] [Google Scholar]
- 124.Horino J, Fujimoto M, Terabe F, Serada S, Takahashi T, Soma Y, Tanaka K, Chinen T, Yoshimura A, Nomura S, Kawase I, Hayashi N, Kishimoto T, Naka T. Suppressor of cytokine signaling-1 ameliorates dextran sulfate sodium-induced colitis in mice. Int. Immunol. 2008;20:753–762. doi: 10.1093/intimm/dxn033. [DOI] [PubMed] [Google Scholar]