Abstract
Marijuana-dependent individuals report using marijuana to alleviate withdrawal, suggesting that pharmacotherapy of marijuana withdrawal could promote abstinence. To identify potential pharmacotherapies for marijuana withdrawal, this study first characterized rimonabant-induced Δ9-tetrahydrocannabinol (Δ9-THC) withdrawal in rhesus monkeys by using drug discrimination and directly observable signs. Second, drugs were examined for their capacity to modify cannabinoid withdrawal. Monkeys receiving chronic Δ9-THC (1 mg/kg/12 h s.c.) discriminated the cannabinoid antagonist rimonabant (1 mg/kg i.v.) under a fixed ratio schedule of stimulus-shock termination. The discriminative stimulus effects of rimonabant were dose-dependent (ED50 = 0.25 mg/kg) and accompanied by head shaking. In the absence of chronic Δ9-THC treatment (i.e., in nondependent monkeys), a larger dose (3.2 mg/kg) of rimonabant produced head shaking and tachycardia. Temporary discontinuation of Δ9-THC treatment resulted in increased responding on the rimonabant lever, head shaking, and activity during the dark cycle. The rimonabant discriminative stimulus was attenuated fully by Δ9-THC (at doses larger than mg/kg/12 h) and the cannabinoid agonist CP 55940 [5-(1,1-dimethylheptyl)-2-[5-hydroxy-2-(3-hydroxypropyl)cyclohexyl]phenol], and partially by the cannabinoid agonist WIN 55212-2 [(R)-(+)-[2, 3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate] and the α2-adrenergic agonist clonidine. In contrast, a benzodiazepine (diazepam) and monoamine agonist (cocaine) did not attenuate the rimonabant discriminative stimulus. Head shaking was attenuated by all test compounds. These results show that the discriminative stimulus effects of rimonabant in Δ9-THC-treated monkeys are a more pharmacologically selective measure of cannabinoid withdrawal than rimonabant-induced head shaking. These results suggest that cannabinoid and noncannabinoid (α2-adrenergic) agonists are potentially useful therapeutics for marijuana dependence inasmuch as they attenuate the subjective experience of Δ9-THC withdrawal.
Pharmacotherapy is a viable strategy for treating dependence on some drugs (e.g., opioid agonists and tobacco). Marijuana-dependent individuals are seeking treatment in increasing numbers, and there is an emerging consensus that a pharmacotherapy could help promote abstinence from marijuana (Compton et al., 2004; Elkashef et al., 2008; Vandrey and Haney, 2009), although currently there are no Food and Drug Administration-approved pharmacotherapies for marijuana dependence. To facilitate identification of a suitable pharmacotherapy, preclinical assays of marijuana (i.e., cannabinoid) dependence are needed. Many people report using marijuana to alleviate withdrawal that emerges upon discontinuation of marijuana use; therefore, a pharmacotherapy could specifically target withdrawal to promote abstinence (Budney et al., 1999). The goals of this study were to use drug discrimination and directly observable signs to characterize cannabinoid withdrawal in rhesus monkeys and, further, to examine cannabinoids and noncannabinoids for their capacity to modify cannabinoid withdrawal.
Dependence and withdrawal to Δ9-tetrahydrocannabinol (Δ9-THC), the cannabinoid primarily responsible for the abuse and dependence liability of marijuana, has been examined in rodents, dogs, and monkeys. In rhesus monkeys, abrupt discontinuation of chronic Δ9-THC treatment resulted in a withdrawal syndrome, evidenced by increased gross movement, eye contact, and tooth baring (Fredericks and Benowitz, 1980) and disruptions in operant responding for food (Beardsley et al., 1986). However, withdrawal was not always detected after discontinuation of chronic Δ9-THC treatment (Harris et al., 1974). Failure to detect withdrawal after discontinuation of chronic Δ9-THC could be caused by the long duration of action and slow elimination of Δ9-THC (Grotenhermen, 2003). With the availability of the CB1 antagonist rimonabant, previous studies were able to demonstrate that rimonabant induces a cannabinoid withdrawal syndrome in rodents and dogs (see Lichtman and Martin, 2002 for review). The directly observable effects of rimonabant in Δ9-THC-treated rodents, including paw tremors and head shaking, were attenuated not only by Δ9-THC but also by clonidine (Lichtman et al., 2001), suggesting that some noncannabinoids have therapeutic potential as treatments for marijuana withdrawal.
Drug discrimination assays have been used to characterize withdrawal from a variety of drug classes (Gellert and Holtzman, 1979; Emmett-Oglesby et al., 1990). Drug discrimination generally is highly sensitive to the effects of drugs and could be uniquely sensitive to cannabinoid withdrawal inasmuch as marijuana withdrawal in humans is evidenced primarily by symptoms (i.e., mood disturbances) and few directly observable signs (Haney et al., 1999b; Budney et al., 2004). One goal of the current study was to treat monkeys with a sufficiently large dose (1 mg/kg/12 h s.c.) of Δ9-THC to produce dependence and, in turn, characterize rimonabant-induced withdrawal by using drug discrimination and directly observable signs. Signs measured in the present study were chosen, in part, from those reported in other species (e.g., head shaking in rodents; Cook et al., 1998) and from previous studies in rhesus monkeys. Δ9-THC decreased heart rate and locomotor activity in rhesus monkeys (Fredericks and Benowitz, 1980; Stark and Dews, 1980; Fredericks et al., 1981; Vivian et al., 1998). Consequently, heart rate and locomotor activity were measured in the current study to examine whether rimonabant-induced withdrawal includes tachycardia and hyperactivity. To help clarify whether the effects of rimonabant during chronic Δ9-THC treatment were related to withdrawal, the effects of rimonabant in nondependent monkeys were examined. Moreover, Δ9-THC treatment was abruptly discontinued in the current study to compare spontaneous withdrawal signs with rimonabant-induced withdrawal signs. A second goal was to examine pharmacologic manipulation of rimonabant-induced cannabinoid withdrawal. Cannabinoid agonists CP 55940 [5-(1, 1-dimethylheptyl)-2-[5-hydroxy-2-(3-hydroxypropyl)cyclohexyl]phenol] and WIN 55212-2 [R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate] were studied to examine the role of cannabinoid receptors in Δ9-THC withdrawal. Clonidine was studied to evaluate α2-adrenergic receptors as targets for pharmacotherapy (Lichtman et al., 2001; Haney et al., 2008), and a benzodiazepine agonist (diazepam) was studied because it reduces anxiety, a symptom of cannabinoid withdrawal (Haney et al., 1999b). Deficits in mesolimbic dopamine neurotransmission were reported in cannabinoid-withdrawn animals (Diana et al., 1998); cocaine was included to test the hypothesis that an indirect-acting dopamine agonist attenuates cannabinoid withdrawal. The results with cocaine and diazepam demonstrate that head shaking and discriminative stimulus effects differ in their pharmacologic selectivity and sensitivity to the withdrawal-reversing effects of drugs.
Materials and Methods
Subjects.
One male and four female rhesus monkeys (Macaca mulatta) discriminated rimonabant (1 mg/kg i.v.) from vehicle while receiving 1 mg/kg/12 h Δ9-THC subcutaneously. Three of these monkeys previously discriminated rimonabant (1 mg/kg i.v.) from vehicle while receiving 0.32 mg/kg/day Δ9-THC intravenously as described previously (McMahon, 2006a). Before treatment with 1 mg/kg/12 h Δ9-THC, treatment with the smaller dose (0.32 mg/kg/day) had been discontinued for at least 1 year and, during this period, the effects of rimonabant in the absence of Δ9-THC treatment were determined. Two other monkeys were experimentally naive before they were treated with 1 mg/kg/12 h Δ9-THC subcutaneously and trained to discriminate rimonabant. The five monkeys discriminating rimonabant were used to generate all of the data reported in this article, with the exception of head shaking data measured in the absence of chronic Δ9-THC treatment, which was measured in a separate group of five rhesus monkeys (one female and four males) discriminating a relatively small dose (0.1 mg/kg) of Δ9-THC (McMahon, 2006b). In those monkeys, rimonabant-induced head shaking was measured at least 48 h after Δ9-THC was administered.
All monkeys were housed individually in stainless-steel cages (33 inches wide × 27 inches deep × 32 inches high) on a 14-h light/10-h dark schedule (lights on at 6:00 AM). They were maintained at 95% free-feeding weight (range 6–11.6 kg) with a diet consisting of primate chow (High Protein Monkey Diet; Harlan Teklad, Madison, WI), fresh fruit, and peanuts, and they were provided water in the home cage. Monkeys were maintained in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Surgery.
Monkeys were anesthetized with ketamine (10 mg/kg i.m.) and isoflurane (1.5–3.0% inhalation). Using sterile techniques, chronic indwelling catheters (heparin coated polyurethane, o.d. = 1.68 mm, i.d. = 1.02 mm; Instech Solomon, Plymouth Meeting, PA) were inserted into a femoral or subclavian vein. Suture silk (coated vicryl; Ethicon Inc., Somerville, NJ) was used to anchor the catheter to the vessel and ligate the section of the vessel proximal to the catheter insertion. The distal end of the catheter was attached to a vascular access port (Mida-cbas-c50; Instech Solomon) positioned subcutaneously at the midscapular region of the back. Telemetry transmitters (CTA-D70; Data Sciences International, St. Paul, MN) were implanted subcutaneously; a skin incision was made approximately 6 cm lateral to the abdominal midline near the flank. The skin over the flank was undermined by using dissection to create a subcutaneous pocket in which the telemetry device was inserted. The skin was closed with a 3-0 absorbable nylon suture. Electrocardiogram leads were placed in line with the long axis of the heart. One electrode was placed in the upper right quadrant of the chest (just below the pectoral muscles against the rib cage near the first interspace), and the other was placed in the lower left quadrant (interspace on the left caudal-lateral thorax).
Operant Conditioning Chambers.
Discriminative stimulus effects and head shaking were measured concurrently in ventilated, sound-attenuating chambers in the presence of white noise. Each chamber contained two levers and two lights, one positioned above each lever. Monkeys were seated in chairs (model R001; Primate Products, Miami, FL), and their feet were placed in shoes containing brass electrodes through which a brief electric stimulus (3 mA, 250 ms) could be delivered from an a.c. generator (Coulbourn Instruments, Allentown, PA). An interface (MedAssociates, St. Albans, VT) connected the chambers to a computer, which controlled and recorded experimental events.
Discrimination Training Procedure.
Each day Δ9-THC (1 mg/kg s.c.) was administered at 6:00 AM and 6:00 PM, and monkeys discriminated rimonabant (1 mg/kg i.v.) from vehicle (at noon) under a fixed ratio schedule of stimulus-shock termination. Experimental sessions consisted of consecutive 20-min cycles. Each cycle began with a 15-min timeout; responding on the levers during the timeout had no programmed consequence. The timeout was followed by illumination of red lights. The lights signaled delivery of an electric stimulus in 40 s; however, five consecutive responses on the correct lever extinguished the lights, prevented delivery of the electric stimulus, and postponed the schedule for 30 s. The correct lever was determined by administration of vehicle or rimonabant at the beginning of the session; determination of correct levers (e.g., left, vehicle; right, rimonabant) varied among monkeys and remained the same for an individual throughout the study. A response on the incorrect lever reset the response requirement on the correct lever. Response periods ended after 5 min or the delivery of four electric stimuli, whichever occurred first. Vehicle training was conducted by administering vehicle intravenously or sham (i.e., dull pressure applied to the skin overlying the vascular access port) during the first minute of each of no more than six cycles. Rimonabant training was conducted by administering the training dose (1 mg/kg i.v.) during the first minute of a cycle followed by vehicle or sham during the first minute of a second cycle. Zero to four vehicle-training cycles preceded the two rimonabant-training cycles.
Discrimination Testing Procedure.
A monkey was tested for the first time when the following criteria were satisfied in every cycle during five consecutive or six of seven training sessions: at least 80% of the total responses occurred on the correct lever and fewer than five responses occurred on the incorrect lever before completion of five consecutive responses on the correct lever. Subsequent tests were conducted when performance for consecutive training sessions, including both vehicle and rimonabant training sessions, satisfied the criteria. The type of training session preceding test sessions varied nonsystematically. Test sessions were identical to training sessions except that five consecutive responses on either lever were reinforced, and animals received vehicle or a dose of drug intravenously during the first minute of a cycle.
To establish a rimonabant dose-effect curve, a dose was administered intravenously during a cycle and then vehicle or sham was administered in a second cycle. The time course of the training dose (1 mg/kg) of rimonabant was established by administering the training dose 2 and 4 h before a multiple-cycle test, during which vehicle was administered intravenously in the first cycle followed by sham in a second cycle.
To examine whether the effects of rimonabant in Δ9-THC-treated monkeys were also observed after abrupt discontinuation of Δ9-THC treatment (i.e., Δ9-THC deprivation), multiple-cycle test sessions were conducted before, during, and after Δ9-THC deprivation for 6 days. Training sessions were not conducted during Δ9-THC deprivation. Test sessions were conducted at noon, and vehicle was administered intravenously in the first cycle followed by sham in subsequent cycles. The first test in the sequence was conducted during Δ9-THC treatment, i.e., 6 h after the morning injection of Δ9-THC (1 mg/kg s.c.). Monkeys were deprived of Δ9-THC that evening (i.e., at 6:00 PM) by administering vehicle subcutaneously instead of Δ9-THC, and monkeys continued to receive vehicle subcutaneously at 6:00 AM and 6:00 PM for the next 5 days and at 6:00 AM on the sixth and last day of Δ9-THC deprivation. Test sessions were conducted on days 1, 2, and 6 of the Δ9-THC deprivation period. On day 6 of deprivation, Δ9-THC treatment was resumed in the evening, i.e., monkeys received 1 mg/kg Δ9-THC at 6:00 PM. The next day, monkeys received 1 mg/kg Δ9-THC at 6:00 AM, and the last test of the sequence was conducted that day at noon.
The effects of test compounds alone and in combination with the training dose of rimonabant were examined during four-cycle test sessions. Vehicle or a dose of test drug was administered in the first cycle followed by sham in a second cycle; rimonabant (1 mg/kg) was administered in a third cycle followed by sham in a fourth cycle. Test drugs were Δ9-THC (0.32–3.2 mg/kg), CP 55940 (0.032–0.32 mg/kg), WIN 55212-2 (1–10 mg/kg), clonidine (0.0032–1 mg/kg), diazepam (0.32–3.2 mg/kg), and cocaine (0.1–1 mg/kg).
Head Shaking.
Cameras mounted inside the operant conditioning chambers provided a frontal view of each monkey. Video was recorded with commercially available software (GV-650; GeoVision, Taipei, Taiwan) and stored on a computer. Frequency of head shaking was quantified by observers blind to treatment. Head shaking was defined as rapid, horizontal, side-to-side oscillation of the head for a minimum of 1 s. Each head shake was separated by at least 1 s of no head shaking. To establish the dose-effect curve of rimonabant and test drugs, the frequency of head shaking was measured immediately after drug administration for 40 min. To establish a time course of rimonabant in Δ9-THC-treated monkeys, the frequency of head shaking was measured for 40 min at 2 and 4 h after administration of the training dose (1 mg/kg). To examine head shaking after the abrupt discontinuation of Δ9-THC treatment, frequency was measured at noon for 80 min. The effects of a test drug on rimonabant-induced head shaking were measured by administering the test drug 40 min before rimonabant; head shaking was measured for 40 min immediately after rimonabant was administered.
Heart Rate and Activity.
Heart rate and activity were measured in four unrestrained monkeys (i.e., in the home cage) via a receiver (RMC-1; Data Sciences International), computer, and commercially available software (Dataquest Acquisition and Analysis System; Data Sciences International). Activity was defined by the position and velocity of movement of the transmitter relative to the receiver; activity was a relative measure (i.e., more active versus less active) and not an absolute measure of distance. During a given observation period, heart rate was measured continuously for 2 min every 5 min and activity was measured continuously. The effects of vehicle and rimonabant in the absence of chronic Δ9-THC treatment were examined in three monkeys that had not received chronic Δ9-THC treatment for at least 6 months and in a fourth monkey that had not previously received Δ9-THC treatment. The effects of vehicle and rimonabant were then reassessed in these same monkeys after they had received Δ9-THC (1 mg/kg/12 h) for 6 months. Vehicle or rimonabant (0.1–3.2 mg/kg i.v.) was administered while monkeys were seated in chairs; they were immediately returned to the home cage at noon for telemetry measurement. Drug discrimination sessions were not conducted on those days. Tests with different doses of rimonabant were separated by at least 3 days. The time course of rimonabant (1 mg/kg i.v.) on heart rate and activity was generated from a single session. For the Δ9-THC deprivation experiment, heart rate and activity were measured throughout the day except during discrimination test sessions (12:00 PM to 2:00 PM).
Drugs.
The following drugs were administered intravenously: rimonabant, Δ9-THC (100 mg/ml in absolute ethanol) (The Research Technology Branch, National Institute on Drug Abuse, Rockville, MD), CP 55940 (Tocris Bioscience, Ellisville, MO), and WIN 55212-2 and diazepam (Sigma-Aldrich, St. Louis, MO). For these drugs, the vehicle consisted of one part absolute ethanol, one part Emulphor-620 (Rhone-Poulenc Inc., Princeton, NJ), and 18 parts physiologic saline. Clonidine hydrochloride (Sigma-Aldrich) and cocaine hydrochloride (National Institute on Drug Abuse) were prepared in distilled sterile water and administered subcutaneously. Ketamine hydrochloride (Bioniche, Athens, GA) was prepared in physiological saline and administered subcutaneously. The volume was 0.03 to 1 ml/kg, and doses (mg/kg) were expressed as the weight of the forms listed above.
Data Analyses.
Discrimination, response rate, and head shaking data were averaged from five monkeys, and telemetry data (heart rate and activity) were averaged from four monkeys. Discrimination data were expressed as a percentage of responses on the rimonabant lever of total responses on the vehicle and rimonabant levers. Rate of responding (responses per s) on both levers was converted to a control, defined as the average response rate in all cycles during the five previous vehicle-training sessions, during which the test criteria were satisfied, immediately preceding that test. Response rate data were expressed as a percentage of the control for individual animals. Discrimination data at a dose were not included for analysis or plotted when the corresponding response rate was less than 20% of control in three or more subjects; however, response rate data were always included for analysis and plotted. Inter-rater reliability for measurement of head shaking was established with linear regression of frequency measured from a training database consisting of 10 separate sessions. To match the temporal parameters used for measurement of head shaking (i.e., 40 min for studies with test drugs and 80 min for the Δ9-THC deprivation study), discrimination and response rate data were averaged from two and four cycles, respectively, for further analysis. Heart rate data were averaged from all values collected during a 40-min period and expressed as a difference from the vehicle (nondrug) control. Activity was cumulated from all values measured for 40 min (studies with rimonabant) or 8 h overnight (Δ9-THC deprivation study). For the rimonabant dose-effect and time course data and the Δ9-THC deprivation study, discrimination, response rate, head shaking, heart rate, and activity data were averaged among monkeys (± S.E.M.) and plotted as a function of dose or time. Dose-effect and time course data were analyzed separately with ANOVA for repeated measures followed by Dunnett's post-hoc test to examine significant differences from the vehicle control (p < 0.05).
Discrimination, response rate, and head shaking data after vehicle or the training dose (1 mg/kg) of rimonabant were determined in three separate test sessions; data from these tests were averaged to provide a single vehicle or rimonabant (1 mg/kg) control for further analysis with test compounds (Δ9-THC, CP 55940, WIN 55212-2, clonidine, diazepam, and cocaine). Head shaking data were converted to a percentage of the rimonabant control for individual subjects. Dose-effect data were analyzed separately per test compound with ANOVA for repeated measures followed by Dunnett's post-hoc test to compare individual doses to the vehicle or rimonabant controls (p < 0.05).
When a drug decreased the effects of rimonabant (1 mg/kg) to less than 50%, potency was calculated by simultaneously fitting straight lines to individual dose-effect data by means of Prism version 5.0 for Windows (GraphPad Software Inc., San Diego, CA) with linear regression. Straight lines were fitted to the linear portion of dose-effect curves, defined by doses producing 20 to 80% of the maximum effect, including not more than one dose producing less than 20% of the maximum effect and not more than one dose producing more than 80% of the maximum effect. Other doses were excluded from linear regression. The slopes of dose-effect curves were compared with an F-ratio test by using GraphPad. If the slopes were not significantly different, then a common, best-fitting slope was used to calculate potency (Kenakin, 1997). Doses corresponding to the 50% level of the effect (ED50), potency ratios, and their 95% confidence limits were calculated by parallel line analysis of data from individual subjects (Tallarida, 2000). Potencies were considered significantly different when the 95% confidence limits of the potency ratio did not include 1.
Results
Discriminative and Rate Effects of Rimonabant in Δ9-THC-Treated Monkeys.
In three monkeys previously trained to discriminate rimonabant (1 mg/kg i.v.) from vehicle in the presence of a relatively small dose (0.32 mg/kg/day i.v.) of Δ9-THC (McMahon, 2006a), stimulus control with rimonabant in the presence of a larger dose (1 mg/kg/12 h) of Δ9-THC was re-established in 8, 8, and 11 sessions in individual monkeys. For two experimentally naive monkeys, stimulus control with rimonabant (1 mg/kg i.v.) during treatment with 1 mg/kg/12 h Δ9-THC was established in 30 and 53 sessions.
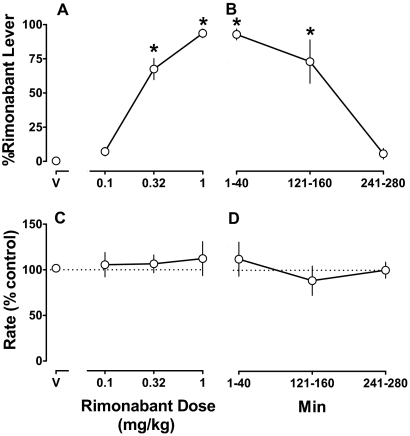
Rimonabant dose-dependently increased responding on the drug-appropriate lever (F3,12 = 142; p < 0.001) (Fig. 1A); doses of 0.32 and 1 mg/kg significantly increased rimonabant-lever responding to a mean of 72 and 93%, respectively. After administration of vehicle, responding was 1% on the rimonabant lever (Fig. 1A, V). The ED50 value (95% confidence limits) of rimonabant to produce discriminative stimulus effects was 0.25 (0.17–0.35) mg/kg. The discriminative stimulus effects of rimonabant decreased as a function of time (Fig. 1B) (F3,9 = 29.6; p < 0.001), with the training dose (1 mg/kg i.v.) producing significant drug-lever responding (73%) for up to 121 to 160 min; percentage responding on the rimonabant lever was no longer significantly different from the vehicle control at 241 to 280 min. Over 10 vehicle training sessions in which the criteria for testing were satisfied average rates of lever pressing for individual monkeys were 0.70, 0.74, 0.80, 1.29, and 1.44 responses per s. Rimonabant, up to the training dose (1 mg/kg), did not significantly modify response rate (p > 0.05) (Fig. 1, C and D).
Fig. 1.
Discriminative stimulus effects of rimonabant (1 mg/kg) in rhesus monkeys receiving chronic Δ9-THC (1 mg/kg/12 h). Abscissae: vehicle (V) or dose in mg/kg body weight (A and C) and time (Min) after administration of the training dose (1 mg/kg) of rimonabant (B and D). Ordinates: mean (± S.E.M.) percentage of responding on the rimonabant lever (A and B) and mean (± S.E.M.) response rate expressed as a percentage of control (V training days) rate [rate (% control)] (C and D). *, p < 0.05 versus V.
The Effects of Rimonabant Alone and during Chronic Δ9-THC: Head Shaking, Heart Rate, and Activity.
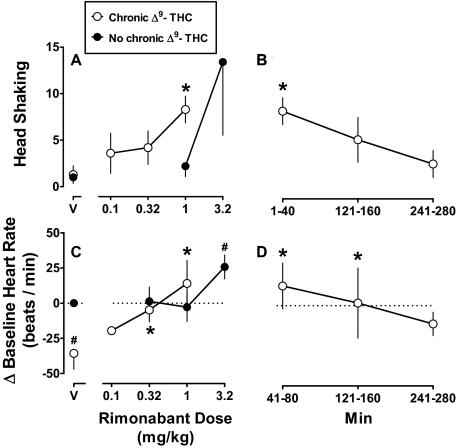
The inter-rater reliability between observers measuring head shaking was relatively high, evidenced by a coefficient of determination of 0.92; the slope of the regression line was not significantly different from unity and the intercept was not significantly different from zero. Administration of vehicle, either during chronic Δ9-THC or not, resulted in a maximum average (± S.E.M.) frequency of 1.3 (± 0.9) head shaking counts (Fig. 2A, V). When administered without chronic Δ9-THC treatment, the mean frequency of head shaking after 1 and 3.2 mg/kg rimonabant was 2.2 and 13.4, respectively (Fig. 2A, ●). Head shaking at 3.2 mg/kg rimonabant was caused by a marked increase in two of five monkeys; the group average data were not statistically significant (F2,12 = 2.18; p = 0.16). During chronic Δ9-THC (1 mg/kg/12 h), rimonabant significantly increased head shaking as function of dose (F3,12 = 12.7; p < 0.001) (Fig. 2A, ○) and time (F3,9 = 9.00; p < 0.01) (Fig. 2B). A dose of 1 mg/kg significantly increased head shaking frequency to 8.3 at 1 to 40 min, an effect that was no longer significantly different from the vehicle control at 121 to 160 min.
Fig. 2.
Head shaking and heart rate produced by rimonabant in combination with chronic Δ9-THC treatment (Chronic Δ9-THC; ○) and without Δ9-THC (No chronic Δ9-THC; ●). Abscissae: vehicle (V) or dose in mg/kg body weight (A and C) and time (Min) after administration of 1 mg/kg rimonabant (B and D). Ordinates: mean (± S.E.M.) frequency of head shaking (A and B) and mean (± S.E.M.) change in heart rate (beats per min) from the nondrug control (C and D). *, p < 0.05 versus V, Chronic Δ9-THC. #, p < 0.05 versus V, No chronic Δ9-THC.
Administration of vehicle intravenously outside the home cage produced an increase in heart rate and activity when monkeys were returned to the home cage for 40 min; therefore, data were analyzed at 41 to 80 min, a period when heart rate and activity were no longer significantly different from data collected at the same time of day when monkeys had not been removed from the home cage. Before chronic Δ9-THC treatment, the average (± S.E.M.) vehicle control heart rate was 132.1 (± 8.1) beats per min; relatively small doses (0.32 and 1 mg/kg) of rimonabant did not significantly modify heart rate, whereas a large dose (3.2 mg/kg) of rimonabant significantly increased heart rate by 25.8 beats per min (F3,9 = 4.98; p < 0.05) (Fig. 2C, ●). Relative to the nondrug control (i.e., no chronic Δ9-THC treatment), heart rate during chronic Δ9-THC (1 mg/kg/12 h) was significantly decreased by 36.0 beats per min (F4,12 = 6.90; p < 0.01) (Fig. 2C, compare ○ and ● above V). Rimonabant dose-dependently attenuated bradycardia produced by chronic Δ9-THC; the significant antagonism produced by 0.32 and 1 mg/kg rimonabant resulted in a heart rate that was not different from the nondrug control (Fig. 2C). Antagonism of Δ9-THC-induced bradycardia decreased as a function of time (F3,9 = 5.36; p < 0.05), i.e., antagonism produced by 1 mg/kg rimonabant was evident at 121 to 160 min and not 241 to 280 min (Fig. 2D).
The nondrug control for activity was 32, 67, 97, and 120 counts per 40-min observation period for four respective monkeys. Before chronic Δ9-THC, there was a tendency for rimonabant to increase activity counts, although not significantly because of relatively large error variance, i.e., average (± S.E.M.) activity after 3.2 mg/kg rimonabant was 169 (± 58.4) counts compared with 78.4 (± 18.6) counts after vehicle. During chronic Δ9-THC treatment, there was a nonsignificant tendency for activity to be decreased relative to the nondrug control and, further, for rimonabant to antagonize Δ9-THC-induced hypoactivity. Mean (± S.E.M.) control activity during chronic Δ9-THC was 20 (± 9.7) counts, whereas activity after rimonabant (1 mg/kg) was relatively high (i.e., 72 ± 20 counts) and similar to the nondrug control.
Temporary Discontinuation of Chronic Δ9-THC Treatment.
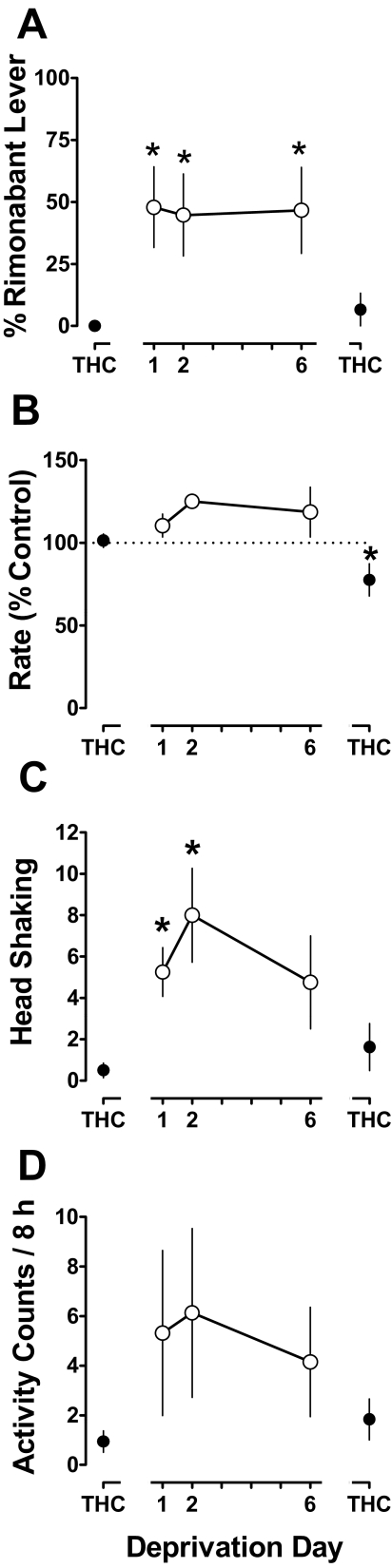
When tested with vehicle the day before discontinuation of Δ9-THC treatment, responding was 0% on the rimonabant lever (Fig. 3A, leftmost THC point). During the same test, response rate was 101% of control and the frequency of head shaking was 0.5 counts (Fig. 3 B and C, respectively, leftmost THC points). Upon discontinuation of Δ9-THC treatment, rimonabant-appropriate responding increased significantly, i.e., to 48% on deprivation day 1 (F4,16 = 5.93; p < 0.01) (Fig. 3A). Response rate during the Δ9-THC deprivation period was not significantly different from the predeprivation control (p > 0.05) (Fig. 3B). Head shaking frequency significantly increased during the deprivation period, to 5.3 and 8.0 counts on deprivation days 1 and 2, respectively (F4,16 = 6.92; p < 0.01) (Fig. 3C). On deprivation day 6, head shaking was no longer significantly different from the predeprivation control. Upon resumption of Δ9-THC treatment, rimonabant-lever responding and head shaking frequency were not significantly different from predeprivation control values (Fig. 3, A and C, respectively, rightmost THC points), whereas the response rate was significantly decreased relative to the predeprivation control (F4,16 = 4.65; p < 0.05), although the decrease was relatively small (Fig. 3B, rightmost THC point).
Fig. 3.
Discriminative and other behavioral effects resulting from abrupt discontinuation of chronic Δ9-THC (1 mg/kg/12 h) treatment. Abscissae: Consecutive days before (leftmost THC), during (Deprivation), and after (rightmost THC) temporary discontinuation of 1 mg/kg/12 h Δ9-THC. Ordinates: percentage of responding on the rimonabant lever (A), mean (± S.E.M.) response rate expressed as a percentage of control (vehicle training days) rate [rate (% control)] (B), mean (± S.E.M.) frequency of head shaking (C), and cumulative home cage activity during the night (D). *, p < 0.05 versus rightmost Δ9-THC point (i.e., predeprivation control).
During the night (i.e., 8-h period beginning 1 h after lights off and ending 1 h before lights on), mean (± S.E.M.) cumulative activity during Δ9-THC treatment was 0.9 (± 0.4) counts (Fig. 3D, leftmost THC point). The nondrug control (i.e., no chronic Δ9-THC treatment) during the same 8-h period was an average (± S.E.M.) of 1.6 (± 0.4) activity counts. Upon discontinuation of chronic Δ9-THC treatment, night activity increased in three of four monkeys. For example, the maximum activity during any one night of the deprivation period was 16, 11, and 3.2 counts for each of three monkeys. Cumulative night activity did not vary before, during, and after the Δ9-THC deprivation period in one of four monkeys, and analysis of the group data did not achieve statistical significance (p > 0.05). Heart rate did not significantly vary as a function of Δ9-THC deprivation status (data not shown).
Effects of Cannabinoid Agonists Alone and in Combination with Rimonabant: Discriminative Stimulus Effects and Head Shaking.
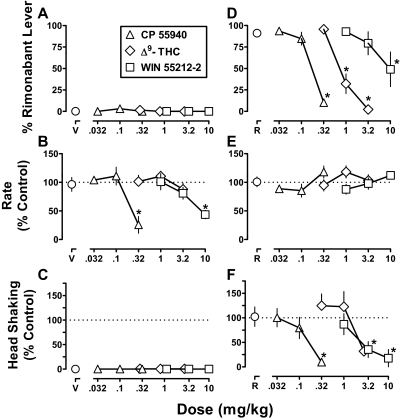
When administered alone, Δ9-THC (0.32–3.2 mg/kg), CP 55940 (0.032–0.32 mg/kg), and WIN 55212-2 (1–10 mg/kg) produced a maximum of 1, 3, and 0% responses on the rimonabant lever, respectively (Fig. 4A). CP 55940 (0.32 mg/kg) and WIN 55212-2 (10 mg/kg) significantly decreased response rate to 26% (F3,12 = 11.9; p < 0.001) and 44% (F3,12 = 6.83; p < 0.01) of the vehicle control, respectively (Fig. 4B). Δ9-THC, up to 3.2 mg/kg, did not significantly modify response rate. The cannabinoid agonists did not significantly increase head shaking (p > 0.05) (Fig. 4C).
Fig. 4.
Discriminative stimulus effects and head shaking after cannabinoid agonists, alone and in combination with rimonabant. Abscissae: dose in mg/kg body weight of cannabinoid agonist in combination with vehicle (V) (A–C) or rimonabant (1 mg/kg; R) (D–F). Ordinates: mean (± S.E.M.) percentage of responding on the rimonabant lever (A and D), mean (± S.E.M.) response rate expressed as a percentage of control (V alone) rate [rate (% control)] (B and E), and mean (± S.E.M.) head shaking expressed as a percentage of control (R alone) (C and F). *, p < 0.05 versus V or R in corresponding panels.
When administered in combination with the training dose (1 mg/kg) of rimonabant, Δ9-THC significantly attenuated rimonabant-lever responding (F3,12 = 40.9; p < 0.001); the effects of the training dose (92% rimonabant-lever responding) were decreased to 2% by 3.2 mg/kg Δ9-THC (Fig. 4D, ◊). Likewise, the discriminative stimulus effects of rimonabant were dose-dependently attenuated by CP 55940 (F3,12 = 89.4; p < 0.001) and WIN 55212-2 (F3,12 = 5.54; p < 0.05) (Fig. 4D, ▵ and □, respectively). However, WIN 55212-2 (up to a dose of 10 mg/kg) produced a smaller decrease (49% rimonabant-lever responding) than that obtained with 0.32 mg/kg CP 55940 (10% rimonabant-lever responding). The slopes of the CP 55940 and Δ9-THC dose-effect curves were not significantly different (p > 0.05), and the ED50 values (95% confidence limits) were 0.14 (0.10–0.20) and 0.85 (0.66–1.1) mg/kg, respectively. CP 55940 was more potent than Δ9-THC, i.e., the potency ratio (95% confidence limits) was 6.0 (4.1–8.6). A slope and ED50 value were not calculated for the WIN 55212-2 dose-effect data because the percentage of rimonabant-lever responding was not decreased to less than 50% in two of five monkeys. Response rate was not significantly modified when the cannabinoid agonists were combined with the training dose of rimonabant (p > 0.05) (Fig. 4E).
Head shaking produced by 1 mg/kg rimonabant was significantly and dose-dependently decreased by Δ9-THC (F3,12 = 4.05; p < 0.05), CP 55940 (F3,12 = 7.14; p < 0.01), and WIN 55212-2 (F3,12 = 17.7; p < 0.001). CP 55940 (0.32 mg/kg), Δ9-THC (3.2 mg/kg), and WIN 55212-2 (10 mg/kg) significantly decreased head shaking to 10, 32, and 18% of control, respectively (Fig. 4F). The slopes of the dose-effect curves for CP 55940, Δ9-THC, and WIN 55212-2 were not significantly different (p > 0.05); the ED50 values (95% confidence limits) were 0.15 (0.07–0.33), 1.8 (0.96–3.5), and 3.0 (1.3–7.1) mg/kg, respectively. CP 55940 attenuated rimonabant-induced head shaking more potently than Δ9-THC and WIN 55212-2, i.e., the potency ratios (95% confidence limits) were 12 (3.1–29) and 17 (5.7–49), respectively. The potencies of Δ9-THC and WIN 55212-2 to attenuate head shaking were not significantly different.
Effects of Noncannabinoids Alone and in Combination with Rimonabant: Discriminative Stimulus Effects and Head Shaking.
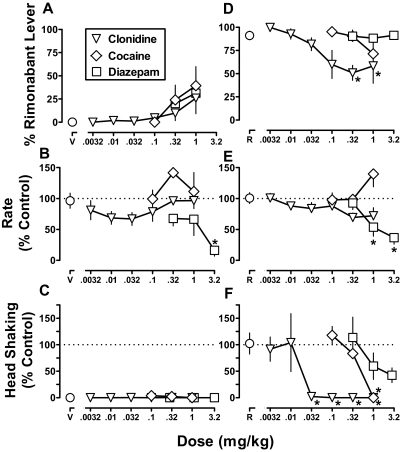
When administered alone, clonidine (0.0032–1 mg/kg), cocaine (0.1–1 mg/kg), and diazepam (0.32–3.2 mg/kg) produced a maximum of 26, 39, and 31% responding on the rimonabant lever, respectively (Fig. 5A). The effect of cocaine was statistically significant (F3,12 = 3.59; p < 0.05), whereas the effects of clonidine and diazepam were not (p > 0.05). Diazepam (3.2 mg/kg) significantly decreased response rate to 37% of the vehicle control (F3,12 = 4.76; p < 0.05), whereas cocaine and clonidine did not significantly modify response rate up to the largest dose (1 mg/kg) studied (p > 0.05) (Fig. 5B). Clonidine, cocaine, and diazepam did not significantly increase head shaking (p > 0.05) (Fig. 5C).
Fig. 5.
Discriminative stimulus effects and head shaking after noncannabinoids, alone and in combination with rimonabant. Abscissae: dose in mg/kg body weight of clonidine, cocaine, or diazepam in combination with vehicle (V) (A–C) or rimonabant (1 mg/kg; R) (D–F). Ordinates: mean (± S.E.M.) percentage of responding on the rimonabant lever (A and D), mean (± S.E.M.) response rate expressed as a percentage of control (V alone) rate [rate (% control)] (B and E), and mean (± S.E.M.) head shaking expressed as a percentage of control (R alone) (C and F). *, p < 0.05 versus V or R in corresponding panels.
When administered in combination with the training dose (1 mg/kg) of rimonabant, drug-lever responding was dose-dependently attenuated by clonidine (F6,18 = 9.18; p < 0.001). The dose-effect curve for clonidine seemed to reach an asymptote with a maximum attenuation to 50% rimonabant-lever responding. Neither cocaine nor diazepam significantly attenuated the discriminative stimulus effects of rimonabant (Fig. 5D, ◊ and □, respectively). When combined with rimonabant (1 mg/kg), diazepam significantly decreased response rate to 54 and 37% of control at doses of 1 and 3.2 mg/kg, respectively (F3,12 = 27.1; p < 0.001). In contrast, cocaine and clonidine (up to 1 mg/kg) did not significantly modify response rate when combined with rimonabant (Fig. 5E).
Rimonabant-induced head shaking was dose-dependently attenuated by clonidine (F6,18 = 7.46; p < 0.001) and cocaine (F3,9 = 23.4; p < 0.001) (Fig. 5F). Although ANOVA of the diazepam dose-effect data was not statistically significant (p > 0.05), diazepam decreased rimonabant-induced head shaking to less than 50% of control in all five monkeys. Furthermore, linear regression demonstrated that the effects of diazepam were dose-dependent (i.e., the slope of the dose-effect curve was significantly different from 0). The slopes of the clonidine, cocaine, and diazepam dose-effect curves to attenuate rimonabant-induced head shaking were not significantly different (p > 0.05). The ED50 values (95% confidence limits) were 0.01 (0.003–0.03) mg/kg for clonidine, 0.59 (0.28–1.2) mg/kg for cocaine, and 1.4 (0.41–5.4) mg/kg for diazepam. Clonidine was significantly more potent than cocaine and diazepam, which were equipotent.
Discussion
Rhesus monkeys receiving chronic Δ9-THC treatment (1 mg/kg/12 h) reliably discriminated the cannabinoid antagonist rimonabant (1 mg/kg). Rimonabant had similar potency for producing discriminative stimulus effects, head shaking, and antagonism of Δ9-THC-induced bradycardia. In the absence of chronic Δ9-THC treatment, a relatively large dose (3.2 mg/kg) of rimonabant produced tachycardia and tended to increase head shaking. When Δ9-THC treatment was abruptly discontinued, there was an increase in both rimonabant-lever responding and head shaking. Moreover, activity during the night, which was typically low in the absence of any drug treatment, tended to increase in monkeys deprived of chronic Δ9-THC treatment. The rimonabant discriminative stimulus was attenuated fully by Δ9-THC and CP 55940 and partially by WIN 55212-2 and clonidine; diazepam and cocaine did not attenuate the rimonabant discriminative stimulus. In contrast, rimonabant-induced head shaking was nonselectively attenuated by the test compounds. These results show that the discriminative stimulus effects of rimonabant in Δ9-THC-treated monkeys are a more pharmacologically selective measure of cannabinoid withdrawal than rimonabant-induced head shaking. In addition to cannabinoid agonists, α2-adrenergic agonists seem to be potential pharmacotherapies for treating marijuana withdrawal.
Rhesus monkeys were physically dependent on Δ9-THC, evidenced by increases in rimonabant-lever responding, head shaking, and night activity when Δ9-THC treatment was abruptly discontinued. The magnitude of rimonabant-lever responding obtained after abrupt discontinuation of Δ9-THC treatment did not achieve the same maximum produced by the training dose of rimonabant during Δ9-THC treatment, suggesting that withdrawal upon abrupt discontinuation of treatment was less robust than rimonabant-induced withdrawal. This result is consistent with previous studies assessing signs of cannabinoid withdrawal in rodents (Aceto et al., 1996) and drug discrimination studies showing that withdrawal induced by abrupt discontinuation of opioid treatment was less robust than antagonist-induced opioid withdrawal (Gellert and Holtzman, 1979). Slow metabolism and elimination of Δ9-THC and its active metabolites (Grotenhermen, 2003) could decrease withdrawal magnitude, as has been shown for other drug classes (i.e., long-acting barbiturates; Boisse and Okamoto, 1978). The presently used dose (1 mg/kg/12 h) of Δ9-THC was comparable with that used previously to produce dependence in rhesus monkeys (Fredericks and Benowitz, 1980), evidenced in one study by a disruption in food-maintained responding when Δ9-THC treatment was abruptly discontinued (Beardsley et al., 1986). That operant responding was not disrupted in the current study might be attributed to the reinforcer (stimulus-shock termination) or route of chronic Δ9-THC treatment (subcutaneous, respectively), which differed from those (food presentation and intravenous) used in the previous study (Beardsley et al., 1986). The current study is the first to indicate that head shaking is a sign of cannabinoid withdrawal in primates. Sleep disruption, a prominent sign of marijuana withdrawal in humans (Haney et al., 1999a,b; Budney et al., 2004), seems likely to be responsible for the increased night activity in three of four rhesus monkeys deprived of chronic Δ9-THC in the current study.
Rimonabant-induced cannabinoid withdrawal in primates was evidenced by dose-dependent and reliable discriminative stimulus effects and head shaking. Several lines of evidence suggest that discriminative stimulus effects measured here reflected rimonabant-induced Δ9-THC withdrawal. First, in the absence of chronic Δ9-THC treatment, rimonabant could not be trained as discriminative stimulus in rhesus monkeys up to a training dose of 3.2 mg/kg i.v. (McMahon, 2006a). Second, the ED50 value for the discriminative stimulus effects of rimonabant was strikingly similar to the apparent affinity (pA2) or dose of rimonabant occupying 50% of cannabinoid receptors, as determined from antagonism of the discriminative stimulus effects of cannabinoid agonists (McMahon, 2006b). The ED50 value of the rimonabant discriminative stimulus also was comparable with doses of rimonabant that antagonized Δ9-THC-induced bradycardia, which was evident even after 6 months of Δ9-THC treatment (see also Kaymakçalan and Sivil, 1974; Fredericks et al., 1981). Third, rimonabant had the same potency for producing discriminative stimulus effects and a sign of withdrawal (head shaking). The discriminative stimulus effects of rimonabant under the present experimental conditions seem to be related to Δ9-THC dependence and withdrawal.
Head shaking was evident not only when rimonabant (1 mg/kg) was administered during Δ9-THC treatment, but also when Δ9-THC treatment was abruptly discontinued, demonstrating that head shaking is a cannabinoid withdrawal sign. However, in the absence of chronic Δ9-THC, a relatively large dose (3.2 mg/kg) of rimonabant produced tachycardia and head shaking, although the latter was not statistically significant. If rimonabant-induced head shaking in Δ9-THC-treated monkeys is a withdrawal sign, then antagonism of Δ9-THC must be the underlying mechanism. In contrast, rimonabant-induced head shaking and tachycardia in the absence of Δ9-THC treatment could be caused by a variety of mechanisms; for example, rimonabant is a CB1 receptor inverse agonist in vitro (Bouaboula et al., 1997). A previous study demonstrated that chronic treatment with a benzodiazepine agonist increased sensitivity to convulsions produced by benzodiazepine inverse agonists (Sannerud et al., 1991). Chronic Δ9-THC treatment increased sensitivity to rimonabant-induced head shaking in monkeys (present study) and rodents (Cook et al., 1998), perhaps implicating CB1 inverse agonism as the mechanism by which rimonabant produces behavioral effects in the absence of Δ9-THC treatment. Alternatively, the behavioral effects of rimonabant could be caused by antagonism of endogenous cannabinoid neurotransmitters (anandamide and 2-AG). However, the apparent affinity of rimonabant determined in the presence of anandamide and Δ9-THC was similar (McMahon, 2009), suggesting that rimonabant should have similar potency for producing behavioral effects regardless of whether antagonism of anandamide or Δ9-THC is the underlying mechanism. The apparent affinity of rimonabant in the presence of other endocannabinoids (2-AG) has not been determined; to the extent that rimonabant antagonizes 2-AG with a lesser potency than that for other agonists, antagonism of 2-AG could be responsible for the effects of rimonabant alone. In Δ9-THC-treated monkeys, rimonabant-induced head shaking was attenuated by every test compound (cannabinoid, α2-adrenergic, benzodiazepine, and monoamine agonists) included for study. Even though head shaking seems to be a cannabinoid withdrawal sign, its utility for establishing the neuropharmacology of cannabinoid dependence and withdrawal is limited by a lack of pharmacologic selectivity.
If the discriminative stimulus effects of rimonabant in Δ9-THC-treated animals were related to withdrawal, then attenuation would be expected from increasing the dose of the dependence-inducing drug (Δ9-THC), which was demonstrated here. Furthermore, to the extent that cannabinoid agonism is the mechanism by which Δ9-THC attenuates rimonabant-induced withdrawal, other cannabinoid agonists (CP 55940) would also be expected to attenuate withdrawal, which was demonstrated here. Although CP 55940 was more potent than Δ9-THC, their individual potencies were similar with respect to attenuation of rimonabant-induced head shaking and discriminative stimulus. In contrast, the potency of WIN 55212-2 varied across the two measures, which can be illustrated by comparing the relative potency of WIN 55212-2 and Δ9-THC. For example, WIN 55212-2 was less potent than Δ9-THC in attenuating the discriminative stimulus effects of rimonabant, whereas the agonists were equipotent in reducing rimonabant-induced head shaking. In monkeys discriminating a relatively small dose of Δ9-THC, the potencies of Δ9-THC and WIN 55212-2 also were similar (McMahon, 2006b). Moreover, in that previous study, surmountable antagonism of Δ9-THC was observed across a broader range of doses of rimonabant than antagonism of WIN 55212-2 (McMahon, 2006b). Collectively, these results show that Δ9-THC and WIN 55212-2 differ in their interaction with rimonabant and suggest that Δ9-THC and WIN 55212-2 differ in receptor mechanism of action; one interpretation is that WIN 55212-2 acts via rimonabant-sensitive (CB1) and rimonabant-insensitive (non-CB1) receptors. Head shaking was a less pharmacologically selective measure of the effects of rimonabant than discriminative stimulus effects; therefore, a drug acting at both CB1 and non-CB1 receptors (WIN 55212-2) might be relatively more potent at attenuating the former (i.e., less pharmacologically selective measure) compared with attenuation of the latter. With regard to therapeutic potential, the current results suggest that attenuation of Δ9-THC or marijuana withdrawal by cannabinoid agonists varies according to the relative activity at CB1 versus other receptors.
A therapeutic with a mechanism of action distinct from marijuana (i.e., a noncannabinoid) might have some advantages over marijuana-like compounds in marijuana-dependent individuals. Cross-tolerance from marijuana to a noncannabinoid, for example, should be less than cross-tolerance to a cannabinoid. Moreover, a noncannabinoid would be expected to not have marijuana-like abuse and dependence liability. In the current study, a benzodiazepine (diazepam) did not attenuate the rimonabant-discriminative stimulus, suggesting that anxiolytic activity is not sufficient to attenuate this particular measure of Δ9-THC withdrawal. In contrast, the α2-adrenergic agonist clonidine attenuated the rimonabant-discriminative stimulus, although attenuation by clonidine was partial (i.e., achieved an asymptote at 50%). Although clonidine was less effective than Δ9-THC and CP 55940, clonidine has relatively low abuse liability (Roehrich and Gold, 1987), which might increase the potential therapeutic utility of α2-adrenergic agonists for marijuana dependence. The present results are consistent with the results of a clinical study reporting that the α2-adrenergic agonist lofexidine attenuated marijuana withdrawal (Haney et al., 2008). In that clinical study, lofexidine was effective when combined with Δ9-THC. Further studies are needed to help determine whether cannabinoid and α2-adrenergic agonists have greater therapeutic utility when they are combined compared with when they are administered separately. The rimonabant-discriminative stimulus in Δ9-THC-treated monkeys seems be an appropriate preclinical measure of cannabinoid withdrawal and might be an especially useful measure of the subjective experience of cannabinoid withdrawal. Studies with cannabinoid and α2-adrenergic agonists, alone and in combination, and studies with novel classes of drugs in this assay have the potential to identify not only the neurobiological underpinnings of cannabinoid dependence, but also medicines that could help marijuana-dependent individuals achieve abstinence.
Acknowledgments
We thank Dr. W. Koek for assistance with statistical analysis; Drs. J. Elliott and M. Leland for assistance with surgical procedures; and D. Aguirre, V. Carlton, L. Hargrove, W. Holbein, D. Schulze, and J. Usrey for technical assistance.
This work was supported by the U.S. Public Health Service, National Institutes of Health, National Institute on Drug Abuse [Grants R01-DA19222 and R01-DA26781].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.168435.
- Δ9-THC
- Δ9-tetrahydrocannabinol
- CB
- cannabinoid
- CP 55940
- 5-(1,1-dimethylheptyl)-2-[5-hydroxy-2-(3-hydroxypropyl)cyclohexyl] phenol
- WIN 55212-2
- (R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate
- 2-AG
- 2-arachidonoylglycerol
- ANOVA
- analysis of variance.
References
- Aceto MD, Scates SM, Lowe JA, Martin BR. (1996) Dependence on Δ9-tetrahydrocannabinol: studies on precipitated and abrupt withdrawal. J Pharmacol Exp Ther 278:1290–1295 [PubMed] [Google Scholar]
- Beardsley PM, Balster RL, Harris LS. (1986) Dependence on tetrahydrocannabinol in rhesus monkeys. J Pharmacol Exp Ther 239:311–319 [PubMed] [Google Scholar]
- Boisse NR, Okamoto M. (1978) Physical dependence to barbital compared to pentobarbital. IV. Influence of elimination kinetics. J Pharmacol Exp Ther 204:526–540 [PubMed] [Google Scholar]
- Bouaboula M, Perrachon S, Milligan L, Canat X, Rinaldi-Carmona M, Portier M, Barth F, Calandra B, Pecceu F, Lupker J, et al. (1997) A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J Biol Chem 272:22330–22339 [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey R. (2004) Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry 161:1967–1977 [DOI] [PubMed] [Google Scholar]
- Budney AJ, Novy PL, Hughes JR. (1999) Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction 94:1311–1322 [DOI] [PubMed] [Google Scholar]
- Compton WM, Grant BF, Colliver JD, Glantz MD, Stinson FS. (2004) Prevalence of marijuana use disorders in the United States: 1991–1992 and 2001–2002. J Am Med Assoc 291:2114–2121 [DOI] [PubMed] [Google Scholar]
- Cook SA, Lowe JA, Martin BR. (1998) CB1 receptor antagonist precipitates withdrawal in mice exposed to Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther 285:1150–1156 [PubMed] [Google Scholar]
- Diana M, Melis M, Muntoni AL, Gessa GL. (1998) Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proc Natl Acad Sci USA 95:10269–10273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkashef A, Vocci F, Huestis M, Haney M, Budney A, Gruber A, el-Guebaly N. (2008) Marijuana neurobiology and treatment. Subst Abus 29:17–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett-Oglesby MW, Mathis DA, Moon RT, Lal H. (1990) Animal models of drug withdrawal symptoms. Psychopharmacology 101:292–309 [DOI] [PubMed] [Google Scholar]
- Fredericks AB, Benowitz NL. (1980) An abstinence syndrome following chronic administration of Δ9-terahydrocannabinol in rhesus monkeys. Psychopharmacology 71:201–202 [DOI] [PubMed] [Google Scholar]
- Fredericks AB, Benowitz NL, Savanapridi CY. (1981) The cardiovascular and autonomic effects of repeated administration of Δ9-tetrahydrocannabinol to rhesus monkeys. J Pharmacol Exp Ther 216:247–253 [PubMed] [Google Scholar]
- Gellert VF, Holtzman SG. (1979) Discriminative stimulus effects of naltrexone in the morphine-dependent rat. J Pharmacol Exp Ther 211:596–605 [PubMed] [Google Scholar]
- Grotenhermen F. (2003) Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 42:327–360 [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. (2008) Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology 197:157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. (1999a) Abstinence symptoms following oral THC administration to humans. Psychopharmacology 141:385–394 [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. (1999b) Abstinence symptoms following smoked marijuana in humans. Psychopharmacology 141:395–404 [DOI] [PubMed] [Google Scholar]
- Harris RT, Waters W, McLendon D. (1974) Evaluation of reinforcing capability of Δ9-tetrahydrocannabinol in rhesus monkeys. Psychopharmacologia 37:23–29 [DOI] [PubMed] [Google Scholar]
- Kaymakçalan S, Sivil S. (1974) Lack of tolerance to the bradycardic effect of Δ9-trans-tetrahydrocannabinol in rats. Pharmacology 12:290–295 [DOI] [PubMed] [Google Scholar]
- Kenakin T. (1997) Pharmacologic Analysis of Drug-Receptor Interaction Lippincott-Raven Publishers, Philadelphia, PA [Google Scholar]
- Lichtman AH, Fisher J, Martin BR. (2001) Precipitated cannabinoid withdrawal is reversed by Δ9-tetrahydrocannabinol or clonidine. Pharmacol Biochem Behav 69:181–188 [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. (2002) Marijuana withdrawal syndrome in the animal model. J Clin Pharmacol 42:20S–27S [DOI] [PubMed] [Google Scholar]
- McMahon LR. (2006a) Discriminative stimulus effects of the cannabinoid CB1 antagonist SR 141716A in rhesus monkeys pretreated with Δ9-tetrahydrocannabinol. Psychopharmacology 188:306–314 [DOI] [PubMed] [Google Scholar]
- McMahon LR. (2006b) Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther 319:1211–1218 [DOI] [PubMed] [Google Scholar]
- McMahon LR. (2009) Apparent affinity estimates of rimonabant in combination with anandamide and chemical analogs of anandamide in rhesus monkeys discriminating Δ9-tetrahydrocannabinol. Psychopharmacology 203:219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (1996) Guide for the Care and Use of Laboratory Animals National Academy Press, Washington, DC [Google Scholar]
- Roehrich H, Gold MS. (1987) Clonidine. Adv Alcohol Subst Abuse 7:1–16 [DOI] [PubMed] [Google Scholar]
- Sannerud CA, Allen M, Cook JM, Griffiths RR. (1991) Behavioral effects of benzodiazepine ligands in non-dependent, diazepam-dependent, and diazepam-withdrawn baboons. Eur J Pharmacol 202:159–169 [DOI] [PubMed] [Google Scholar]
- Stark P, Dews PB. (1980) Cannabinoids. I. Behavioral effects. J Pharmacol Exp Ther 214:124–130 [PubMed] [Google Scholar]
- Tallarida RJ. (2000) Drug Synergism and Dose-Effect Data Analysis, pp 44–50, Chapman and Hall/CRC, Boca Raton, FL [Google Scholar]
- Vandrey R, Haney M. (2009) Pharmacotherapy for cannabis dependence: how close are we? CNS Drugs 23:543–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Kishioka S, Butelman ER, Broadbear J, Lee KO, Woods JH. (1998) Analgesic, respiratory, and heart rate effects of cannabinoid and opioid agonists in rhesus monkeys: antagonist effects of SR 141716A. J Pharmacol Exp Ther 286:697–703 [PubMed] [Google Scholar]