Abstract
Lubiprostone activates ClC-2 chloride channels in epithelia. It is approved for treatment of chronic idiopathic constipation in adults and constipation-predominate irritable bowel syndrome in women. We tested a hypothesis that lubiprostone can reverse the constipating action of morphine and investigated the mechanism of action. Short-circuit current (Isc) was recorded in Ussing chambers as a marker for chloride secretion during pharmacological interactions between morphine and lubiprostone. Measurements of fecal wet weight were used to obtain information on morphine-lubiprostone interactions in conscious mice. Morphine decreased basal Isc, with an IC50 of 96.1 nM. The action of dimethylphenylpiperazinium (DMPP), a nicotinic receptor agonist that stimulates neurogenic Isc, was suppressed by morphine. Lubiprostone applied after pretreatment with morphine reversed morphine suppression of both basal Isc and DMPP-evoked chloride secretion. Electrical field stimulation (EFS) of submucosal neurons evoked biphasic increases in Isc. Morphine abolished the first phase and marginally suppressed the second phase. Lubiprostone reversed, in concentration-dependent manner, the action of morphine on the first and second phases of the EFS-evoked responses. Subcutaneous lubiprostone increased fecal wet weight and numbers of pellets expelled. Morphine significantly reduced fecal wet weight and number of pellets. Injection of lubiprostone, 30-min after morphine, reversed morphine-induced suppression of fecal wet weight. We conclude that inhibitory action of morphine on chloride secretion reflects suppression of excitability of cholinergic secretomotor neurons in the enteric nervous system. Lubiprostone, which does not directly affect enteric neurons, bypasses the neurogenic constipating effects of morphine by directly opening chloride channels in the mucosal epithelium.
Constipation is a side effect of pain treatment with morphine and other opioid agonists (Ruan, 2007; Droney et al., 2008). Constipation is induced by stimulating nonpropulsive motility patterns and suppressing mucosal secretion in the intestinal tract, both of which reflect suppression of neuronal excitability in the enteric nervous system (ENS) (Morita and North, 1981, 1982; Liu et al., 2001).
Suppression of intestinal transit, which prolongs the time available for absorption of electrolytes and water, was invoked previously as an explanation for the constipating effect of morphine (Daniel, 1968). This is an incomplete explanation in view of advances in understanding of ENS control of mucosal secretion and the function of secretomotor neurons in the submucosal division of the ENS (Cooke, 1987). Firing of secretomotor neurons evokes secretion of H2O, electrolytes, and mucus. Morphine acts to hyperpolarize and silence action potential discharge by secretomotor neurons (Wood and Galligan, 2004). Suppression of firing in the secretomotor neuronal pool reduces secretion and results in lowered liquidity of the small and large intestinal contents and drier, harder stool in the large intestine.
Lubiprostone.
Lubiprostone belongs to the prostone family of compounds. Members of this family are naturally occurring bicyclic fatty acids formed by enzymatic oxidation of the 15-hydroxyl group of prostaglandins to a keto group by 15-hydroxyprostaglandin dehydrogenase (Anggård, 1966).
The mechanism of lubiprostone action is thought to be opening of chloride (Cl−) channels in the small and large intestinal epithelium (Fei et al., 2009) and bicarbonate (HCO3−) secretory pathways in the duodenum (Mizumori et al., 2009). Even so, clear-cut identification of the Cl− channels through which lubiprostone stimulates intestinal Cl− secretion is unresolved. The cystic fibrosis transmembrane conductance regulator (CFTR) and ClC-2 channels both are nominees for activation by lubiprostone.
Bijvelds et al. (2009) concluded that opening of CFTR accounted for lubiprostone action. Conversely, Cuppoletti et al. (2004) reported that lubiprostone opened ClC-2 channels exclusively, and Bao et al. (2008) found that lubiprostone opened both CFTR and ClC-2. The EC50 values for lubiprostone in the work of Cuppoletti et al. (2004) and Bao et al. (2008) were in the low nanomolar range (i.e., 17–18 nM and less than 100 nM, respectively). Bijvelds et al. (2009) applied lubiprostone in the low micromolar range. Concentrations, in the micromolar range, open both ClC-2 and CFTR (Bao et al., 2008). Lubiprostone opens both channels but at a lower effective concentration for ClC-2 than for CFTR. The low concentrations required for clinical efficacy in humans (8–24 μg/day) are consistent with therapeutic action primarily at the ClC-2 channel.
Exposure of flat-sheet preparations from guinea pig small or large intestinal preparations in Ussing flux chambers to lubiprostone stimulates chloride secretion, with a low EC50 value of 43.5 nM in the small intestine and an EC50 value of 31.7 nM in the colon (Fei et al., 2009). Lubiprostone stimulates chloride secretion, with an EC50 value of 24.3 nM across flat-sheet preparations of human jejunum in Ussing chambers (Sun et al., 2008). These low EC50 values for stimulation of chloride secretion are reminiscent of the low EC50 value for stimulation of chloride movement across T84 and A6 monolayers in the work of Cuppoletti et al. (2004) and Bao et al. (2008), but they are different from the EC50 values in the micromolar range of concentrations reported by Bijvelds et al. (2009).
The selective CFTR channel blocker CFTR(inh)-172 does not suppress lubiprostone stimulation of chloride secretion (Fei et al., 2009). In contrast, Bijvelds et al. (2009) reported that stimulation by lubiprostone of chloride movement across T84 cell monolayers was blocked by CFTR(inh)-172, which supported action at CFTR rather than ClC-2. An abbreviated study with CFTR-null mice by Bijvelds et al. (2009) found no stimulation of chloride secretion by lubiprostone, which further supported their conclusion that lubiprostone action was at CFTR, not ClC-2.
Perfusion of lubiprostone in rat duodenal loops in situ evokes secretion of Cl−, HCO3−, and H2O. Relatively high concentrations of lubiprostone, in the micromolar range, dose-dependently increases HCO3− secretion in these preparations, whereas only concentrations less than 100 nM effectively stimulate chloride secretion (Mizumori et al., 2009). Similar to results for guinea pig preparations in Ussing chambers, CFTRinh-172 does not suppress lubiprostone-evoked Cl− secretion in rat duodenum (Mizumori et al., 2009).
Prostaglandins.
The structural similarity of lubiprostone to prostaglandins underlies a contradictory suggestion that lubiprostone acts through a G protein-coupled adenyl cyclase-cAMP-protein kinase A enterocyte signal transduction pathway (Bassil et al., 2008; Bijvelds et al., 2009). Results from T84 cell monolayers, gastric smooth muscle strips and duodenal loops in situ have been invoked as supporting a mechanism of action linked through prostaglandin receptors (Bijvelds et al., 2009; Mizumori et al., 2009). On the contrary, results from flat-sheet preparations of guinea pig small and large intestine in Ussing chambers and intracellular electrophysiological recording of secretomotor neuronal activity do not support prostaglandin receptor stimulation as a mechanism of lubiprostone action (Fei et al., 2009).
The present study used changes in short-circuit current (Isc) across the mucosa of small and large intestinal preparations in Ussing flux chambers as a marker for morphine- or lubiprostone-evoked changes in chloride secretion. Chloride is the predominate anion involved in morphine-evoked changes in Isc (Fondacaro et al., 1990; Sheldon et al., 1990). We aimed to test a hypothesis that stimulation of electrogenic Cl− secretion by lubiprostone offsets the inhibitory action of morphine on secretion and might reverse the constipating effects of morphine. Some of the results have been published in abstract form (Fei et al., 2007).
Materials and Methods
Animals.
Canonical methods for study of intestinal mucosal secretion in Ussing chambers were the same as described previously (Fang et al., 2008; Fei et al., 2009). Adult male Hartley strain guinea pigs (300–350 g) and male C57BL/6J mice (20–30 g) were used. Guinea pigs were stunned by a sharp blow to the head and exsanguinated from the cervical vessels, and mice were euthanized by cervical dislocation according to a protocols approved by The Ohio State University Laboratory Animal Care and Use Committee and United States Department of Agriculture Veterinary Inspectors. Segments of ileum between 10 and 20 cm proximal to the ileocecal junction were removed from the guinea pigs, and the midcolon was removed from the mice, flushed with ice-cold Krebs' solution, and opened along the mesenteric border. Flat-sheet preparations, from which the longitudinal and circular muscle layers together with the myenteric plexus were removed by microdissection, were obtained from the opened segments. The submucosal plexus remained intact with the mucosa. Composition of the Krebs' solution was 120 mM NaCl, 6 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 1.35 mM NaH2PO4, 14.4 mM NaHCO3, and 11.5 mM glucose. The Krebs' solution in the Ussing chambers was gassed with 95% O2, 5% CO2 and buffered at pH 7.4.
Ussing Flux Chambers.
Tissues from each animal were studied simultaneously in four standard Ussing flux chambers. Each chamber was equipped with pairs of silver/silver chloride electrodes via Krebs' agar bridges connected to calomel half-cells for measurement of transmural potential difference. A second pair of electrodes connected to WPI DVC-1000 or EVC-4000 voltage/current clamp amplifiers (World Precision Instruments, Inc., Sarasota, FL) compensated for the solution resistance between the transmural potential difference-sensing bridges. Guinea pig and mouse flat-sheet preparations were mounted between halves of Ussing chambers, which had chamber openings with a total cross-sectional area of 0.64 or 0.72 cm2. The tissues were bathed on both sides from circulation reservoirs containing 10 ml of Krebs' solution and maintained at 37°C by circulation from a temperature-controlled water bath. The current necessary to change the transepithelial potential difference by 2.5 mV was used to monitor tissue conductance, calculated according to Ohm's law, as a measure of tissue viability. Isc was monitored by the WPI DVC-1000 or ECV-4000 in voltage-clamp mode. Concentration-response relations were obtained by adding the drugs to either the chamber compartment bathing the mucosa (mucosal side) or the compartment that bathed the muscle-stripped side (submucosal side). Drug-evoked changes in Isc were measured and expressed as ΔIsc. The data were normalized to the cross-sectional area of the preparations (i.e., 0.64 or 0.72 cm2). Morphine and dimethylphenylpiperazinium (DMPP) were dissolved in Krebs' solution or 0.9% NaCl and lubiprostone in dimethyl sulfoxide (DMSO). Volumes not exceeding 10 μl were added to the 10-ml circulation reservoirs.
Transmural electrical field stimulation of intramural neurons was done, according to the method of Cooke et al. (1983) by passing electrical current between a pair of aluminum foil electrodes placed on the submucosal surface at the intersection between the two halves of the Ussing chamber. Current was supplied by Grass SD 88 stimulators (Grass Instruments, Quincy, MA).
Fecal Wet Weight.
Mice were housed in groups of five in Plexiglas chambers, with food and water available ad libitum before testing. The mice were maintained on a 12-h light/dark cycle under controlled temperature and humidity. Fecal pellet weight was assessed as a surrogate for an integrated combination of mucosal secretion and transit. This involved determination of the fresh weight of fecal pellets expelled at 15-min intervals over a 6-h collection period. The data collected at each 15-min time point were an integral of the number of fecal pellets expelled and the H2O content of the pellets.
Subcutaneous injections of lubiprostone, morphine, 0.9% NaCl, or DMSO were administered with 1-ml syringes and 30-gauge needles at a volume of 10 ml/kg. Each mouse initially received an injection of 0.9% NaCl, 2.6% DMSO, or morphine (3 or 10 mg/kg) before placement into a Plexiglas chamber (15.2 × 16.5 × 12.0 cm) lined with filter paper. Thirty minutes after placement in the chamber, each mouse was injected either with vehicle (2.6% DMSO) or lubiprostone (0.1 or 0.2 mg/kg) and returned to the chamber. The first fecal sample was collected for each mouse at 15 min after initial placement in the chamber, and consecutive samples were then collected for 360 min at 15-min intervals (see Fig. 7).
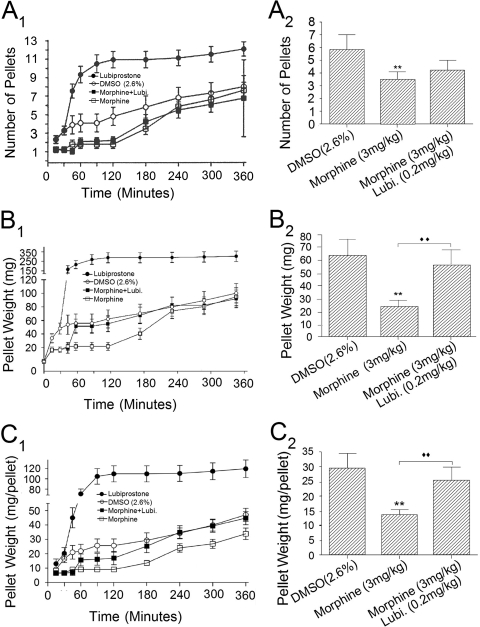
Fig. 7.
Morphine-induced suppression of defecation in conscious mice was reversed by lubiprostone. Mice were injected with morphine (3 mg/kg), and after 30 min they received a second injection of either 2.6% DMSO or 0.2 mg/kg lubiprostone. All injections were subcutaneous. Fecal pellets were collected counted and weighed at 30- or 60-min intervals over a time period of 6 h. A, 1, lubiprostone treatment (●) significantly elevated (P < 0.001) the cumulative number of fecal pellets compared with controls (○; 2.6% DMSO). Morphine (□) suppressed fecal pellet output compared with controls (P < 0.001). Morphine effects started to return toward control levels 120 min after morphine treatment. Injection of lubiprostone (■) 30 min after administration of morphine did not reverse the action of morphine on fecal output. The data represent the sum total number of fecal pellets accumulated at the end of each test period. For example, if three pellets had appeared at the end of the first 30 min and four pellets during the next 15 min, and then the data point on the graph at 45 min was entered as seven pellets (3 + 4 = 7). 2, quantitative data for the action of lubiprostone (Lubi.) on morphine suppression of fecal output after 180 min. B, 1, lubiprostone treatment (●) significantly elevated (P < 0.001) the cumulative wet weight of fecal pellets compared with 2.6% DMSO (○). Morphine (□) suppressed fecal pellet wet weight compared with controls (P < 0.001). Morphine effects started to return toward control levels 120 min after morphine treatment. Injection of lubiprostone (■) 30 min after administration of morphine restored fecal wet weight to control levels within an hour of injection. 2, quantitative data for the action of lubiprostone (Lubi.) on morphine-induced suppression of fecal wet weight in the time interval between lubiprostone injection and suppression of fecal wet weight 120 min after injection of morphine. C, 1 and 2, wet weights of single fecal pellets varied between 2.8 and 103 g. Because wet weight of each fecal pellet was greatly elevated for lubiprostone-treated animals, we normalized fecal pellet weight to pellet number and expressed the data as weight per pellet. Lubiprostone treatment (●) significantly elevated (P < 0.001) the cumulative wet weight of individual fecal pellets relative to 2.6% DMSO (○). Morphine (□) suppressed wet weight per pellet compared with DMSO controls (P < 0.001). Morphine effects started to return toward control levels approximately 120 min after morphine treatment. Injection of lubiprostone (■) 30 min after administration of morphine rapidly reversed the effect of morphine on weight per pellet. 2, quantitative data for the action of lubiprostone (Lubi.) on morphine suppression of weight per pellet in the time interval between lubiprostone injection and suppression of fecal wet weight 120 min after injection of morphine. Values are expressed as means ± S.E.M., with n = 8–38 mice (**, ♦♦, P < 0.001). Two-way analysis of variance with Bonferroni post hoc analysis.
Chemicals.
Lubiprostone, free of any prostaglandin contamination, was synthesized by R-Tech Ueno, Ltd., Tokyo, Japan) (>99.8% purity) and obtained as frozen aliquots of 2 mM solutions in 100% DMSO. DMSO, obtained as frozen aliquots from the same source, was used to dilute lubiprostone and for testing vehicle effects. Morphine sulfate pentahydrate, tetrodotoxin, and DMPP were purchased from Sigma-Aldrich (St. Louis, MO).
Statistical Analysis.
Ussing chamber data are presented as means ± S.E., with n values referring to numbers of animals or preparations. Continuous curves for concentration-response relationships were constructed with the following least-squares fitting routine using SigmaPlot software (SPSS Inc., Chicago, IL): V = Vmax/[1 + (EC50/C)nH], where V is the observed increased Isc, Vmax is the maximal response, C is the corresponding drug concentration, EC50 is the concentration that induces the half-maximal response, and nH is the apparent Hill coefficient. Student's t test or paired t test was used to determine significance, with P < 0.05 considered to be significant. For whole animal studies, time course curves for accumulation of feces were constructed for the 6-h test period and analyzed with two-way analysis of variance and Bonferroni post hoc analysis. Significance of differences in accumulated fecal pellets between the different treatments (i.e., lubiprostone and morphine) was analyzed after 2 h with Student's t test.
Results
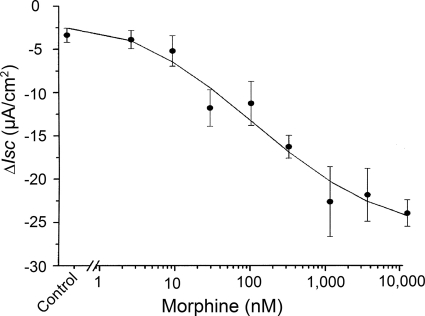
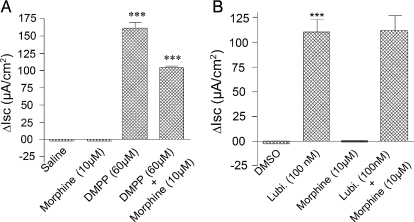
Exposure to morphine reduced basal Isc in a concentration-dependent manner that was reminiscent of the findings of others (Fondacaro et al., 1990; Sheldon et al., 1990). Morphine was added to the submucosal side of four muscle-striped small intestinal preparations from each of six guinea pigs, and changes in basal Isc were recorded 10 min later. Morphine in concentrations between 1 and 10,000 nM suppressed basal Isc, with an IC50 value of 96.1 nM (Fig. 1). Maximal suppression was by 23 μA/cm2 at concentrations between 1 and 10 μM. Pretreatment with 1 μM tetrodotoxin suppressed basal Isc, as we described previously (Fei et al., 2009), and it suppressed or abolished the inhibitory action of morphine on basal Isc (data not shown). Morphine suppression of basal Isc in four preparations from the midcolon of each of six mice was small and not significantly different from the saline carrier alone (Fig. 2A). Application of DMPP, which acts at excitatory nicotinic receptors on secretomotor neurons, stimulated Isc in the mouse preparations. Morphine, applied in the presence of DMPP, suppressed the DMPP-evoked increases in Isc (Fig. 2A).
Fig. 1.
Concentration-response relationship for morphine-induced decrease in basal Isc. Morphine or 0.9% NaCl (control) was applied to the submucosal side of the chamber, and changes in basal Isc were evaluated after 10 min. Morphine decreased the basal Isc in a concentration-dependent manner, with an IC50 value of 96.1 nM. Values are expressed as means ± S.E.M., with n = 4 preparations from each of six animals.
Fig. 2.
Inhibitory action of morphine on neurally stimulated Isc was reversed by lubiprostone in mouse colon. A, DMPP, which acts at nicotinic receptors to excite secretomotor neurons, stimulated Isc. Application of morphine, 10 min before addition of DMPP, significantly suppressed DMPP stimulation of neurogenic Isc. B, lubiprostone (Lubi.) alone significantly stimulated Isc relative to DMSO control and morphine alone suppressed basal Isc. Unlike the neurally mediated stimulation of Isc by DMPP, pretreatment with morphine for 10 min did not suppress stimulation of Isc by lubiprostone. All agents were applied to the submucosal side of the chambers. Values are expressed as means ± S.E.M., with n = 4 preparations from each of six animals (***, P < 0.001).
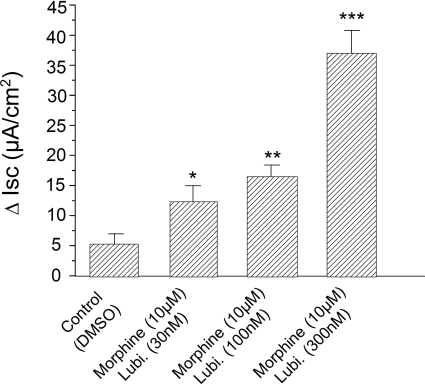
Morphine (10 μM) was added to the submucosal side of four small intestinal preparations from each of six guinea pigs 5 min before applying lubiprostone in a range of concentrations between 30 and 300 nM. Lubiprostone reduced, in concentration-dependent manner, morphine-induced suppression of basal Isc. Application of lubiprostone at 30, 100, or 300 nM in the submucosal bath, 5 min after pretreatment with morphine, increased Isc by 12.2 ± 2.8, 16.4 ± 2.1, and 37.2 ± 3.7 μA/cm2, respectively (Fig. 3). Lubiprostone stimulation of Isc in the mouse colon was unaffected by 10 μM morphine (Fig. 2B).
Fig. 3.
Effects of lubiprostone (Lubi.) on suppression, by morphine, of basal Isc secretion in guinea pig ileum. Morphine (10 μM) was applied to the submucosal side of the chambers 5 min before applying lubiprostone on the same side. Lubiprostone reversed morphine-induced decreases in basal Isc in concentration-dependent manner. Application of lubiprostone, 5 min after pretreatment with morphine, increased Isc in concentration-dependent manner. Values are expressed as means ± S.E.M., with n = 4 preparations from each of six animals (*, P < 0.05; **, P < 0.01; and ***, P < 0.001).
Transmural Electrical Field Stimulation.
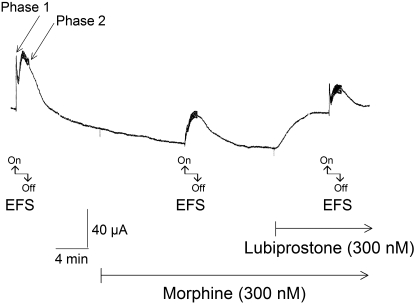
Transmural EFS evoked characteristic biphasic increases in Isc, such as those reported by Cooke et al. (1983), Fang et al. (2008), and Fie et al. (2009) (Fig. 4). The first phase (i.e., phase 1) mainly reflects stimulation of cholinergic submucosal secretomotor neurons and action of ACh at muscarinic receptors expressed by the enterocytes (Cooke, 1984). The second phase (i.e., phase 2) reflects Cl− secretion evoked by release of peptidergic neurotransmitters; primarily vasoactive intestinal peptide (VIP) (Cooke, 1987). The cholinergic component, but not the putative VIP component, is enhanced by lubiprostone (Fei et al., 2009). The protocol was to evoke the biphasic increases in Isc, add 300 nM morphine to the bathing solution on the submucosal side, and apply EFS a second time 10 min later. Morphine suppressed both phase 1 and phase 2 of the EFS-evoked Isc in four preparations from each of six guinea pigs. Morphine decreased the first phase of the EFS-evoked response in a concentration-dependent manner, with an IC50 of 40.7 nM (Figs. 4 and 5). Suppression of the second phase was weaker, with a maximal decrease in Isc of 33% in the presence of 300 nM morphine (Fig. 5).
Fig. 4.
Transmural EFS of guinea pig ileum preparations evoked biphasic changes in Isc, which are labeled by arrows as phase 1 and phase 2. Morphine (300 nM), applied in the submucosal chamber, decreased both phases of the EFS-evoked response. Suppression of the second phase was weaker, with a maximal decrease in Isc of 33% in the presence of 300 nM morphine (see Fig. 5). Application of 300 nM lubiprostone, after 10 min in the presence of morphine, elevated Isc. Lubiprostone, in the continued presence of morphine, restored phase 1 and suppressed phase 2. Transmural EFS was done with stimulus-pulse parameters of 0.5-ms duration, 10-Hz frequency, and 3.5-mA strength.
Fig. 5.
Effects of morphine on Isc, evoked by EFS of guinea pig ileum, were different for phases 1 and 2. Morphine significantly decreased phase 1 of the response in a concentration-dependent manner, with an IC50 value of 40.7 nM. Suppression of phase 2 was weak, with a maximal decrease in Isc of 33% in the presence of 300 nM morphine. Values are expressed as means ± S.E.M., with n = 4 preparations from each of six animals.
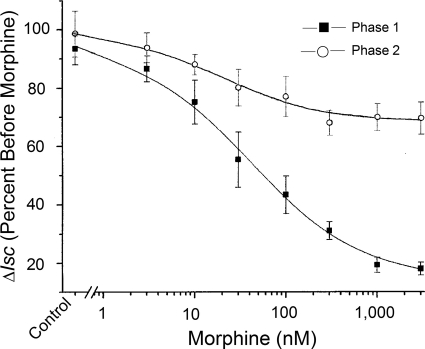
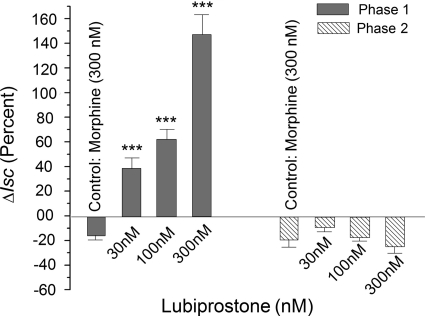
The presence of lubiprostone on the submucosal side of four preparations from each of six guinea pigs reversed the morphine-induced suppression of the first phase of the biphasic EFS-evoked response (Figs. 4 and 6). Application of 30, 100, or 300 nM lubiprostone in the presence of 300 nM morphine caused a concentration-dependent increase in the amplitude of the morphine-suppressed phase 1 (Fig. 6).
Fig. 6.
Effects of lubiprostone on morphine suppression of Isc, evoked by EFS were different for phases 1 and 2 of the evoked responses. Morphine (300 nM) suppressed phase 1 of the EFS-evoked Isc response and this action was reversed in concentration-dependent manner by application of lubiprostone in concentrations of 30 nm, 100 nM, and 300 nm after 5 min in the presence of morphine. The effect of lubiprostone on phase 2 in the presence of morphine was diametrically opposed to its action on phase 1. In the presence of morphine, lubiprostone suppressed phase 2 of the EFS-evoked responses in concentration-dependent manner. Transmural EFS was done with stimulus pulse parameters of 0.5-ms duration, 10-Hz frequency, and 3.5-mA strength. Values are expressed as means ± S.E.M., with n = 4 preparations from each of six animals (***, P < 0.001).
The effect of lubiprostone on morphine suppression of phase 2 of the EFS-evoked responses was opposite to the effect for phase 1 (Fig. 6). Suppression of phase 2 by 300 nM morphine was enhanced in concentration-dependent manner by the addition of 30, 100, or 300 nM lubiprostone. The reduction in the amplitude of phase 2 after addition of 300 nM lubiprostone was 1.3-fold greater than the reduction produced by morphine alone (Fig. 6).
Defecation.
Subcutaneous injection of lubiprostone (0.2 mg/kg), in the mouse, increased the numbers of fecal pellets collected at 15-min intervals relative to controls, which had received injections of 2.6% DMSO (Fig. 7A, 1 and 2). This action of lubiprostone was sustained at nearly constant levels over a 5.5-h collection period. In contrast to lubiprostone, subcutaneous morphine (3 mg/kg) reduced the numbers of fecal pellets collected at 15-min intervals (Fig. 7A1). Unlike the action of lubiprostone to enhance fecal output, suppression of fecal output by morphine was not sustained. The beginning of recovery from morphine suppression was apparent at approximately 120 min after administration and had returned to the values for 0.9% NaCl or DMSO alone (i.e., control) after approximately 240 min (Fig. 7A1). Subcutaneous injection of lubiprostone (0.2 mg/kg), 30 min after subcutaneous injection of 0.2 mg/kg morphine, reversed morphine-induced suppression of fecal pellet weight, which returned to near control levels within 30 min of the injection (Fig. 7A1). Injection of lubiprostone 30 min after administration of morphine did not reverse the action of morphine to suppress fecal output (Fig. 7A, 1 and 2).
Subcutaneous injection of lubiprostone (0.2 mg/kg), in the mouse, increased the wet weight of fecal pellets collected at 15-min intervals relative to controls, which had received injections of 0.9% NaCl or 2.6% DMSO (Fig. 7B, 1 and 2). Elevation of fecal wet weight by lubiprostone was sustained at nearly constant levels over a 5.5-h collection period (Fig. 7B1). Unlike lubiprostone, subcutaneous morphine (3 mg/kg) reduced the weight of fecal pellets, which were collected and analyzed in the same manner as for lubiprostone (Fig. 7B1). In contrast to the prolonged action of lubiprostone to enhance fecal weight, suppression of fecal weight by morphine was not sustained for extended time (Fig. 7B1) The beginning of recovery from morphine suppression was apparent at 120 min after administration and had returned to the values for 0.9% NaCl or DMSO alone (i.e., control) after approximately 240 min (Fig. 7B1). Subcutaneous injection of lubiprostone (0.2 mg/kg), 30 min after subcutaneous injection of 0.2 mg/kg morphine, reversed morphine-induced suppression of fecal pellet weight, which returned to near control levels within 30 min of the injection (Fig. 7B, 1 and 2).
The wet weight of individual fecal pellets released by lubiprostone-treated animals varied greatly between 2.8 and 103 g. In view of this, we divided pellet weight by the number of pellets and expressed the data as weight per pellet (Fig. 7C, 1 and 2). Injection of lubiprostone significantly elevated (P < 0.001) the cumulative wet weight of individual fecal pellets relative to 2.6% DMSO or 0.9% NaCl. Morphine treatment suppressed the wet weight per pellet compared with controls (P < 0.001). Morphine effects began to return toward control levels approximately 120 min after morphine treatment (Fig. 7C1) Injection of lubiprostone, 30 min after administration of morphine, rapidly reversed the effect of morphine on weight per pellet (7C, 1 and 2).
Discussion
Morphine Suppression of Isc.
Guinea pig or mouse flat-sheet intestinal preparations in Ussing flux chambers generate a persistent basal Isc, which can reflect a combination of neurogenic Cl− secretion, evoked by ongoing discharge of secretomotor neurons in the submucosal plexus, and non-neurogenic Na+ absorption. Nicotinic receptors on secretomotor neurons, which when stimulated by ACh or nicotinic agonists (e.g., DMPP), evoke Cl− secretion (Cooke, 1987). A decrease in basal Isc and DMPP-stimulated activity occurs in parallel with blockade of secretomotor neurons by tetrodotoxin (Carey and Cooke, 1989). We found that morphine behaved in similar manner to tetrodotoxin in suppressing basal or DMPP-evoked Isc. This probably reflected an action of morphine to suppress ongoing discharge of secretomotor neurons. Morphine action of this nature is mediated by μ-type opioid receptors, which are expressed by enteric neurons in the guinea pig and mouse intestine (Minnis et al., 2003). Morphine and other opioids act at these receptors to increase potassium conductance and hyperpolarize the membrane potential of the neuron (Morita and North, 1982). Hyperpolarization of the membrane potential and consequent suppression of action potential generation by secretomotor neurons is postulated to be a mechanism by which opiates suppress mucosal secretion and induce a constipated state in animals and humans (Wood and Galligan, 2004; Wood, 2007).
Lubiprostone.
Action of lubiprostone to reverse the inhibitory effect of morphine on basal Isc is unlikely to result from any action on secretomotor neurons because blockade of conduction of enteric neuronal action potentials by tetrodotoxin has no significant effect on stimulation of Isc by lubiprostone (Fei et al., 2009). Moreover, application of lubiprostone to secretomotor neurons in preparations from the guinea pig ileum does not change their electrophysiological behavior (Fei et al., 2009).
Lubiprostone-reversal of the inhibitory action of morphine on basal Isc was probably a result of its documented action to open ClC-2 channels expressed by mucosal enterocytes (Cuppoletti et al., 2004; Bao et al., 2008; Fei et al., 2009). The basal Isc, recorded in the absence of morphine, reflected ongoing stimulation of Cl− secretion by neurotransmitters released from tonically active secretomotor neurons. Morphine suppression of secretomotor neuronal excitability eliminated this component of basal Isc (Figs. 3 and 4). Direct action of lubiprostone to open epithelial Cl− channels in the mucosa, at the time when neuronal excitability is inhibited by morphine, can account for the reversal by lubiprostone of morphine suppression of basal Isc.
Transmural Electrical Field Stimulation.
Transmural electrical stimulation of guinea pig intestinal preparations in Ussing chambers evoked increases in Isc much like those found in a variety of other species, including mice (Carey and Cooke, 1984), rabbit (Hubel, 1978), and humans (Hubel and Shirazi, 1982). The biphasic responses to EFS in the guinea pig reflect the release of two or more neurotransmitters from secretomotor neurons at their junctions with the secretory epithelium and release of excitatory transmitters from interneurons neurons that are synaptically connected to the secretomotor neurons. Secretomotor neurons release ACh and VIP as primary neurotransmitters (Cooke, 1984). Phase 1 of the biphasic response to EFS in the guinea pig is mediated mainly or entirely by the release of ACh and is mimicked by application of the muscarinic agonist carbachol. Phase 2 has a peptidergic component, which includes VIP as the primary transmitter (Cooke, 1987).
Morphine, at maximal concentrations in our study, abolished the first phase of the EFS-evoked response, whereas a small reduction in the second phase occurred. In view of the evidence that the first phase is cholinergically mediated (Cooke, 1987) and the evidence that morphine acts to suppress release of ACh from enteric neurons (Paton, 1957; North and Tonini, 1977), a reasonable conclusion is that morphine suppression of the first phase reflected inhibition of ACh release. Alternatively, opiates and opioid peptides are reported to also decrease Isc due to suppression of Cl− or H2CO3− secretion and stimulation of Na+ and Cl− absorption (Cooke and Reddix, 1994). We did not determine the extent to which these factors might be involved in the morphine-induced suppression of Isc.
The limited effectiveness of morphine in suppressing the second phase suggests that expression of the μ-opioid receptor subtype is mainly by cholinergic secretomotor neurons rather than by a subpopulation that releases VIP or a related peptide. The evidence hints that VIPergic secretomotor neurons in the guinea pig do not express μ-opioid receptors, and this could account for lack of effectiveness of morphine on the second phase. Evidence for this can be found in a report from Surprenant and North (1985) that submucosal neurons, which express α2-noradrenergic receptors, do not express μ-opioid receptors and from Bornstein et al. (1986, 1988) that VIPergic, but rarely cholinergic, secretomotor neurons receive noradrenergic inhibitory input.
Lubiprostone reversed the action of morphine to suppress the first phase, but not the second phase, of the biphasic EFS-evoked Isc responses. The second phase of the EFS-evoked responses, which was only marginally suppressed by morphine, was suppressed further when lubiprostone was added in the presence of morphine (Fig. 6). These discordant effects probably reflect a direct action of lubiprostone on the epithelium because lubiprostone has no action on secretomotor neurons (Fei et al., 2009). Fei et al. (2009) suggested an explanation for the augmenting action of lubiprostone on phase 1 of the EFS-evoked Isc responses might be direct opening of apical ClC-2 channels adding a Cl− secretory component that becomes synergistic with a separate Ca2+-activated Cl− component in a postreceptor muscarinic signal cascade, both of which would enhance cholinergically evoked Cl− secretion.
The opposing actions of lubiprostone on the two phases of EFS-evoked responses and differential action of morphine on the two phases might be related to signaling by ACh and VIP on two separate populations of enterocytes. ACh action might be on enterocytes that express muscarinic and μ-opioid receptors, but not inhibitory noradrenergic receptors, whereas VIP acts on enterocytes that express inhibitory noradrenergic receptors but not μ-opioid receptors. We reported previously that lubiprostone, in the presence of neural blockade with tetrodotoxin, suppresses stimulation of Isc by VIP in similar manner to its suppression of phase 2 of the neurally mediated response evoked by EFS (Fei et al., 2009).
Suppression by lubiprostone of VIP stimulation of Isc might be explained by opening of the same set of epithelial ClC-2 channels by both agents. Both lubiprostone and VIP open ClC-2 channels in the apical membranes. Lubiprostone does this by directly opening the channels (Cuppoletti et al., 2004; Bao et al., 2008). Opening of Cl− channels by VIP is by a different metabotropic mechanism operating through stimulation of protein kinase A and channel phosphorylation (Dharmasathaporn and Pandol, 1986; McCabe and Dharmsathaphorn, 1988). Activation of transfected ClC-2 channels by lubiprostone is independent of protein kinase A in human embryonic kidney-293 cells (Cuppoletti et al., 2004). Lubiprostone-opening of the same ClC-2 channels, which mediate stimulation of Isc by VIP, is expected to dampen the response to VIP, whether it is released as a neurotransmitter or exogenously applied. Limited expression of μ-opioid receptors by the VIPergic secretomotor neurons responsible for phase 2 of the EFS-evoked responses can account in part for the minimal action of morphine on phase 2.
Defecation.
The effect of morphine to suppress fecal pellet output and wet weight was characteristic of many previous observations of the constipating action of opioids. It might have resulted from inhibition of propulsive motility, suppression of mucosal secretion, or a combination of both; and the site of action could have been in the ENS, the central nervous system, or both.
The effect of lubiprostone to increase the wet weight and numbers of rodent fecal pellets is assumed to be analogous to increased stool liquidity and frequency in humans. This would be consistent with the reported efficacy of lubiprostone in the treatment of chronic constipation and constipation-predominant irritable bowel syndrome in humans (Sweetser et al., 2009). Elevation of fecal weight by lubiprostone in the conscious mice probably reflected its action to stimulate mucosal secretion of H2O and electrolytes. Stimulation of secretion by lubiprostone in this case appears to reflect a direct action on the mucosal epithelium because lubiprostone stimulation of Cl− secretion is independent of any action in the ENS (Fei et al., 2009). Whether the observed increases in fecal pellet output might involve a motility component occurring in concert with stimulated secretion is open to question. Lubiprostone is reported not to cause accelerated colonic transit in humans (Sweetser et al., 2009).
Lubiprostone reversal of morphine-induced suppression of fecal wet weight can be attributed to a direct action on the mucosal epithelium. Lack of any effects of lubiprostone on enteric neurons makes it unlikely that this resulted from interference with the known inhibitory actions of morphine in the ENS. A direct mucosal action is supported by evidence that lubiprostone stimulates Cl− secretion by directly opening ClC-2 channels in the apical membranes of epithelial cells (Bao et al., 2008; Cuppoletti et al., 2004). Our results support the hypothesis that lubiprostone bypasses the constipating action of morphine by directly stimulating mucosal secretion and thereby increasing the liquidity of the intestinal contents.
Acknowledgments
We are indebted to Profs. Helen J. Cooke and Xiucai Fang for helpful direction in the conduct of the Ussing chamber studies and preparation of the manuscript.
This work was funded by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants R01-DK37238, R01-DK57075 (both to J.D.W.), K08-DK60468 (to Y.X.)]; National Institutes of Health National Institute on Drug Abuse [Grants R01-DA18860, K01-DA14600 (both to L.M.B.), F32-DA21592 (to K.R.)]; a Pharmaceutical Manufacturers of America Foundation postdoctoral fellowship (to S.L.); and a grant-in-aid from Sucampo Pharmaceuticals, Inc.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.166116.
- ENS
- enteric nervous system
- CFTR
- cystic fibrosis transmembrane conductance regulator
- Isc
- short-circuit current
- DMPP
- dimethylphenylpiperazinium
- DMSO
- dimethyl sulfoxide
- ACh
- acetylcholine
- EFS
- electrical field stimulation
- VIP
- vasoactive intestinal peptide.
References
- Anggård E. (1966) The biological activities of three metabolites of prostaglandin E1. Acta Physiol Scand 66:509–510 [DOI] [PubMed] [Google Scholar]
- Bao HF, Liu L, Self J, Duke BJ, Ueno R, Eaton DC. (2008) A synthetic prostone activates apical chloride channels in A6 epithelial cells. Am J Physiol Gastrointest Liver Physiol 295:G234–G251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil AK, Borman RA, Jarvie EM, McArthur-Wilson RJ, Thangiah R, Sung EZ, Lee K, Sanger GJ. (2008) Activation of prostaglandin EP receptors by lubiprostone in rat and human stomach and colon. Br J Pharmacol 154:126–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijvelds MJ, Bot AG, Escher JC, De Jonge HR. (2009) Activation of intestinal Cl- secretion by lubiprostone requires the cystic fibrosis transmembrane conductance regulator. Gastroenterology 137:976–985 [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Costa M, Furness JB. (1986) Synaptic inputs to immunohistochemically identified neurones in the submucous plexus of the guinea-pig small intestine. J Physiol 381:465–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC, Costa M, Furness JB. (1988) Intrinsic and extrinsic inhibitory synaptic inputs to submucous neurones of the guinea-pig small intestine. J Physiol 398:371–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey HV, Cooke HJ. (1989) Tonic activity of submucosal neurons influences basal ion transport. Life Sci 44:1083–1088 [DOI] [PubMed] [Google Scholar]
- Carey HV, Cooke HJ. (1984) Influence of enteric nerves on jejunal function of the prebald lethal mouse. Gastroenterology 86:A1040 [Google Scholar]
- Cooke HJ. (1984) Influence of enteric cholinergic neurons on mucosal transport in guinea pig ileum. Am J Physiol 246:G263–G267 [DOI] [PubMed] [Google Scholar]
- Cooke HJ. (1987) Neural and humoral regulation of small intestinal electrolyte transport, in Physiology of the Gastrointestinal Tract, pp 1307–1350, Raven Press, New York [Google Scholar]
- Cooke HJ, Reddix RA. (1994) Neural regulation of intestinal electrolyte transport, in Physiology of the Gastrointestinal Tract, pp 2083–2132, Raven Press, New York [Google Scholar]
- Cooke HJ, Shonnard K, Wood JD. (1983) Effects of neuronal stimulation on mucosal transport in guinea pig ileum. Am J Physiol 245:G290–G296 [DOI] [PubMed] [Google Scholar]
- Cooke HJ, Shonnard K, Highison G, Wood JD. (1983) Effects of neurotransmitter release on mucosal transport in guinea pig ileum. Am J Physiol 245:G745–G750 [DOI] [PubMed] [Google Scholar]
- Cuppoletti J, Malinowska DH, Tewari KP, Li QJ, Sherry AM, Patchen ML, Ueno R. (2004) SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol 287:C1173–C1183 [DOI] [PubMed] [Google Scholar]
- Daniel EE. (1968) Pharmacology of the gastrointestinal tract, in Handbook of Physiology, Alimental Canal (pp 2267–2324), American Physiological Society, Washington, DC [Google Scholar]
- Dharmsathaphorn K, Pandol SJ. (1986) Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest 77:348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droney J, Ross J, Gretton S, Welsh K, Sato H, Riley J. (2008) Constipation in cancer patients on morphine. Support Care Cancer 16:453–459 [DOI] [PubMed] [Google Scholar]
- Fang X, Liu S, Wang XY, Gao N, Hu HZ, Wang GD, Cook CH, Needleman BJ, Mikami DJ, Xia Y, et al. (2008) Neurogastroenterology of tegaserod (HTF 919) in the submucosal division of the guinea-pig and human enteric nervous system. Neurogastroenterol Motil 20:80–93 [DOI] [PubMed] [Google Scholar]
- Fei G, Liu S, Wang G-D, Qu M-H, Wang X-Y, Zou F, Du Y, Xia Y, Bohn L, Wood JD. (2007) Stimulation of mucosal secretion by lubiprostone in guinea-pig small intestine and colon. Gastroenterology 132:A683–W1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei G, Wang YZ, Liu S, Hu HZ, Wang GD, Qu MH, Wang XY, Xia Y, Sun X, Bohn LM, et al. (2009) Stimulation of mucosal secretion by lubiprostone (SPI-0211) in guinea pig small intestine and colon. Am J Physiol Gastrointest Liver Physiol 296:G823–G832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondacaro JD, Kolpak DC, Burnham DB, McCafferty GP. (1990) Cecectomized rat. A model of experimental secretory diarrhea in conscious animals. J Pharmacol Methods 24:59–71 [DOI] [PubMed] [Google Scholar]
- Hubel KA. (1978) The effects of electrical field stimulation and tetrodotoxin on ion transport by the isolated rabbit ileum. J Clin Invest 62:1039–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel KA, Shirazi S. (1982) Human ileal ion transport in vitro: changes with electrical field stimulation and tetrodotoxin. Gastroenterology 83:63–68 [PubMed] [Google Scholar]
- McCabe RD, Dharmsathaphorn K. (1988) Mechanism of VIP-stimulated chloride secretion by intestinal epithelial cells. Ann NY Acad Sci 527:326–345 [DOI] [PubMed] [Google Scholar]
- Minnis JG, Patierno S, Kohlmeier SE, Brecha NC, Tonini M, Sternini C. (2003) Ligand-induced mu opioid receptor endocytosis and recycling in enteric neurons. Neuroscience 119:33–42 [DOI] [PubMed] [Google Scholar]
- Mizumori M, Akiba Y, Kaunitz JD. (2009) Lubiprostone stimulates duodenal bicarbonate secretion in rats. Dig Dis Sci 54:2063–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, North (1981) Opiates and enkephalin reduce the excitability of neuronal processes. Neuroscience 6:1943–1951 [DOI] [PubMed] [Google Scholar]
- Morita K, North RA. (1982) Opiate activation of potassium conductance in myenteric neurons: inhibition by calcium ion. Brain Res 242:145–150 [DOI] [PubMed] [Google Scholar]
- North RA, Tonini M. (1977) The mechanism of action of narcotic analgesics in the guinea-pig ileum. Br J Pharmacol 61:541–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton WD. (1957) The action of morphine and related substances on contraction and on acetylcholine output of coaxially stimulated guinea-pig ileum. Br J Pharmacol Chemother 12:119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan X. (2007) Drug-related side effects of long-term intrathecal morphine therapy. Pain Physician 10:357–366 [PubMed] [Google Scholar]
- Sheldon RJ, Rivière PJ, Malarchik ME, Moseberg HI, Burks TF, Porreca F. (1990) Opioid regulation of mucosal ion transport in the mouse isolated jejunum. J Pharmacol Exp Ther 253:144–151 [PubMed] [Google Scholar]
- Sun X, Xia Y, Liu S, Wang YZ, Qu MH, Wang GD, Wang XY, Zou F, Ren W, Needleman BJ, et al. (2008). Lubiprostone reverses the inhibitory action of morphine on mucosal secretion in the human jejunum. Gastroenterology A122:844 [Google Scholar]
- Surprenant A, North RA. (1985) mu-Opioid receptors and alpha 2-adrenoceptors coexist on myenteric but not on submucous neurones. Neuroscience 16:425–430 [DOI] [PubMed] [Google Scholar]
- Sweetser S, Busciglio IA, Camilleri M, Bharucha AE, Szarka LA, Papathanasopoulos A, Burton DD, Eckert DJ, Zinsmeister AR. (2009) Effect of a chloride channel activator, lubiprostone, on colonic sensory and motor functions in healthy subjects. Am J Physiol Gastrointest Liver Physiol 296:G295–G301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JD. (2005) Opioids, the enteric nervous system and postoperative ileus. Semin Colon Rectal Surg 16:188–196 [Google Scholar]
- Wood JD, Galligan JJ. (2004) Function of opioids in the enteric nervous system. Neurogastroenterol Motil 16 (Suppl 2):17–28 [DOI] [PubMed] [Google Scholar]