Abstract
We review the definitions, determinants, and ways of enhancing successful cognitive and emotional aging. Objective definitions of successful aging based on physical health emphasize outcomes including freedom from disability and disease, whereas subjective definitions center on well-being, social connectedness, and adaptation. Most older people do not meet objective criteria for successful aging, while a majority meet the subjective criteria. Older people with severe mental illness are not excluded from successful aging. The determinants of successful aging include complex interactions of lifestyle behaviors and social environment with genes. Depression interferes with nearly all determinants of successful aging. Evidence-based means of enhancing successful aging include calorie restriction, physical exercise, cognitive stimulation, social support, and optimization of stress. Future directions for successful aging research and implications for geriatric psychiatry are discussed.
Keywords: Successful aging, physical exercise, cognitive stimulation, social support
While most of the focus of psychiatry is rightfully placed on the definitions, mechanisms, and treatment of mental disorders, we believe it is equally worthwhile to investigate positive states of mental health, including successful cognitive and emotional aging.
Aging will become increasingly more relevant to psychiatry in the years to come, by virtue of the unprecedented global demographic redistribution toward older adults – for example, in the United States there are more older adults than children younger than 14 for the first time in recorded history 1. There will be a disproportionately greater rise in the numbers of older adults with psychiatric disorders 2. Preventing or slowing the progression of brain illnesses, including psychiatric disorders, represents one of the major challenges in the coming decades. Broadening our understanding of processes involved in successful aging can potentially help us develop innovative approaches to prevention of psychiatric illness and promotion of mental health.
In this review, we discuss the various ways in which successful aging has been defined, the evidence for predictors and mechanisms of successful aging, and interventions that may positively alter the course of aging in people with and without psychiatric disorders.
HOW IS SUCCESSFUL AGING DEFINED?
Although “successful aging” was not an explicit theme in the biomedical literature until the early 1960s 3, there have long been efforts to understand how to promote longevity and positive states of health in later life. The writings of ancient philosophers reveal disagreements in views of positive emotional outcomes in later life. Aging has been described as a largely intractable process 4 versus one involving possibilities for adaptation to new roles 5. Modern psychiatrists and psychologists considered later life either as a product of early developmental tasks 6 or as a period of continued growth and conflicts that had to be negotiated 7, 8.
In the 1970s and 1980s, formal models of successful aging emerged. In their influential 1987 article, Rowe and Kahn 9 noted that research on aging was historically dominated by efforts to discriminate between pathological and “normal” aging, with little effort being devoted to understanding the upper end of the continuum (i.e., successful aging). Successful aging was characterized as involving three components: a) freedom from disease and disability, b) high cognitive and physical functioning, and c) social and productive engagement. The MacArthur Network on Successful Aging operationalized these criteria, and followed over a period of seven years a sample of 1000 older adults who met the criteria. Another prominant model of successful aging proposed around the same time period was that of Baltes 10, who described successful aging in terms of lifespan developmental trajectories, with a focus on behavioral and psychological adaptation to losses.
During the subsequent two decades, there have been a number of epidemiological studies that have examined the population frequency and predictors of successful aging using various operationalized definitions. Depp and Jeste 11 identified 28 studies with sample sizes greater than 100, published in English-language journals, and including adults over age 60. Across the operational definitions provided in these studies, there were 14 components of successful aging used. Physical functioning and freedom from disability were included in nearly every definition, but no other component was present in more than 50% of the studies. Overall, in 28 studies there were 29 different definitions used for successful aging. Therefore, little agreement existed among researchers regarding the elements of successful aging, beyond physical functioning.
A smaller subset of studies has used qualitative methods (e.g., focus groups, surveys, personal interviews) to identify the components of successful aging 12-14. These studies provide an interesting contrast to quantitative studies, which focused more on physical and functional attributes. In qualitative studies, older adults were much more likely to emphasize adaptation to illnesses and other psychological traits (e.g., optimism; sense of purpose) as well as engagement (e.g., social relationships) in their concepts of successful aging. Among qualitative studies, the perspectives of older adults appeared to differ somewhat by the method used (e.g., focus groups emphasized shared experiences related to aging 13, whereas individual interviews focused more on developmental trajectories) and by culture of origin (e.g., older Japanese people cited belonging vs. American emphasis on independence 15).
Just as successful aging defies consensus definition, parallel efforts to define positive states in psychiatric conditions have also proven challenging. As with successful aging, there is a tension between models of “sustained remission” in chronic mental illness and those of “recovery” 16. The former term corresponds to freedom from syndromal levels of symptoms associated with functional impairments for a period of time (e.g., 2 years), whereas definitions of recovery center on adaptation to enable attainment of goals (e.g., “a journey of healing and transformation enabling a person … to live a meaningful life in a community of his or her choice while striving to achieve his or her full potential” 17. Recovery, like subjectively defined successful aging, is less of an outcome than a process, is more personalized, and does not require an absence of symptoms or illness.
The limited consensus on successful aging or recovery from psychiatric disorders reflects some of the difficulties in defining positive states. In part this difficulty stems from a lack of clinical or policy imperatives to attain consensus such as those required to define diagnostic terms. Another difficulty in delineating positive states from others is that some individuals are excluded from being categorized as “successful”. Nevertheless, there are some areas of agreement. Definitions of successful aging, remission, and recovery are all multi-dimensional and integrate multiple domains (e.g., physical, cognitive, emotional, and social functioning). Subjective definitions tend to represent processes and emphasize attainment or maintenance of goals, positive attitudes toward the self and future, and attainment of social milestones and connectedness. Objective definitions tend to emphasize freedom from disease and disability. In terms of trajectories, successful aging definitions tend to emphasize mitigating deterioration, whereas recovery or remission represents lengthening periods of inter-episode wellness.
HOW COMMON IS SUCCESSFUL AGING?
Given the lack of consensus on what constitutes successful aging, it is of little surprise that estimates of its frequency in the community vary widely. Nevertheless, there are interesting trends in the reported rates of successful aging depending on the components of the definitions as well as the source of the assessment. In the review of 28 studies described above, the rate of successful aging in researcher-defined studies 11 ranged from 0.4% to 96%. The median percentage of people who met criteria for successful aging was 35%. This is similar to that seen in the MacArthur Network on Successful Aging, in which one third of older adults met the operationalized Rowe and Kahn criteria for successful aging 18. Aggregating across the studies reviewed, the more components included in the model, the lower the rate of successful aging. In examining the contribution of individual components of successful aging to overall rate, it is apparent that the presence of disability or chronic disease is more often the rate-limiting factor, whereas most older adults sampled were socially engaged and had relatively unimpaired cognitive functioning.
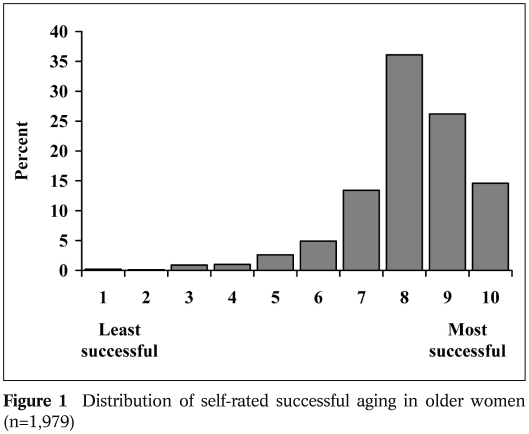
Relatively few studies have asked older adults to rate themselves in terms of successful aging. In such a study of 205 community dwelling older adults, Montross et al 19 noted that most older adults viewed themselves as aging successfully, despite having physical illnesses and disability. In a study that expanded on this finding, we administered a survey questionnaire to a sample of 1,979 women over age 60, who were enrolled in the San Diego site of the Women’s Health Initiative 20. Respondents were asked to rate themselves on a scale from 1 (not successful) to 10 (very successful). As seen in Figure 1, the vast majority of older people rated themselves with a score of 7 or higher, with only a small percentage of people rating themselves as “unsuccessful”. That most people rated themselves as aging successfully, even when they did not meet objective criteria for successful aging (Table 1), is consistent with several other studies 14,21.
Figure 1.
Table 1.
Table 1 Percent of sample (n=1,979) meeting criteria for domains of successful aging
| Domain | Operational definition | % of sample meeting criteria |
| Absence of diseasea | Absence of self-reported cancer, diabetes, high blood pressure, heart attacks, other heart disease, stroke, osteoporosis, Parkinson disease, and respiratory diseaseb | 15% |
| Freedom from disability | SF-36 scores of “no limitation” in the ability to a) lift or carry groceries, b) climb one flight of stairs, c) bend/kneel/stoop, d) walk one block, or e) bath/dress oneselfb | 38% |
| Normal cognitive functioning | Score of 18 or higher on self-administered Cognitive Assessment Screening Test | 71% |
| Active engagement with life | Visiting family and/or friends at least once a week and having three or more close friendsb | 74% |
| Mastery/growth | Score of “often true” or “true nearly all of the time” on the item “I am in control of my life”c | 81% |
| Positive adaptation | Score of “often true” or “true nearly all of the time” on the following two items: a) “I am able to adapt to change,” and b) “I tend to bounce back after illness or hardship”c | 81% |
| Life satisfaction | Score of at least 73 on the SF-36 emotional health/well-being subscale | 84% |
| Self-rated successful aging | Score ranging from 7–10 on a 1–10 scale item asking “Where do you rate yourself in terms of successful aging?”b | 90% |
| Independent living | Living independently in own home or retirement community; not residing in a skilled nursing facilityd | 94% |
| SF-36 – Short Form 36 | ||
| a As outlined by Phelan and Larson 64 literature review of successful aging | ||
| b Modeled after the Strawbridge et al. 21 operational definition of successful aging; % is reported from Montross et al 19 sample | ||
| c Items derived from the Connor-Davidson Resilience Scale (CD-RISC) 36 | ||
| d Living independently used by Roos and Havens 65 | ||
Little is known about rate of successful aging in persons with severe mental illness. In schizophrenia, long-term follow up studies led by Bleuler 22, Harding 23, and Ciompi 24 indicate that, in contrast to earlier assumptions about progressive deterioration, a majority of patients experience significant improvement in later life. More recently, Bellack estimated that 50% of people with schizophrenia attain at least short-lasting recovery during their lifetime 16.
This estimate is higher than that associated with sustained remission (i.e., freedom from symptoms for two years or longer): in a sample of 251 older adults with schizophrenia, Auslander et al 25 found a sustained remission rate of about 10%. In a study of older persons with schizophrenia, Cohen et al 26 compared outcomes using five separate positive constructs: recovery, remission, community integration, subjective successful aging, and objective successful aging. In this study, the authors compared schizophrenia patients with an age-matched community dwelling control group of older persons without major mental illness. In the schizophrenia group, 23% met criteria for community integration (vs. 41% of comparison group), 13% met criteria for subjective successful aging (vs. 27% of comparison group), and only 2% met full criteria for objective successful aging (vs. 19% of comparison group).
On the basis of this evidence, it seems likely that only a small proportion of older adults are aging successfully according to objective criteria based on physical health, whereas a remarkably high percentage believe they are aging successfully and meet other psychosocial criteria for successful aging. Similarly, while a minority of older people with schizophrenia experience sustained remission from symptoms, far fewer appear to meet objective criteria for successful aging.
WHAT ARE THE DETERMINANTS AND MODIFIERS OF SUCCESSFUL AGING?
In epidemiological studies, the predictors of successful aging, as defined by objective criteria, appear to correspond to predictors of chronic medical illness 11. This is consistent with the reliance on physical functioning in the objective definitions. Similarly, in longitudinal epidemiological studies, the best predictors of successful aging include younger age, freedom from arthritis or diabetes, and not smoking. However, in predicting self-rated successful aging, somewhat different responses are revealed. For example, when examining the sample of 1,979 women described above (Table 2), we identified several significant predictors of self-rated successful aging. Depression emerged as a potent negative correlate with self-rated successful aging. Positive correlates included optimism, resilience, cognitive ability, and physical and mental health-related quality of life. However, chronological age was not associated with self-ratings of successful aging, whereas income and education were minimally related to successful aging.
Table 2.
Table 2 Correlates of self-rated successful aging in older women (n=1,979)
| Variable | Pearson correlation coefficient |
| Chronological age | -0.044** |
| Level of education | 0.081** |
| Income | 0.060** |
| Attitude toward aging (Philadelphia Geriatric Morale Scale) | 0.302** |
| Physical activity participation (Godin Leisure Activity Scale) | 0.156** |
| SF-36 Mental Health Composite | 0.161** |
| SF-36 Physical Health Composite | 0.266** |
| Cognitive Ability Screening Test | 0.098** |
| Cognitive Failures Questionnaire | -0.149** |
| Connor-Davidson Resilience Scale | 0.274** |
| Optimism (Life Orientation Test) | 0.229** |
| Depressive Symptoms (CES-D) | -0.275** |
| Perceived Stress Scale | -0.225** |
| *p<0.01; ** p<0.001 | |
| SF-36 – Short Form 36; CES-D – Center for Epidemiological Studies Depression Scale | |
The contribution of genetic factors to successful aging is an emerging field of research. Glatt et al 27 reviewed studies that examined the influence of genes on multi-dimensional definitions of successful aging in samples of older people. In case-control studies examining single nucleotide polymorphisms (SNPs), allelic variation that was significantly different across “successful” vs. comparison groups in two or more reports included six genes: APOE, GSTT1, IL6, IL10, PON1, and SIRT3. Although there were only 29 studies with limited consensus on phenotypes of successful aging, these genes have plausible relationships to biological processes and risk factors for disease in aging. Nevertheless, there is clearly a substantial degree to which variation in aging phenotypes stems from non-heritable influences. In twin studies, lifespan is approximately 20-30% attributed to heritability and the proportion of variation due to heritability in functioning in an older sample was 22% 28. Both longevity and functioning appear less heritable than cognitive ability (30-50%) 29
The goal of altering the fundamental biological processes that govern the rate of aging represents a shift from the focus on specific diseases 30. Although there is no unifying theory of aging, and it remains unclear what the nature of mechanisms of aging is, there is a great deal of interest in the role of inflammation and oxidative stress. In humans, chronic exposure to stress is associated with chromosomal alterations, damage to brain structures, and early mortality 31. Caloric restriction, which may result in lengthening the lifespan in mice and in humans, appears to reduce the levels of inflammatory markers 32. Greater social integration in older adults is also associated with reduced inflammation 33. Yet, the relationship with stress and biology is not monotonic. Mild levels of stress, such as those produced by exercise, cognitive activity, or caloric restriction, may stimulate tropic factors which lead to greater resistance to stress (a process called hormesis) 34. Thus, interventions targeting multiple domains may have shared pathways (e.g., reducing inflammation; stimulating increased stress resistance). These studies also point to the need to quantify resilience, in addition to stress. There are self-report measures of resilience that evidence good psychometric properties in older adults 35,36, yet there is a need for objective and experimental paradigms assessing resilience to be developed for use with older adults.
In a deviation from older concepts, it now appears that the window of opportunity for modifying processes regulating brain aging is not restricted to early life, but extends into later adulthood. The brains of older animals provided with enriched environments show evidence of synaptogenesis 37 and neurogenesis in select brain regions (e.g., dentate gyrus of the hippocampus). There is evidence from functional neuroimaging investigations that high-performing older adults exhibit greater bilateral activation on cognitive tasks, suggesting that “successful” brain aging may involve reorganization and compensation for deterioration 38. In the largest study of its kind, the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) randomized controlled trial found that, among older adults without dementia, brief cognitive training in a variety of modalities improved performance on cognitive tests 39. Similar improvements in cognitive ability have been seen with cognitive training in schizophrenia, associated with a number of efforts to develop pharmacologic and non-pharmacologic interventions 40.
Beyond the individual, social networks and social engagement, as shown by novel approaches to network analyses, appear to be vectors for positive states of health such as happiness 41 as well as negative ones such as loneliness, obesity, and smoking 42,43. In older adults, loneliness predicts increased risk for Alzheimer’s disease 44. Overlaying the social network, the built environment also influences access to health behaviors, socialization, and cognitive activities 45. Therefore, the influences on successful aging are complex and operate on multiple levels, from genes to neighborhoods.
Positive psychological traits have remarkable effects on mortality, with a number of longitudinal studies indicating that, even after controlling for other relevant variables, higher sense of purpose in life 46, optimism 47, and more positive attitude toward aging 48 are associated with longer lifespans. To understand how these traits modify outcomes in aging, it will be imperative to refine these broad constructs and to learn how they may relate to brain function and development.
Wisdom is a complex trait often associated with aging. It is notable that the modern Western construct of wisdom is largely similar to that found in ancient religious and philosophical texts, including the Bhagavad Gita – an Indian religious/philosophical text probably written around 2000 B.C. (e.g., rich knowledge about life, emotional regulation, insight, acting in face of uncertainty, and a focus on common good/compassion) 49.
In unpublished work, we studied associations between domains of wisdom in a community dwelling sample of 1,973 older women described above. We constructed measures for the domains of social/pragmatic decision making, emotional homeostasis, management of uncertainty, self-reflection/understanding, and spirituality using items drawn from multiple scales measuring cognition, emotion, and positive personality traits. Using item response theory, we found that measures for each domain had acceptable internal consistency and reliability, and that the domains of social decision making, emotional homeostasis, and management of uncertainty were strongly associated with each other. Self reflection/understanding was also significantly associated with other three domains, but to a lesser degree. Spirituality, as measured in our study was, however, not significantly associated with the other domains of wisdom.
Wisdom maps onto neurobiological structures 50. Emotional regulation, decision making, value relativism may involve top-down regulation of limbic and striatal brain regions. The lateral prefrontal cortex facilitates calculated, reason-based decision making, whereas the medial prefrontal cortex is implicated in emotional valence and prosocial attitudes/behaviors. Reward neurocircuitry (ventral striatum, nucleus accumbens) is also important for promoting prosocial attitudes/behaviors. The characteristics of wisdom seem to be impaired by specific brain lesions. For example, fronto-temporal dementia is characterized by impulsivity, diminished empathy, and emotional reactivity. The same brain structures (e.g., prefrontal cortex) are implicated in both wisdom and frontal lobe deficits, harkening back to the changes in character exhibited by the historic case of Phineas Gage 51, as well as more recent reports outlining cognitive deficits secondary to ventromedial prefrontal lobe damage 51. Hence it may be possible to study wisdom as a neurobiologically determined trait.
WHAT ARE THE IMPLICATIONS OF THE DETERMINANTS OF SUCCESSFUL AGING FOR LATE-LIFE PSYCHIATRIC DISORDERS?
Consistent with the large negative correlation between self-rated successful aging and depression, nearly all of the determinants of successful aging reviewed above are negatively impacted by depression. Depression is associated with lower rates of exercise and worse nutrition, greater social isolation and diminished engagement in productive activity, and negative outlook on the future and the self. Even subsyndromal symptoms of depression relate to broad negative effects across aging-related phenotypes 20. Inflammation and stress-related biological processes are implicated as a shared pathway to both depressive symptoms and cognitive impairment in older people 52. These relationships are likely to be bi-directional; for example, diminished social engagement leads to greater depression, and vice versa 53. It is also evident that, while trends in some health behaviors are improving (e.g., reduction in smoking), others such as healthy diets, physical activity, and social integration may be declining despite all of the evidence of their benefits 54.
At the same time, it appears that interventions to improve lifestyle behaviors or social engagement, while not specifically targeting depression, may have anti-depressant effects in older people. Indeed, randomized controlled trials support the role of exercise in treating late-life depression 55, and there is emerging evidence that cognitive training targeting speed-of-processing 56 as well as dietary patterns 57 may reduce or prevent depressive symptoms. Multi-component interventions aimed at increasing healthy lifestyles appear to produce changes in brain function as detected with neuroimaging 58. This suggests that the armamentarium of geriatric psychiatric treatments may need to expand to include lifestyle interventions.
WHERE DO WE GO FROM HERE? FUTURE DIRECTIONS IN SUCCESSFUL AGING
Although there is clearly great public interest and imperative to define and promote successful aging, its definition remains controversial. How can we attain greater consensus about successful aging? As in the definition of recovery versus remission from psychiatric disorders 16, there is a gulf between researcher and lay definitions – the former describes freedom from disease and disability, and the latter focuses on adaptation, meaningfulness, and connection. It should be possible to better integrate these perspectives, incorporating both subjective and objective elements into definitions. Moreover, some constructs included in subjective definitions, such as resilience and wisdom, are not yet adequately operationalized; better instruments to measure such constructs will enable them to be incorporated into epidemiological studies. “Toolbox” initiatives that unify the measurement of constructs and use more dimensional ratings could also advance the consistency among studies 59. Many studies have reported age effects in cross-sectional studies, though the real interest is in understanding the causal and dynamic processes in aging. Methodological advances that enable more efficient collection of longitudinal data, such as accelerated longitudinal designs 60), could aid in increasing the power to detect processes rather than outcomes. Studies of this kind could link broad phenotypes (e.g., social engagement) with intermediate phenotypes that can be measured more objectively (e.g., extroversion) and with biomarkers (e.g., oxytocin). The operationalization of frailty represents a useful example of defining a complex phenotype based on its basic biological processes 61, and could provide a model in this regard. We have taken steps in this direction, by deconstructing wisdom into its collection of putative neurobiological constituents 50.
Fortunately, even with the difficulty in defining successful aging, there is remarkable convergence in some of the components and their environmental influences. In particular, there are many shared pathways between stress and inflammation, obesity and sedentary behavior, and risk for impaired cognitive ability, depression, and cardiovascular disease. Interventions such as caloric restriction may work at the beginning of this pathway. Alternatively, interventions addressing multiple targets, such as those that combine physical activity and cognitive stimulation, may have a synergistic effect on basic biological processes. New technologies, such as exergames that use video games to combine physical activity, pleasant activity, and cognitive stimulation, may reduce late-life subsyndromal depression 62. Multi-level interventions, for example those that target the individual and the built environment, are also promising routes to behavior change 63.
Given the leverage that depression has on successful aging, as well as the increasing prominence of brain health as a public health issue, psychiatric treatments could impact the likelihood of successful aging for many people. Psychiatry, including geriatric psychiatry, should broaden its scope to include enhancement of lifestyles, social functioning, and other aspects of recovery. Given that the peak ages for most physiological functions occur in late adolescence 30, altering age-related trajectories should begin early. Conversely, given what we now know about the plasticity of the aging brain, it is never too late to strive for successful aging in people with and without mental illnesses.
Acknowledgements
This work was supported, in part, by National Institute of Mental Health grants K23MH077225 and P30MH066248, and by the Sam and Rose Stein Institute for Research on Aging.
References
- 1.Administration on Aging. The road to an aging policy for the 21st century. Washington: White House Conference on Aging; 1996. [Google Scholar]
- 2.Jeste DV, Alexopoulos GS, Bartels SJ. Consensus statement on the upcoming crisis in geriatric mental health: research agenda for the next two decades. Arch Gen Psychiatry. 1999;56:848–853. doi: 10.1001/archpsyc.56.9.848. [DOI] [PubMed] [Google Scholar]
- 3.Havighurst RJ. Successful aging. Gerontologist. 1961;1:8–13. [Google Scholar]
- 4.Chandler AR. Aristotle on mental aging. J Gerontol. 1948;3:220–224. doi: 10.1093/geronj/3.3.220. [DOI] [PubMed] [Google Scholar]
- 5.Cicero MT. On old age. Kila: Kessinger; 2004. [Google Scholar]
- 6.Freud S. Standard edition of the complete works of Sigmund Freud. Vol. 7. London: Hogarth Press; 1905/1953. [Google Scholar]
- 7.Jung CG. Modern man in search of a soul. New York: Harcourt, Brace & World; 1933. [Google Scholar]
- 8.Erikson E. Eight ages of man. Int J Psychoanal. 1966;2:281–300. [PubMed] [Google Scholar]
- 9.Rowe JW, Kahn RL. Human aging: usual and successful. Science. 1987;237:143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- 10.Baltes PB, Kahn RL. On the incomplete architecture of human ontogeny: selection, optimization, and compensation as foundation of developmental theory. Am Psychol. 1997;52:366–380. doi: 10.1037//0003-066x.52.4.366. [DOI] [PubMed] [Google Scholar]
- 11.Depp CA, Jeste DV. Definitions and predictors of successful aging: a comprehensive review of larger quantitative studies. Am J Geriatr Psychiatry. 2006;14:6–20. doi: 10.1097/01.JGP.0000192501.03069.bc. [DOI] [PubMed] [Google Scholar]
- 12.Knight T, Ricciardelli LA. Successful aging: perceptions of adults aged between 70 and 101 years. Int J Aging Hum Dev. 2003;56:223–245. doi: 10.2190/CG1A-4Y73-WEW8-44QY. [DOI] [PubMed] [Google Scholar]
- 13.Reichstadt J, Depp CA, Palinkas LA. Building blocks of successful aging: a focus group study of older adults’ perceived contributors to successful aging. Am J Geriatr Psychiatry. 2007;15:194–201. doi: 10.1097/JGP.0b013e318030255f. [DOI] [PubMed] [Google Scholar]
- 14.von Faber M, Bootsma-van der Wiel A, van Exel E. Successful aging in the oldest old: who can be characterized as successfully aged? Arch Intern Med. 2001;161:2694–2700. doi: 10.1001/archinte.161.22.2694. [DOI] [PubMed] [Google Scholar]
- 15.Phelan EA, LaCroix AZ. Older adults’ views of «successful aging» - How do they compare with researchers’ definitions? J Am Geriatr Soc. 2004;52:211–216. doi: 10.1111/j.1532-5415.2004.52056.x. [DOI] [PubMed] [Google Scholar]
- 16.Bellack AS. Scientific and consumer models of recovery in schizophrenia: concordance, contrasts, and implications. Schizophr Bull. 2006;32:432–442. doi: 10.1093/schbul/sbj044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services, Substance Abuse and Mental Health Services Administration. National consensus statement on mental health recovery. www.mentalhealth.samhsa.gov.
- 18.Berkman LF, Seeman TE, Albert M. High, usual and impaired functioning in community-dwelling older men and women: findings from the MacArthur Foundation Research Network on Successful Aging. J Clin Epidemiol. 1993;46:1129–1140. doi: 10.1016/0895-4356(93)90112-e. [DOI] [PubMed] [Google Scholar]
- 19.Montross LP, Depp C, Daly J. Correlates of self-rated successful aging among community-dwelling older adults. Am J Geriatr Psychiatry. 2006;14:43–51. doi: 10.1097/01.JGP.0000192489.43179.31. [DOI] [PubMed] [Google Scholar]
- 20.Vahia IV, Meeks TW, Thompson WK. Subsyndromal depression and successful aging in older women. Am J Geriatr Psychiatry. 2010;18:212–220. doi: 10.1097/JGP.0b013e3181b7f10e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strawbridge WJ, Wallhagen MI, Cohen RD. Successful aging and well-being: self-rated compared with Rowe and Kahn. Gerontologist. 2002;42:727–733. doi: 10.1093/geront/42.6.727. [DOI] [PubMed] [Google Scholar]
- 22.Bleuler M. Die spatschizophrenen krankheitsbilder. Fortschr Neurol Psychiatrie. 1943;15:259–290. [Google Scholar]
- 23.Harding CM. Changes in schizophrenia across time: paradoxes, patterns and predictors. In: Carl I, Cohen M, editors. Schizophrenia into later live. Washington: American Psychiatric Publishing Inc; 2003. pp. 19–42. [Google Scholar]
- 24.Ciompi L. Catamnestic long-term study on the course of life and aging of schizophrenics. Schizophr Bull. 1980;6:606–618. doi: 10.1093/schbul/6.4.606. [DOI] [PubMed] [Google Scholar]
- 25.Auslander LA, Lindamer LL, Delapena J. A comparison of community-dwelling older schizophrenia patients by residential status. Acta Psychiatr Scand. 2001;103:380–386. doi: 10.1034/j.1600-0447.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- 26.Cohen CI, Pathak R, Ramirez PM. Outcome among community dwelling older adults with schizophrenia: results using five conceptual models. Commun Ment Health J. 2009;45:151–156. doi: 10.1007/s10597-008-9161-8. [DOI] [PubMed] [Google Scholar]
- 27.Glatt SJ, Chayavichitsilp P, Depp C. Successful aging: from phenotype to genotype. Biol Psychiatry. 2007;62:282–293. doi: 10.1016/j.biopsych.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Gurland BJ, Page WF, Plassman BL. A twin study of the genetic contribution to age-related functional impairment. J Gerontol A Biol Sci Med Sci. 2004;59:859–863. doi: 10.1093/gerona/59.8.m859. [DOI] [PubMed] [Google Scholar]
- 29.Read S, Pedersen NL, Gatz M. Sex differences after all those years? Heritability of cognitive abilities in old age. J Gerontol B Psychol Sci Soc Sci. 2006;61:137–143. doi: 10.1093/geronb/61.3.p137. [DOI] [PubMed] [Google Scholar]
- 30.Cutler RG, Mattson MP. The adversities of aging. Ageing Res Rev. 2006;5:221–238. doi: 10.1016/j.arr.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216. doi: 10.1176/appi.ajp.161.2.195. [DOI] [PubMed] [Google Scholar]
- 32.Martin B, Mattson MP, Maudsley S. Caloric restriction and intermittent fasting: two potential diets for successful brain aging. Ageing Res Rev. 2006;5:332–353. doi: 10.1016/j.arr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loucks EB, Berkman LF, Gruenewald TL. Social integration is associated with fibrinogen concentration in elderly men. Psychosom Med. 2005;67:353–358. doi: 10.1097/01.psy.0000160482.89163.e8. [DOI] [PubMed] [Google Scholar]
- 34.Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamond AJ, Depp CA, Allison M. Measurement and predictors of resilience among community-dwelling older women. J Psychiatr Res. 2008;43:148–154. doi: 10.1016/j.jpsychires.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC) Depress Anxiety. 2003;18:76–82. doi: 10.1002/da.10113. [DOI] [PubMed] [Google Scholar]
- 37.Milgram NW, Head E, Zicker SC. Long-term treatment with antioxidants and a program of behavioral enrichment reduces age-dependent impairment in discrimination and reversal learning in beagle dogs. Exp Gerontol. 2004;39:753–765. doi: 10.1016/j.exger.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Cabeza R, Anderson ND, Locantore JK. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- 39.Willis SL, Tennstedt SL, Marsiske M. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296:2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGurk SR, Twamley EW, Sitzer DI. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164:1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fowler JH, Christakis NA. Dynamic spread of happiness in a large social network: longitudinal analysis over 20 years in the Framingham Heart Study. BMJ. 2008;337:278–294. doi: 10.1136/bmj.a2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357:370–379. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 43.Christakis NA, Fowler JH. The collective dynamics of smoking in a large social network. N Engl J Med. 2008;358:2249–2258. doi: 10.1056/NEJMsa0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson RS, Krueger KR, Arnold SE. Loneliness and risk of Alzheimer disease. Arch Gen Psychiatry. 2007;64:234–240. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- 45.Gordon-Larsen P, Nelson MC, Page P. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics. 2006;117:417–424. doi: 10.1542/peds.2005-0058. [DOI] [PubMed] [Google Scholar]
- 46.Krause N. Meaning in life and mortality. J Gerontol B Psychol Sci Soc Sci. 2009;64:517–527. doi: 10.1093/geronb/gbp047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giltay EJ, Geleijnse JM, Zitman FG. Dispositional optimism and all-cause and cardiovascular mortality in a prospective cohort of elderly Dutch men and women. Arch Gen Psychiatry. 2004;61:1126–1135. doi: 10.1001/archpsyc.61.11.1126. [DOI] [PubMed] [Google Scholar]
- 48.Levy BR. Mind matters: cognitive and physical effects of aging self-stereotypes. J Gerontol B Psychol Sci Soc Sci. 2003;58:203–211. doi: 10.1093/geronb/58.4.p203. [DOI] [PubMed] [Google Scholar]
- 49.Jeste DV, Vahia I. Comparison of the conceptualization of wisdom in ancient Indian literature with modern views: focus on the Bhagavad Gita. Psychiatry. 2008;71:3–3. doi: 10.1521/psyc.2008.71.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meeks TW, Jeste DV. Neurobiology of wisdom?: an overview. Arch Gen Psychiatry. 2009;66:355–365. doi: 10.1001/archgenpsychiatry.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cato MA, Delis DC, Abildskov TJ. Assessing the elusive cognitive deficits associated with ventromedial prefrontal damage: a case of a modern-day Phineas Gage. J Int Neuropsychol Soc. 2004;10:453–465. doi: 10.1017/S1355617704103123. [DOI] [PubMed] [Google Scholar]
- 52.O’Hara R. Stress, aging, and mental health. Am J Geriatr Psychiatry. 2006;14:295–298. doi: 10.1097/01.JGP.0000216710.07227.5c. [DOI] [PubMed] [Google Scholar]
- 53.Taylor MG, Lynch SM. Trajectories of impairment, social support, and depressive symptoms in later life. J Gerontol B Psychol Sci Soc Sci. 2004;59:S238–S246. doi: 10.1093/geronb/59.4.s238. [DOI] [PubMed] [Google Scholar]
- 54.Olshansky SJ, Passaro DJ, Hershow RC. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 55.Blumenthal JA, Babyak MA, Moore KA. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 56.Wolinsky FD, Vander Weg MW, Martin R. The effect of speed-of-processing training on depressive symptoms in ACTIVE. J Gerontol A Biol Sci Med Sci. 2009;64:468–472. doi: 10.1093/gerona/gln044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanchez-Villegas A, Delgado-Rodriguez M, Alonso A. Association of the Mediterranean dietary pattern with the incidence of depression: the Seguimiento Universidad de Navarra/University of Navarra follow-up (SUN) cohort. Arch Gen Psychiatry. 2009;66:1090–1098. doi: 10.1001/archgenpsychiatry.2009.129. [DOI] [PubMed] [Google Scholar]
- 58.Small GW, Silverman DH, Siddarth P. Effects of a 14-day healthy longevity lifestyle program on cognition and brain function. Am J Geriatr Psychiatry. 2006;14:538–545. doi: 10.1097/01.JGP.0000219279.72210.ca. [DOI] [PubMed] [Google Scholar]
- 59.Zerhouni EA. US biomedical research: basic, translational, and clinical sciences. JAMA. 2005;294:1352–1358. doi: 10.1001/jama.294.11.1352. [DOI] [PubMed] [Google Scholar]
- 60.Tucker-Drob EM, Johnson KE, Jones RN. The cognitive reserve hypothesis: a longitudinal examination of age-associated declines in reasoning and processing speed. Dev Psychol. 2009;45:431–446. doi: 10.1037/a0014012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fried LP, Tangen CM, Walston J. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 62.Rosenberg D, Depp CI, Vahia IV. Exergames for subsyndromal depression in older adults: a pilot study. Am J Geriatr Psychiatry. 2010;18:221–226. doi: 10.1097/JGP.0b013e3181c534b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sallis JF, Cervero RB, Ascher W. An ecological approach to creating active living communities. Annu Rev Public Health. 2006;27:297–322. doi: 10.1146/annurev.publhealth.27.021405.102100. [DOI] [PubMed] [Google Scholar]
- 64.Phelan EA, Larson EB. “Successful aging” – where next? J Am Geriatr Soc. 2002;50:1306–1308. doi: 10.1046/j.1532-5415.2002.t01-1-50324.x. [DOI] [PubMed] [Google Scholar]
- 65.Roos NP, Havens B. Predictors of successful aging: a twelve-year study of Manitoba elderly. Am J Publ Health. 1991;81:63–68. doi: 10.2105/ajph.81.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andresen EM, Bowley N, Rothenberg BM. Test-retest performance of a mailed version of the Medical Outcomes Study 36-Item Short-Form Health Survey among older adults. Med Care. 1996;34:1165–1170. doi: 10.1097/00005650-199612000-00001. [DOI] [PubMed] [Google Scholar]