Abstract
NAD(P)H:quinone oxidoreductase 1 (NQO1) deficiency resulting from a homozygous NQO1*2 polymorphism has been associated with an increased risk of benzene-induced myeloid toxicity and a variety of de novo and therapy-induced leukemias. Endothelial cells in human bone marrow form one of the two known hematopoietic stem cell microenvironments and are one of the major cell types that express NQO1 in bone marrow. We have used a transformed human bone marrow endothelial cell (TrHBMEC) line to study the potential impact of a lack of NQO1 activity on adhesion molecule [endothelial leukocyte adhesion molecule 1 (E-selectin), vascular cell adhesion molecule (VCAM)-1, and intercellular adhesion molecule (ICAM)-1] expression and functional adhesion to bone marrow progenitor cells. We used both 5-methoxy-1,2-dimethyl-3-[(4-nitrophenoxy)methyl]indole-4,7-dione (ES936), a mechanism-based inhibitor of NQO1, and anti-NQO1 small interfering RNA to abrogate NQO1 activity. Real-time reverse transcription-polymerase chain reaction data demonstrated a significant inhibition of tumor necrosis factor (TNF)α-induced E-selectin mRNA levels after ES936 pretreatment. Immunoblot assays demonstrated a significant reduction in TNFα-stimulated E-selectin, VCAM-1, and ICAM-1 proteins after inhibition or knockdown of NQO1. The mechanisms underlying this effect remain undefined, but modulation of nuclear factor-κB (p65), c-Jun, and activating transcription factor 2, transcriptional regulators of adhesion molecules, were observed after inhibition or knockdown of NQO1. Decreased level of E-selectin, VCAM-1, and ICAM-1 also resulted in a functional deficit in adhesion. A parallel plate flow chamber study demonstrated a marked reduction in CD34+ cell (KG1a) adhesion to NQO1-deficient TrHBMECs relative to controls. The reduced adhesive ability of TrHBMECs may affect the function of the vascular stem cell niche and also may contribute to the increased susceptibility of polymorphic individuals lacking NQO1 to leukemias and hematotoxicants such as benzene.
NAD(P)H:quinone oxidoreductase 1 (NQO1, DT-diaphorase) is a flavin-containing quinone reductase (Ernster, 1967; Bianchet et al., 2004) that is polymorphic in humans (Traver et al., 1997). The NQO1*2 polymorphism is a single-nucleotide polymorphism, defined as a C-to-T substitution at position 609 of the human NQO1 cDNA, corresponding to a proline-to-serine change at position 187 of the protein (Traver et al., 1997). The mutant NQO1*2 protein is rapidly degraded by the ubiquitin proteasomal system, resulting in an absence or only trace levels of NQO1 protein in individuals carrying the NQO1*2/*2 genotype (Moran et al., 1999). Benzene is an occupational and environmental pollutant, and chronic exposure to benzene can induce aplastic anemia, myelodysplasia, and acute myeloid leukemia (Travis et al., 1994). Benzene requires metabolism to exert toxicity, and benzene-derived quinones are considered to play a major role in its toxicity (Ross, 2000). NQO1 can metabolize benzene-derived quinones and with respect to benzene-induced toxicity, there is convincing evidence that NQO1 acts as a detoxification enzyme. NQO1 knockout mice demonstrated increased benzene-induced hematotoxicity (Bauer et al., 2003), and an increased risk of benzene poisoning associated with the NQO1*2 polymorphism has been demonstrated in individuals occupationally exposed to benzene (Rothman et al., 1997). However, increased risks of a variety of de novo and therapy-induced leukemias also have been associated with the NQO1*2 polymorphism (Larson et al., 1999; Wiemels et al., 1999; Naoe et al., 2000; Krajinovic et al., 2002; Smith et al., 2002; Ross and Siegel, 2004), and unchallenged NQO1 knockout mice demonstrate myeloid hyperplasia (Long et al., 2002). The mechanisms whereby a lack of NQO1 due to the NQO1*2 polymorphism predisposes to both benzene-induced myeloid toxicity and a variety of leukemias not associated with quinone exposure remain unclear. However, NQO1 has multiple functions and is part of a coordinated response to stress (Ross and Siegel, 2004), stabilizes proteins such as p53 against proteasomal degradation (Asher et al., 2002), and can function in an antioxidant role (Siegel et al., 2004).
Bone marrow stroma and particularly endothelial cells are intimately associated with developing blood cells, and up-regulation of cell adhesion molecules in response to cytokine induction is an important endothelial cell function (Mantovani et al., 1992). Adhesion molecules expressed by endothelial cells are important regulators of hematopoiesis and contribute to stem cell/progenitor cell homing and mobilization (Schweitzer et al., 1997; Wright et al., 2001; Avecilla et al., 2004; Kopp et al., 2005). Altered hematopoiesis was reported in mice deficient in both P- and E-selectin (Frenette et al., 1996). The administration of antibodies against VCAM-1 ligand VLA-4 or CD44 [a major E-selectin ligand on hematopoietic progenitor cells (HPCs); Dimitroff et al., 2001] in mice led to a significant increase in circulating stem cells (Vermeulen et al., 1998). In preliminary microarray studies, we demonstrated that inhibition of NQO1 led to decreased VCAM-1 expression (Zhou et al., 2007). Because cytokines and adhesion molecules play key roles in regulating hematopoiesis, we hypothesized that NQO1 may influence cytokine-stimulated hematopoietic cell adhesion to bone marrow endothelial cells via modulation of adhesion molecule expression.
In this study, we have used transformed human bone marrow endothelial cells (TrHBMECs) as a model system to investigate the effect of a lack of NQO1 on adhesion molecule expression and adhesion of CD34+ cells. TrHBMEC is a bone marrow sinusoidal endothelial cell line that has been used in studies of the adhesion and homing of HPCs (Schweitzer et al., 1997). TNFα stimulates adhesion molecule production, and we have characterized adhesion molecule responses to TNFα induction as a result of mechanism-based inhibition of NQO1 activity by ES936 (Winski et al., 2001) or genetic knockdown of NQO1 in TrHBMECs. We have focused on E-selectin, critical for HPC rolling and adhering on TrHBMECs (Schweitzer et al., 1997), and on both VCAM-1 and ICAM-1, which contribute to firm adhesion of HPCs to TrHBMECs (Schweitzer et al., 1997). We have also examined the functional consequences of modulation of NQO1 activity on adhesion of CD34+ cells to TrHBMECs and explored potential mechanisms by which NQO1 deficiency might down-regulate adhesion molecule expression in response to TNFα induction.
Materials and Methods
Materials.
NADH, 2,6-dichlorophenol-indophenol, dicumarol, and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide were obtained from Sigma-Aldrich (St. Louis, MO). The mechanism-based NQO1 inhibitor ES936 was synthesized by Prof. C. J. Moody (School of Chemistry, University of Nottingham, Nottingham, UK) based on published procedures (Naylor et al., 1998). RNeasy kit and QuantiTect SYBR Green PCR kits were obtained from QIAGEN (Valencia, CA). NE-PER nuclear and cytoplasmic extraction reagents were purchased from Pierce Chemical (Rockford, IL). Transfection reagent Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA). siRNA targeting human NQO1 and scrambled siRNA control were both purchased from Dharmacon RNA Technologies (Lafayette, CO). Mouse anti-E-selectin primary antibodies good for nonreducing conditions were obtained from Abcam Inc. (Cambridge, MA). Mouse anti-VCAM-1 and ICAM-1 monoclonal antibodies and rabbit anti-IκBα, p65, and phospho-p65 (Ser529) polyclonal antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Mouse anti-phospho-IκBα (Ser32/36) monoclonal antibody and rabbit anti-PARP, phospho-c-Jun (Ser63), and phospho-ATF2 (Thr71) antibodies were purchased from Cell Signaling Technology (Danvers, MA). Mouse anti-NQO1 monoclonal antibody (clone A180) was generated in our laboratory. Mouse anti-β-actin antibody was obtained from Sigma-Aldrich. TNFα was purchased from R&D Systems (Minneapolis, MN). Parallel plate flow chamber was obtained from Glycotech Corporation (Gaithersburg, MD).
Cell Lines.
TrHBMECs were a generous gift from Dr. Babette B. Weksler (Weill Medical College, Cornell University, Ithaca, NY). Cells were cultured as described previously (Zhou et al., 2009) on 0.2% (w/v) collagen-coated cell culture plates in Dulbecco's modified Eagle's medium (low glucose) supplemented with 5% (v/v) fetal bovine serum, penicillin (100 units/ml), streptomycin (100 units/ml), 3 mM l-glutamine, 10 mM HEPES, and 1% (v/v) BME vitamins (Sigma-Aldrich). Cells were maintained at 37°C in 5% CO2. Cells with passage number ranging from 19 to 25 were used in this study. KG1a hematopoietic cell line was obtained from American Type Culture Collection (Manassas, VA). Cells were propagated in RPMI 1640 medium with 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C in 5% CO2. KG1a cells with passage number ranging from 3 to 7 were used in the cell-cell adhesion study.
NQO1 Activity Assay.
Cell culture medium was aspirated, and cells were washed twice with phosphate-buffered saline and scraped into 25 mM Tris-HCl, pH 7.4, containing 250 mM sucrose and 5 μM FAD, followed by probe sonication on ice. Sonicates were then centrifuged at 13,000 rpm for 10 min at 4°C, and the supernatant was assayed for NQO1 activity and protein concentration. NQO1 activity assays were performed as described by Ernster (1967) and modified by Benson et al. (1980) using 2,6-dichlorophenol-indophenol as a substrate. Reactions (1 ml) contained 25 mM Tris-HCl, pH 7.4, 0.7 mg/ml bovine serum albumin, 0.2 mM NADH, and 40 μM 2,6-dichlorophenol-indophenol. Reactions were started by the addition of a small volume (2–5 μl) of cell supernatant. Reactions were performed in the absence and presence of 20 μM dicumarol. NQO1 activity is defined as the dicumarol-inhibitable reduction of 2,6-dichlorophenol-indophenol measured at 600 nm at 25°C.
siRNA Transfection.
TrHBMECs were transfected with siRNA targeting human NQO1 or scrambled siRNA control (Dharmacon RNA Technologies) using Lipofectamine 2000 (Invitrogen) transfection reagent. The procedure was performed as suggested in the manufacturer's protocol. Knockdowns were extremely efficient as indicated by immunoblotting (see appropriate figures), and no knockdown of the closely related protein NQO2 was detected in TrHBMECs.
Quantitative Real-Time RT-PCR of the E-selectin Gene.
The relative amount of E-selectin mRNA was assessed using Quantitect SYBR Green Real-Time PCR kit and primer set QT00015358 (QIAGEN) with an ABI 7500 system (Applied Biosystems, Foster City, CA). β-2-Macroglobulin was used as the internal control. DMSO was used as vehicle control for ES936 treatment. All of the reactions were run in triplicate. The fold increase of E-selectin mRNA in TrHBMECs after ES936 and TNFα treatment, normalized to an endogenous β-2-macroglobulin reference and relative to DMSO and TNFα control, is given by 2−ΔΔΔCT, where ΔΔΔCT = ΔΔCTES936 + TNFα − ΔΔCTDMSO + TNFα, ΔΔCT = ΔCTTNFα − ΔCTNo TNFα control, and ΔCT = threshold cycles for target E-selectin − threshold cycles for reference β-2-macroglobulin.
Immunoblot Analysis.
Nuclear extracts preparation was performed as suggested in the manufacturer's protocol (Pierce Chemical). For the whole-cell lysate preparation, TrHBMECs were scraped into complete medium, and cells were collected by centrifugation, washed in phosphate-buffered saline, and repelleted by centrifugation. Cell pellets were resuspended in radioimmunoprecipitation assay buffer with protease inhibitors added and sonicated on ice for 15 s. Sonicates were centrifuged at 13,000 rpm for 5 min to remove cellular debris. After centrifugation, the supernatant was assayed for protein concentrations using the method of Lowry (Lowry et al., 1951). Protein samples were diluted in 5× Laemmli sample buffer, heated to 90°C for 5 min, and then separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes in 25 mM Tris, 192 mM glycine containing 20% (v/v) methanol at 110 V for 1 h. After transfer, membranes were placed overnight in blocking buffer [10 mM Tris-HCl, pH 8, containing 125 mM NaCl, 0.2% (v/v) Tween 20, and 5% (w/v) nonfat dry milk or 5% (w/v) bovine serum albumin]. Antibody dilutions were based on manufacturer's recommendations. For all analyses, horseradish peroxidase-conjugated goat anti-mouse or anti-rabbit IgG were diluted at 1:5000 in blocking buffer for 0.5 to 1 h at room temperature. Protein bands were visualized using luminol-based enhanced chemiluminescence as described by the manufacturer (PerkinElmer Life and Analytical Sciences, Boston, MA). For nonreducing immunoblotting, dithiothreitol and/or β-mercaptoethanol were removed from the sample buffer; primary antibodies were used at the dilutions of 1:1000 for anti-E-selectin. Densitometry analysis software used was Photoshop 7.0 (Adobe Systems, Mountain View, CA), and experimental data were normalized to loading controls.
Flow Chamber Adhesion Assay.
Under defined shear stress, adhesion was measured under flow conditions in a parallel plate flow perfusion chamber. The assay was performed as described previously (Schweitzer et al., 1997), with minor modifications. In brief, TrHBMECs were pretreated with siRNA for 72 h or ES936 for 2 h to abrogate NQO1 protein or activity, and the near confluent gelatin-coated 35-mm plates were used in the circular flow chamber system (Glycotech Corporation) at 37°C. Before adhesion experiments, TrHBMECs were stimulated with TNFα (10 ng/ml) for 4 h to induce adhesion molecule expression. CD34+ KG1a cells were used in a concentration of 0.5 million/ml resuspended in HEPES buffer (20 mM HEPES, 132 mM NaCl, 6 mM KCl, 1.2 mM KH2PO4, 1 mM MgSO4, 5 mM glucose, and 1 mM CaCl2, pH 7.4). KG1a cells were perfused through the system for 5 min at a shear stress of 1.0 dyne/cm2. After cell perfusion, a relatively high-shear stress of 6.0 dyne/cm2 1-min wash was applied using HEPES buffer to detach rolling or very weakly adhering cells. Cells remaining adhered after this wash were considered firmly adhered. To quantify the firm adhesion results, typically at least 10 randomized high-power fields (total surface ≥1 mm2) in each plate were chosen to count the firmly adhered KG1a cells. Chamber corner areas were avoided, and only the portions of the plates that were covered with greater than 95% endothelial cells were evaluated. For each treatment at least three to five independent experiments were performed to evaluate cell-cell adhesion.
Statistical Analysis.
Values are presented as the mean ± S.D. Statistical significance was evaluated using the Student's t test for comparisons between two mean values. A value of p < 0.05 was considered significant. All experiments were performed at least three times.
Results
Inhibition of NQO1 Activity by ES936 in TrHBMECs.
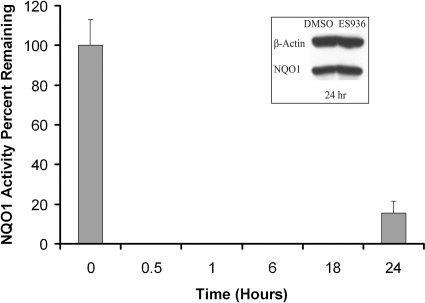
To determine concentrations of ES936 that were not overtly cytotoxic in TrHBMECs, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide growth inhibition assays were performed. The IC50 value for ES936 in TrHBMECs was found to be greater than 1 μM, and initial dose-response studies determined that a concentration of ES936 of 100 nM would be an optimal dose for NQO1 inhibition without adverse effects on cell viability (data not shown). Time course studies (Fig. 1) were then performed to test the efficiency of inhibition of NQO1 activity in TrHBMECs. NQO1 catalytic activity was totally inhibited in TrHBMECs by 100 nM ES936 within 30 min and remained inhibited for up to 18 h after a single dose (Fig. 1). Immunoblot analysis for NQO1 indicated that ES936 treatment for 24 h did not inhibit NQO1 protein expression in TrHBMECs (Fig. 1, inset). Loss of NQO1 activity was due to mechanism-based inhibition of enzyme activity by ES936 and not to down-regulation of NQO1 protein expression.
Fig. 1.
Inhibition of NQO1 activity by ES936 in TrHBMECs. NQO1 catalytic activity was measured in TrHBMECs after a single treatment with ES936 (100 nM) for the indicated times. Results were calculated as the percentage of NQO1 activity remaining compared with DMSO-treated controls. Results are expressed as the mean ± S.D. of three independent experiments. Inset, NQO1 protein levels after 24-h treatment was measured by immunoblot analysis; no decrease of NQO1 expression was observed.
ES936 Treatment Inhibited TNFα-Induced E-selectin mRNA Expression in TrHBMECs.
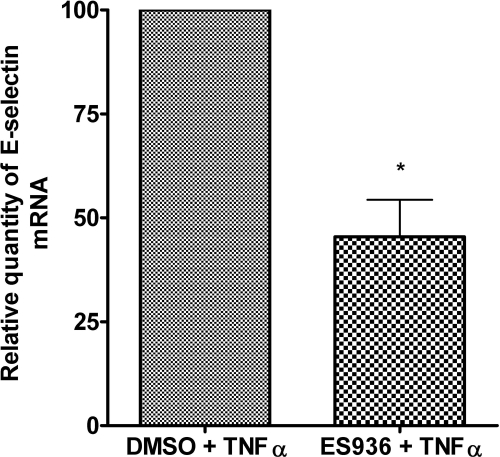
Treatment with TNFα for 4 h up-regulated E-selectin protein to its peak in TrHBMECs and significantly enhanced CD34+ HPC rolling and adhering on TrHBMECs (Schweitzer et al., 1997). To examine ES936 modulation of TNFα-stimulated E-selectin mRNA level, TrHBMECs were pretreated with ES936 or DMSO for 2 h and then stimulated with TNFα for an additional 2 h. As shown in Fig. 2, quantitative real time RT-PCR results demonstrated that TNFα induced up-regulation of E-selectin mRNA was inhibited by approximately 60% by pretreatment with ES936.
Fig. 2.
Pretreatment of TrHBMECs with ES936 inhibited TNFα-stimulated E-selectin mRNA expression. TrHBMECs were treated with ES936 (100 nM) for 2 h and stimulated with TNFα (10 ng/ml) for an additional 2 h. E-selectin mRNA was analyzed using real-time RT-PCR and normalized to β-2-macroglobulin internal control. DMSO was used as vehicle control for ES936 treatment. The percentage of decrease of E-selectin mRNA in TrHBMECs after ES936 and TNFα treatment, normalized to an endogenous β-2-macroglobulin reference and relative to DMSO and TNFα treatment, is given by 2−ΔΔΔCT. Results are expressed as the mean ± S.D. of three independent experiments. n = 3; *, p < 0.05.
ES936 Treatment Inhibited TNFα-Induced E-selectin Protein Up-Regulation in TrHBMECs.
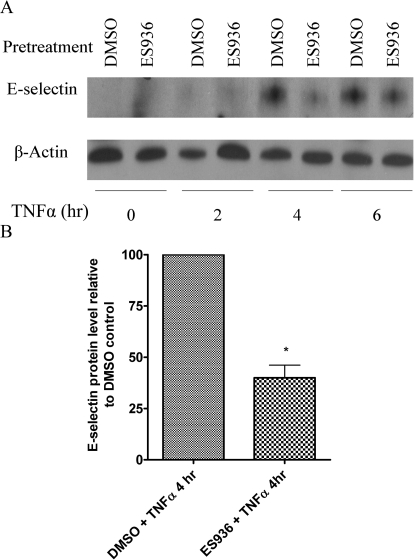
We performed immunoblot assays to determine E-selectin protein levels in TrHBMECs after TNFα stimulation. As shown in Fig. 3, after 4 h of TNFα induction, E-selectin protein expression reaches a peak. However, ES936 pretreatment resulted in a marked decrease in TNFα-stimulated E-selectin protein levels after 4 h (Fig. 3, A and B), and protein levels were still decreased at 6 h.
Fig. 3.
Pretreatment of TrHBMECs with ES936 inhibited TNFα-induced E-selectin protein expression. A, TrHBMECs were pretreated with ES936 (100 nM) for 2 h and then exposed to TNFα (10 ng/ml) for the indicated times. E-selectin protein levels were examined in TrHBMEC sonicates (15 μg) by nonreducing immunoblot analysis. β-Actin was included as a loading control. The blot is representative of at least three independent experiments. B, semiquantification (as described under Materials and Methods) of relative E-selectin protein levels after 4 h (peak protein level) of TNFα induction. n = 3; *, p < 0.05.
ES936 Treatment Inhibited TNFα-Induced VCAM-1 and ICAM-1 Protein Up-Regulation in TrHBMECs.
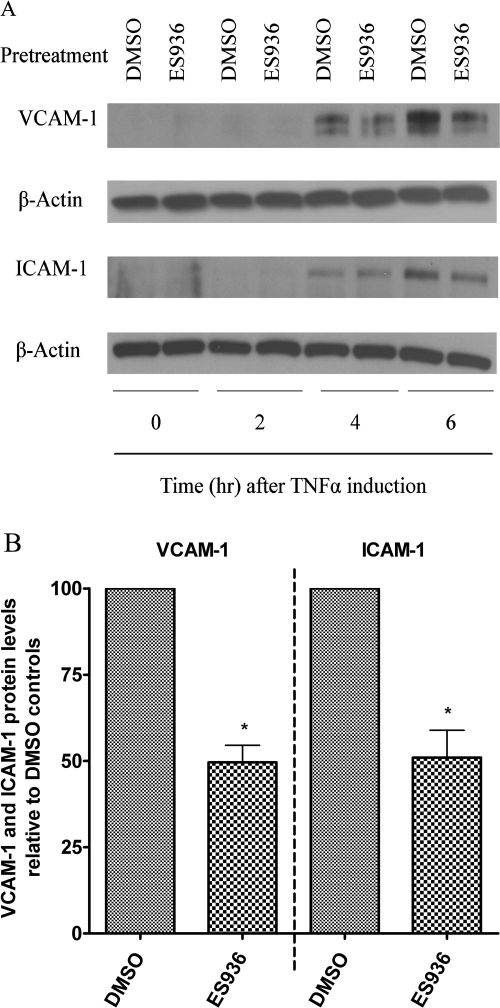
We extended our studies to test the effect of inhibition of NQO1 activity on TNFα-induced VCAM-1 and ICAM-1 expression. VCAM-1 and ICAM-1 expression reaches a peak 6 h after TNFα induction (Schweitzer et al., 1997; Fig. 4A). Immunoblot analysis of VCAM-1 and ICAM-1 protein levels demonstrated a decrease of VCAM-1 and ICAM-1 proteins in ES936-pretreated samples, particularly 6 h after TNFα stimulation (Fig. 4, A and B).
Fig. 4.
Pretreatment of TrHBMECs with ES936 inhibited TNFα-induced VCAM-1 and ICAM-1 protein expression. A, TrHBMECs were pretreated with ES936 (100 nM) or DMSO for 2 h and then exposed to TNFα (10 ng/ml) for the indicated times. VCAM-1 and ICAM-1 protein levels were examined in TrHBMEC extracts (15 μg) by immunoblot analysis. β-Actin was included as a loading control. The blot is representative of at least three independent experiments. B, semiquantification (as described under Materials and Methods) of relative VCAM-1 and ICAM-1 protein levels after 6 h (peak protein levels) of TNFα induction. n = 3; *, p < 0.05.
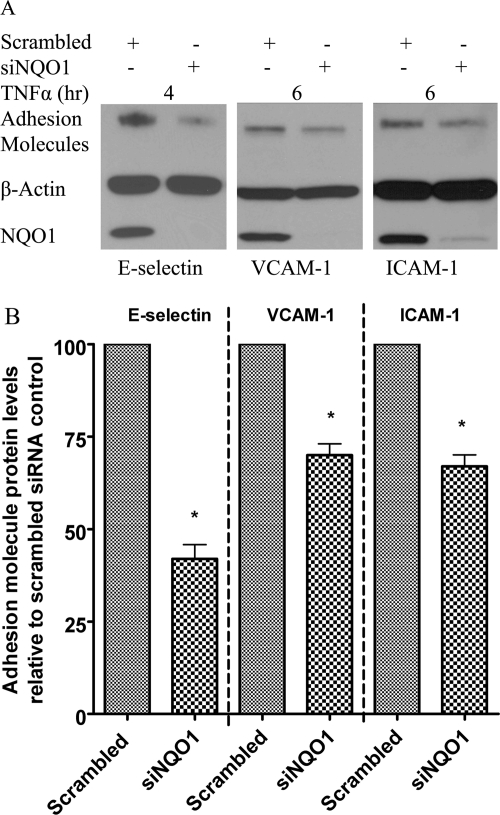
Knockdown of NQO1 Protein in TrHBMECs by Use of siRNA Also Inhibits TNFα-Induced Up-Regulation of E-selectin, VCAM-1, and ICAM-1.
To further confirm the regulation of adhesion molecule expression by NQO1, anti-NQO1 siRNA was used to knockdown NQO1 protein. Seventy-two hours after siRNA transfection, TNFα was then added to induce adhesion molecule expression. Immunoblot assays demonstrated the efficient knockdown of NQO1 and marked reduction of E-selectin, VCAM-1, and ICAM-1 protein levels after TNFα stimulation as a result of NQO1 knockdown (Fig. 5, A and B).
Fig. 5.
Pretreatment of TrHBMEC with siNQO1 down-regulated TNFα induced E-selectin, VCAM-1, and ICAM-1 proteins. A, TrHBMECs were transfected with siRNAs (either siRNA targeting NQO1 or scrambled siRNA control) for 72 h and then exposed to TNFα (10 ng/ml) for the indicated times. E-selectin, VCAM-1, and ICAM-1 protein levels were examined in TrHBMEC extracts (15 μg) by immunoblot analysis. β-Actin was included as a loading control. The blot is representative of at least three independent experiments. B, semiquantification (as described under Materials and Methods) of relative E-selectin, VCAM-1, and ICAM-1 protein levels after 4 h (peak E-selectin level) or 6 h (peak VCAM-1 and ICAM-1 protein levels) of TNFα induction. n = 3; *, p < 0.05.
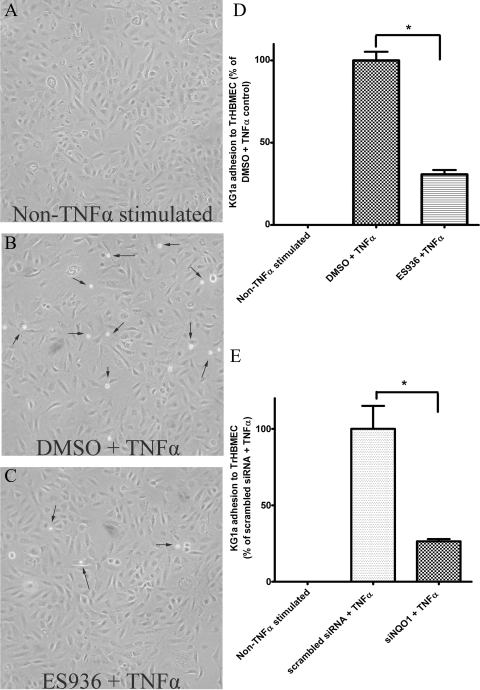
Inhibition or Knockdown of NQO1 Resulted in Impaired Adhesion of KG1a CD34+ Hematopoietic Cells to TrHBMECs after TNFα Stimulation.
To examine a functional endpoint of impaired adhesion molecule expression, we explored whether adhesion of CD34+ KG1a cells would be impaired in NQO1-compromised TrHBMECs. Treatment of TrHBMECs with TNFα alone markedly increased KG1a cell adhesion to TrHBMEC as shown in Fig. 6, A and B (smaller adhered KG1a cells indicated by arrows). However, pretreatment of TrHBMECs with ES936 followed by TNFα stimulation resulted in markedly decreased adhesion of CD34+ KG1a cells to TrHBMECs (Fig. 6, B and C). Adherent cells were counted as described under Materials and Methods. Statistical analysis (Fig. 6D) demonstrated a significant decrease in KG1a CD34+ cell adhesion to TrHBMECs as a result of inhibition of NQO1. We then extended these experiments to knockdown of NQO1 in TrHBMECs, and pretreatment with siRNA against NQO1 significantly reduced TNFα-stimulated adhesion of KG1a cells to TrHBMECs (Fig. 6E). Both inhibition and knockdown of NQO1 in TrHBMECs led to a reduction of TNFα-stimulated adhesion of approximately 60 to 70% (Fig. 6, A–E).
Fig. 6.
Inhibition or knockdown of NQO1 in TrHBMECs inhibited KG1a cell adhesion to TrHBMECs. KG1a (CD34+ hematopoietic) cells were perfused over TrHBMECs in a parallel plate flow chamber as described under Materials and Methods. Stimulation with TNFα (10 ng/ml for 4 h) significantly increased KG1a cell adherence to TrHBMECs (A, B, and D), whereas inhibition of NQO1 by ES936 dramatically decreased KG1a cell adhesion to TrHBMECs (B–D) in the presence of TNFα (10 ng/ml for 4 h) stimulation. Knockdown of NQO1 by siRNA also dramatically decreased TNFα (10 ng/ml, 4 h) induced KG1a cell adhesion to TrHBMECs (E). Results shown were determined from at least three independent experiments (n = 5 for ES936 pretreatment). *, p < 0.05. Arrows delineate smaller KG1a cells that have adhered to TrHBMECs.
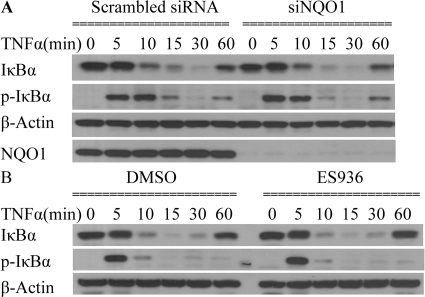
Inhibition or Knockdown of NQO1 Did Not Inhibit TNFα-Induced IκBα Phosphorylation and Degradation in TrHBMECs.
An important regulatory pathway of TNFα-induced adhesion molecule expression is the NF-κB signaling pathway (Collins et al., 1995). Classical NF-κB activation relies on phosphorylation followed by degradation of its bound inhibitors belonging to the IκB family and translocation of free NF-κB from cytosol to the nucleus. IκBα is a well characterized inhibitor of NF-κB, and its up-regulation can significantly inhibit TNFα-induced gene expression (Kordula et al., 2000; Son et al., 2004). We therefore examined total IκBα and phosphorylated IκBα levels after TNFα stimulation in TrHBMECs. However, little difference in the kinetics of either phosphorylation of IκBα or degradation/formation of IκBα could be observed in NQO1-compromised cells (Fig. 7, A and B). Levels of IκBα and its phosphorylated form were similar in anti-NQO1 siRNA-treated cells relative to scrambled siRNA-treated controls and in ES936-treated cells relative to vehicle controls (DMSO) (Fig. 7, A and B).
Fig. 7.
Inhibition or knockdown of NQO1 did not inhibit TNFα-induced IκBα phosphorylation and degradation in TrHBMECs. A, TrHBMECs were transfected with siRNAs (either siRNA targeting NQO1 or scrambled siRNA control) for 72 h and then exposed to TNFα (10 ng/ml) for the indicated times. IκBα, phospho-IκBα, and NQO1 protein levels were examined in TrHBMEC extracts by immunoblot analysis. β-Actin was included as a loading control. The blot is representative of at least three independent experiments. B, TrHBMECs were pretreated with ES936 for 2 h and then exposed to TNFα (10 ng/ml) for the indicated times; IκBα and phospho-IκBα protein levels were examined in TrHBMEC extracts by immunoblot analysis. β-Actin was included as a loading control. The blot is representative of at least three independent experiments.
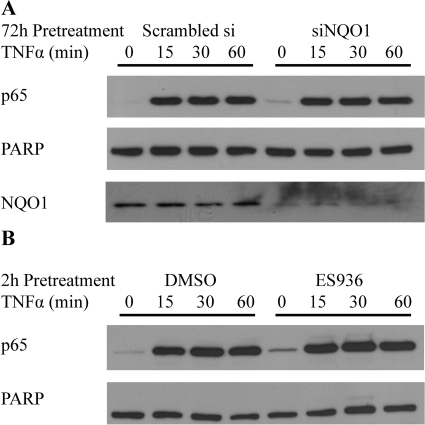
Inhibition or Knockdown of NQO1 Did Not Block TNFα-Induced Nuclear Translocation of NF-κB Subunit p65 in TrHBMECs.
The induction of IκBα phosphorylation and degradation indicated that NQO1 deficiency did not alter the upstream events of the NF-κB signaling pathway. We then examined total nuclear p65 levels in TrHBMECs to determine whether NQO1 deficiency had any effects on p65 translocation. As shown in Fig. 8, TNFα significantly induced p65 nuclear translocation; however, little difference could be observed in NQO1-compromised cells. Levels of nuclear p65 after TNFα induction were similar in anti-NQO1 siRNA-treated cells relative to scrambled siRNA treated controls and in ES936-treated cells relative to vehicle controls (DMSO) (Fig. 8, A and B).
Fig. 8.
Inhibition or knockdown of NQO1 did not block TNFα-induced nuclear translocation of NF-κB subunit p65 in TrHBMECs. A, TrHBMECs were transfected with siRNAs (either siRNA targeting NQO1 or scrambled siRNA control) for 72 h and then exposed to TNFα (10 ng/ml) for the indicated times. Nuclear extracts were prepared, and p65 and NQO1 protein levels were examined by immunoblot analysis. PARP was included as a loading control. The blot is representative of at least three independent experiments. B, TrHBMECs were pretreated with ES936 for 2 h and then exposed to TNFα (10 ng/ml) for the indicated times. Nuclear extracts were prepared and p65 protein level was examined by immunoblot analysis. PARP was included as a loading control. The blot is representative of at least three independent experiments.
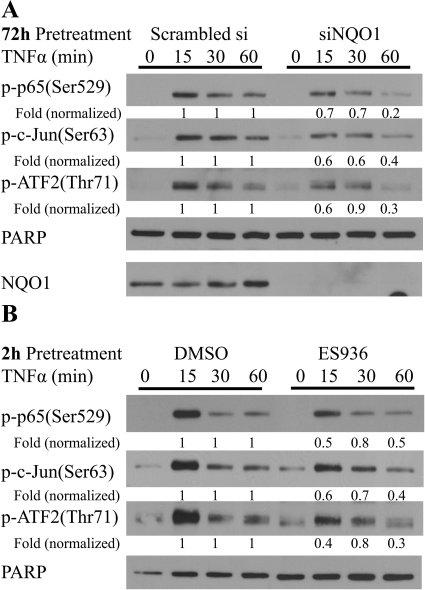
Inhibition or Knockdown of NQO1 Resulted in Lower Phospho-p65, c-Jun, and ATF2 Levels in TrHBMEC Nucleus in Response to TNFα Induction.
Although NQO1 did not inhibit TNFα-induced nuclear translocation of NF-κB subunit p65, NQO1 deficiency could alter nuclear NF-κB activity by altering events such as p65 phosphorylation, degradation, and DNA binding. We therefore examined phospho-p65 levels in TrHBMECs (Fig. 9, A and B). Inhibition or knockdown of NQO1 led to decreased amounts of phospho-p65 in TrHBMEC nuclear fractions (as determined by the same time point pairwise comparison between the control and treatment groups normalized to the PARP loading control). Because TNFα also regulates E-selectin expression via parallel c-Jun and ATF2 pathways and because phosphorylation of both c-Jun and ATF2 contribute to TNFα-induced E-selectin promoter activity in human endothelial cells (Min and Pober, 1997; Read et al., 1997), we also examined phospho-c-Jun and ATF2 levels. NQO1-deficient TrHBMECs exhibit lower amounts of both nuclear phospho-c-Jun and ATF2 proteins in response to TNFα stimulation (Fig. 9, A and B). These findings may provide a potential mechanism by which NQO1 modulates adhesion molecule expression.
Fig. 9.
Inhibition or knockdown of NQO1 resulted in lower phospho-p65, c-Jun, and ATF2 levels in TrHBMEC nucleus in response to TNFα induction. A, TrHBMECs were transfected with siRNA (either siRNA targeting NQO1 or scrambled siRNA control) for 72 h and then exposed to TNFα (10 ng/ml) for the indicated times. Nuclear extracts were prepared, and phospho-p65, c-Jun, ATF2, and NQO1 protein levels were examined by immunoblot analysis. PARP was included as a loading control. The blot is representative of at least three independent experiments. Fold change is a same time point pairwise comparison between the control and siNQO1 treatment groups normalized to the PARP loading control. B, TrHBMECs were pretreated with ES936 for 2 h and then exposed to TNFα (10 ng/ml) for the indicated times. Nuclear extracts were prepared and phospho-p65, c-Jun, and ATF2 protein levels were examined by immunoblot analysis. PARP was included as a loading control. The blot is representative of at least three independent experiments. Fold change is a same time point pairwise comparison between the control and treatment groups normalized to the PARP loading control.
Discussion
In this work, we used a transformed human bone marrow endothelial cell line to study the consequences of a lack of NQO1 activity in this important bone marrow compartment. Our data demonstrated that inhibition of NQO1 activity led to a reduction in TNFα-stimulated E-selectin, VCAM-1, and ICAM-1 protein levels in TrHBMECs. It is important to note that decreased adhesion molecule levels also led to altered functional adhesion. Inhibition or knockdown of NQO1 and decreased adhesion molecule levels led to a marked decrease in TNFα-induced CD34+ hematopoietic cell adhesion to TrHBMECs in the order of 60 to 70%.
The main hematopoietic system resides in bone marrow and is in close contact with the stromal microenvironment, which supports hematopoietic stem cell growth and differentiation. The bone marrow stroma is composed of a variety of different cell types providing structural and functional support for hematopoiesis: endothelial cells, adipocytes, smooth muscle cells, reticular cells, osteoblasts, and stromal fibroblasts. Among these cell types, endothelial cells have a particular biological relevance. Recent advances reveal the identity of two niches that play an important role in the homeostatic regulation of HSCs: the osteoblastic niche (osteoblasts and hematopoietic cells) and the vascular niche (hematopoietic cells and endothelial cells) (Caplan and Dennis, 2006; Li and Li, 2006). The vascular niche offers an alternative niche for mobilized stem cells that promotes proliferation and further differentiation or maturation and release into the circulatory system (Avecilla et al., 2004; Kopp et al., 2005). Because blood cells can easily penetrate sinusoids that are formed by a single layer of endothelial cells, residence of HSCs in the vascular niche enables robust and rapid response to hematopoietic stress and immediate release of HSCs into the bloodstream upon cytokine or chemokine exposure (Wright et al., 2001). Adhesion of HSCs to endothelial cells is an important part of these regulatory mechanisms (Frenette et al., 1996; Vermeulen et al., 1998; Li and Li, 2006).
In this study, we used both a mechanism-based inhibitor and siRNA to inhibit or knockdown NQO1 in TrHBMECs. NQO1-compromised TrHBMECs served as a model system to determine the effects of a lack of NQO1 activity on adhesion molecule expression in human bone marrow endothelial cells. Previous work in TrHBMECs using blocking antibodies has shown critical contributions of E-selectin to rolling and adhesion of CD34+ cells, whereas a combination of VCAM-1 and ICAM-1 antibodies markedly reduced firm adhesion of CD34+ cells to TrHBMECs (Schweitzer et al., 1997). We demonstrated that either mechanism-based inhibition or siRNA-mediated knockdown of NQO1 resulted in down-regulation of TNFα-induced E-selectin, VCAM-1, and ICAM-1 expression in TrHBMECs. Similar down-regulation of TNFα-induced E-selectin after inhibition of NQO1 activity also was observed in human umbilical vein endothelial cells (data not shown). Furthermore, inhibition or knockdown of NQO1 was demonstrated to inhibit TNFα-induced CD34+ hematopoietic cell adhesion to TrHBMECs. In the hematopoietic system, many cell-surface and adhesion molecules, including E-selectin and VCAM-1, are implicated in the cross-talk between HSCs and their niches, resulting in modulation of HSC mobilization, recruitment to the vascular niche, or homing to the bone marrow (Frenette et al., 1996; Vermeulen et al., 1998; Avecilla et al., 2004; Kopp et al., 2005; Li and Li, 2006). Consequently, the altered expression of E-selectin, VCAM-1, and ICAM-1 after inhibition of NQO1 catalytic activity or NQO1 knockdown and the subsequent functional deficit in adhesion of CD34+ cells to human bone marrow endothelial cells may affect proliferation and differentiation of HSCs. It is also conceivable that this impaired functioning of the vascular stem cell niche may provide a unifying hypothesis for the increased incidence of leukemias and the more pronounced toxic effects of benzene in individuals lacking NQO1 due to the presence of the homozygous NQO1*2 polymorphism. Polymorphisms in both TNFα and VCAM-1 also have been associated with increased susceptibility to benzene poisoning in occupationally exposed individuals (Lan et al., 2005; Lv et al., 2007).
Mechanisms whereby inhibition or knockdown of NQO1 might result in decreased adhesion molecule expression remain undefined, but some clues are provided by our own and previous work (Ahn et al., 2006). The NF-κB and JNK signaling pathways have been shown to be critical in TNFα-induced up-regulation of adhesion molecules (Collins et al., 1995; Min and Pober, 1997; Read et al., 1997). It is important to note that NQO1 deficiency also was reported to be able to regulate the TNFα signaling pathway in mouse keratinocytes and HCT116 cells by inhibiting IκBα phosphorylation and JNK activation (Ahn et al., 2006). Our data also suggest a role for NQO1 in the NF-κB and JNK signaling pathways, but it is interesting that we did not find altered IκBα phosphorylation and p65 translocation in TrHBMECs as demonstrated previously in keratinocytes and in colon cancer cells (Ahn et al., 2006). However, our data demonstrate decreased nuclear levels of phospho-p65, c-Jun, and ATF2 in TrHBMECs after inhibition or knockdown of NQO1 (Fig. 9, A and B). There is not an absolute inhibition of TNFα-stimulated transcription factor levels in the nucleus, but this is in agreement with the degree of reduction in adhesion molecule expression observed after NQO1 inhibition or knockdown (Figs. 3–5). Whether the observed reduction in nuclear levels of phospho-p65, c-Jun, and ATF2 resulted from decreased phosphorylation, enhanced degradation, or accelerated shuttling back to cytoplasm needs further investigation. Although the relative importance of each of these regulators remains unclear, the collective changes in the nuclear levels of such key transcription factors may provide a potential mechanism whereby knockdown or inhibition of NQO1 could influence adhesion molecule expression. It is interesting to note that the benzene metabolite hydroquinone also has been shown to inhibit NF-κB activation in human bone marrow progenitor cells (Kerzic et al., 2003).
In summary, our data have demonstrated that inhibition or knockdown of NQO1 abrogated TNFα-induced E-selectin, VCAM-1, and ICAM-1 expression in TrHBMECs, leading to reduced CD34+ hematopoietic cell adhesion. Given the documented roles of these adhesion molecules in HSC development and differentiation, it is possible that such alterations may play a role in hematopoietic pathology and the increased susceptibility of polymorphic individuals lacking NQO1 to hematotoxicants such as benzene.
This work was supported by National Institutes of Health National Institute of Environmental Health Sciences [Grant ES09554].
Parts of this work were presented previously: Ross D and Zhou H (2009) Benzene: Health Effects and Mechanisms of Bone Marrow Toxicity. Implications for t-AML and the Mode of Action Framework; 2009 Sept 7–11; Technical University of Munich, Munich, Germany.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.167841.
- NQO1
- NAD(P)H:quinone oxidoreductase 1
- E-selectin
- endothelial leukocyte adhesion molecule 1
- HPC
- hematopoietic progenitor cell
- TrHBMEC
- transformed human bone marrow endothelial cell
- TNF
- tumor necrosis factor
- ES936
- 5-methoxy-1,2-dimethyl-3-[(4-nitrophenoxy)methyl]indole-4,7-dione
- VCAM
- vascular cell adhesion molecule
- ICAM
- intercellular cell adhesion molecule
- siRNA
- small interfering RNA
- IκB
- inhibitor of nuclear factor-κB
- PARP
- poly(ADP-ribose) polymerase
- RT-PCR
- reverse transcription-polymerase chain reaction
- DMSO
- dimethyl sulfoxide
- NF-κB
- nuclear factor-κB
- ATF
- activating transcription factor
- HSC
- hematopoietic stem cell
- JNK
- c-Jun NH2-terminal kinase.
References
- Ahn KS, Sethi G, Jain AK, Jaiswal AK, Aggarwal BB. (2006) Genetic deletion of NAD(P)H:quinone oxidoreductase 1 abrogates activation of nuclear factor-kappaB, IkappaBalpha kinase, c-Jun N-terminal kinase, Akt, p38, and p44/42 mitogen-activated protein kinases and potentiates apoptosis. J Biol Chem 281:19798–19808 [DOI] [PubMed] [Google Scholar]
- Asher G, Lotem J, Kama R, Sachs L, Shaul Y. (2002) NQO1 stabilizes p53 through a distinct pathway. Proc Natl Acad Sci USA 99:3099–3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, et al. (2004) Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med 10:64–71 [DOI] [PubMed] [Google Scholar]
- Bauer AK, Faiola B, Abernethy DJ, Marchan R, Pluta LJ, Wong VA, Roberts K, Jaiswal AK, Gonzalez FJ, Butterworth BE, et al. (2003) Genetic susceptibility to benzene-induced toxicity: role of NADPH: quinone oxidoreductase-1. Cancer Res 63:929–935 [PubMed] [Google Scholar]
- Benson AM, Hunkeler MJ, Talalay P. (1980) Increase of NAD(P)H:quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc Natl Acad Sci USA 77:5216–5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchet MA, Faig M, Amzel LM. (2004) Structure and mechanism of NAD[P]H:quinone acceptor oxidoreductases (NQO). Methods Enzymol 382:144–174 [DOI] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. (2006) Mesenchymal stem cells as trophic mediators. J Cell Biochem 98:1076–1084 [DOI] [PubMed] [Google Scholar]
- Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. (1995) Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J 9:899–909 [PubMed] [Google Scholar]
- Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. (2001) CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol 153:1277–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernster L. (1967) DT-diaphorase. Methods Enzymol 10:309–317 [DOI] [PubMed] [Google Scholar]
- Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD. (1996) Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell 84:563–574 [DOI] [PubMed] [Google Scholar]
- Kerzic PJ, Pyatt DW, Zheng JH, Gross SA, Le A, Irons RD. (2003) Inhibition of NF-kappaB by hydroquinone sensitizes human bone marrow progenitor cells to TNF-alpha-induced apoptosis. Toxicology 187:127–137 [DOI] [PubMed] [Google Scholar]
- Kopp HG, Avecilla ST, Hooper AT, Rafii S. (2005) The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 20:349–356 [DOI] [PubMed] [Google Scholar]
- Kordula T, Bugno M, Rydel RE, Travis J. (2000) Mechanism of interleukin-1- and tumor necrosis factor alpha-dependent regulation of the alpha 1-antichymotrypsin gene in human astrocytes. J Neurosci 20:7510–7516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajinovic M, Sinnett H, Richer C, Labuda D, Sinnett D. (2002) Role of NQO1, MPO and CYP2E1 genetic polymorphisms in the susceptibility to childhood acute lymphoblastic leukemia. Int J Cancer 97:230–236 [DOI] [PubMed] [Google Scholar]
- Lan Q, Zhang L, Shen M, Smith MT, Li G, Vermeulen R, Rappaport SM, Forrest MS, Hayes RB, Linet M, et al. (2005) Polymorphisms in cytokine and cellular adhesion molecule genes and susceptibility to hematotoxicity among workers exposed to benzene. Cancer Res 65:9574–9581 [DOI] [PubMed] [Google Scholar]
- Larson RA, Wang Y, Banerjee M, Wiemels J, Hartford C, Le Beau MM, Smith MT. (1999) Prevalence of the inactivating 609C→T polymorphism in the NAD(P)H:quinone oxidoreductase (NQO1) gene in patients with primary and therapy-related myeloid leukemia. Blood 94:803–807 [PubMed] [Google Scholar]
- Li Z, Li L. (2006) Understanding hematopoietic stem-cell microenvironments. Trends Biochem Sci 31:589–595 [DOI] [PubMed] [Google Scholar]
- Long DJ, 2nd, Gaikwad A, Multani A, Pathak S, Montgomery CA, Gonzalez FJ, Jaiswal AK. (2002) Disruption of the NAD(P)H:quinone oxidoreductase 1 (NQO1) gene in mice causes myelogenous hyperplasia. Cancer Res 62:3030–3036 [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275 [PubMed] [Google Scholar]
- Lv L, Kerzic P, Lin G, Schnatter AR, Bao L, Yang Y, Zou H, Fu H, Ye X, Gross SA, et al. (2007) The TNF-alpha 238A polymorphism is associated with susceptibility to persistent bone marrow dysplasia following chronic exposure to benzene. Leuk Res 31:1479–1485 [DOI] [PubMed] [Google Scholar]
- Mantovani A, Bussolino F, Dejana E. (1992) Cytokine regulation of endothelial cell function. FASEB J 6:2591–2599 [DOI] [PubMed] [Google Scholar]
- Min W, Pober JS. (1997) TNF initiates E-selectin transcription in human endothelial cells through parallel TRAF-NF-kappa B and TRAF-RAC/CDC42-JNK-c-Jun/ATF2 pathways. J Immunol 159:3508–3518 [PubMed] [Google Scholar]
- Moran JL, Siegel D, Ross D. (1999) A potential mechanism underlying the increased susceptibility of individuals with a polymorphism in NAD(P)H:quinone oxidoreductase 1 (NQO1) to benzene toxicity. Proc Natl Acad Sci USA 96:8150–8155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naoe T, Takeyama K, Yokozawa T, Kiyoi H, Seto M, Uike N, Ino T, Utsunomiya A, Maruta A, Jin-nai I, et al. (2000) Analysis of genetic polymorphism in NQO1, GST-M1, GST-T1, and CYP3A4 in 469 Japanese patients with therapy-related leukemia/ myelodysplastic syndrome and de novo acute myeloid leukemia. Clin Cancer Res 6:4091–4095 [PubMed] [Google Scholar]
- Naylor MA, Swann E, Everett SA, Jaffar M, Nolan J, Robertson N, Lockyer SD, Patel KB, Dennis MF, Stratford MR, et al. (1998) Indolequinone antitumor agents: reductive activation and elimination from (5-methoxy-1-methyl-4,7-dioxoindol-3-yl)methyl derivatives and hypoxia-selective cytotoxicity in vitro. J Med Chem 41:2720–2731 [DOI] [PubMed] [Google Scholar]
- Read MA, Whitley MZ, Gupta S, Pierce JW, Best J, Davis RJ, Collins T. (1997) Tumor necrosis factor alpha-induced E-selectin expression is activated by the nuclear factor-kappaB and c-JUN N-terminal kinase/p38 mitogen-activated protein kinase pathways. J Biol Chem 272:2753–2761 [DOI] [PubMed] [Google Scholar]
- Ross D. (2000) The role of metabolism and specific metabolites in benzene-induced toxicity: evidence and issues. J Toxicol Environ Health A 61:357–372 [DOI] [PubMed] [Google Scholar]
- Ross D, Siegel D. (2004) NAD(P)H:quinone oxidoreductase 1 (NQO1, DT-diaphorase), functions and pharmacogenetics. Methods Enzymol 382:115–144 [DOI] [PubMed] [Google Scholar]
- Rothman N, Smith MT, Hayes RB, Traver RD, Hoener B, Campleman S, Li GL, Dosemeci M, Linet M, Zhang L, et al. (1997) Benzene poisoning, a risk factor for hematological malignancy, is associated with the NQO1 609C–>T mutation and rapid fractional excretion of chlorzoxazone. Cancer Res 57:2839–2842 [PubMed] [Google Scholar]
- Schweitzer KM, Vicart P, Delouis C, Paulin D, Dräger AM, Langenhuijsen MM, Weksler BB. (1997) Characterization of a newly established human bone marrow endothelial cell line: distinct adhesive properties for hematopoietic progenitors compared with human umbilical vein endothelial cells. Lab Invest 76:25–36 [PubMed] [Google Scholar]
- Siegel D, Gustafson DL, Dehn DL, Han JY, Boonchoong P, Berliner LJ, Ross D. (2004) NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Mol Pharmacol 65:1238–1247 [DOI] [PubMed] [Google Scholar]
- Smith MT, Wang Y, Skibola CF, Slater DJ, Lo Nigro L, Nowell PC, Lange BJ, Felix CA. (2002) Low NAD(P)H:quinone oxidoreductase activity is associated with increased risk of leukemia with MLL translocations in infants and children. Blood 100:4590–4593 [DOI] [PubMed] [Google Scholar]
- Son DS, Roby KF, Terranova PF. (2004) Tumor necrosis factor-alpha induces serum amyloid A3 in mouse granulosa cells. Endocrinology 145:2245–2252 [DOI] [PubMed] [Google Scholar]
- Traver RD, Siegel D, Beall HD, Phillips RM, Gibson NW, Franklin WA, Ross D. (1997) Characterization of a polymorphism in NAD(P)H: quinone oxidoreductase (DT-diaphorase). Br J Cancer 75:69–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis LB, Li CY, Zhang ZN, Li DG, Yin SN, Chow WH, Li GL, Dosemeci M, Blot W, Fraumeni JF, Jr, Hayes RB, Linet MS. (1994) Hematopoietic malignancies and related disorders among benzene-exposed workers in China. Leuk Lymphoma 14:91–102 [DOI] [PubMed] [Google Scholar]
- Vermeulen M, Le Pesteur F, Gagnerault MC, Mary JY, Sainteny F, Lepault F. (1998) Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood 92:894–900 [PubMed] [Google Scholar]
- Wiemels JL, Pagnamenta A, Taylor GM, Eden OB, Alexander FE, Greaves MF. (1999) A lack of a functional NAD(P)H:quinone oxidoreductase allele is selectively associated with pediatric leukemias that have MLL fusions. United Kingdom Childhood Cancer Study Investigators. Cancer Res 59:4095–4099 [PubMed] [Google Scholar]
- Winski SL, Faig M, Bianchet MA, Siegel D, Swann E, Fung K, Duncan MW, Moody CJ, Amzel LM, Ross D. (2001) Characterization of a mechanism-based inhibitor of NAD(P)H:quinone oxidoreductase 1 by biochemical, X-ray crystallographic, and mass spectrometric approaches. Biochemistry 40:15135–15142 [DOI] [PubMed] [Google Scholar]
- Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. (2001) Physiological migration of hematopoietic stem and progenitor cells. Science 294:1933–1936 [DOI] [PubMed] [Google Scholar]
- Zhou H, Dehn D, Kepa JK, Siegel D, Ross D. (2007) Proc Am Assoc Cancer Res 48:5371 [Google Scholar]
- Zhou H, Kepa JK, Siegel D, Miura S, Hiraki Y, Ross D. (2009) Benzene metabolite hydroquinone up-regulates chondromodulin-I and inhibits tube formation in human bone marrow endothelial cells. Mol Pharmacol 76:579–587 [DOI] [PMC free article] [PubMed] [Google Scholar]