Abstract
Incorporation of the α5 nicotinic acetylcholine receptor (nAChR) subunit can greatly influence nAChR function without altering receptor number. Although few animal studies have assessed the role of the α5 nAChR in nicotine-mediated behaviors, recent evidence suggests an association between polymorphisms in the α5 nAChR gene and nicotine dependence phenotypes in humans. Thus, additional studies are imperative to elucidate the role and function of the α5 nAChR subunit in nicotine dependence. Using α5(−/−) mice, the current study aimed to examine the role of α5 nAChRs in the initial pharmacological effects of nicotine, nicotine reward using the conditioned place preference model, and the discriminative effects of nicotine using a two-lever drug discrimination model. 86Rb+ efflux and 125I-epibatidine binding assays were conducted to examine the effect of α5 nAChR subunit deletion on expression and activity of functional nAChRs. Results show that α5(−/−) mice are less sensitive to the initial effects of nicotine in antinociception, locomotor activity, and hypothermia measures and that the α5 nAChR is involved in nicotine reward. Alternatively, α5(−/−) mice did not differ from wild-type littermates in sensitivity to the discriminative stimulus effects of nicotine. Furthermore, deletion of the α5 nAChR subunit resulted in a statistically significant decrease in function in the thalamus and hindbrain, but the decreases noted in spinal cord were not statistically significant. Receptor number was unaltered in all areas tested. Taken together, results of the study suggest that α5 nAChRs are involved in nicotine-mediated behaviors relevant to development of nicotine dependence.
Nicotine, the primary addictive component of tobacco, exerts its effects by binding to nicotinic acetylcholine (ACh) receptors (nAChRs). These receptors are pentameric ligand-gated ion channels that exist as homomeric or heteromeric complexes of α and β subunits. To date, 12 neuronal subunits (α2–α10 and β2–β4) have been identified in mammals (for review, see Gotti et al., 2007). The α5 nAChR subunit is expressed in discrete regions of the mammalian central nervous system, including the cerebral cortex (Gerzanich et al., 1998), cerebellum, thalamus (Flora et al., 2000), striatum (Zoli et al., 2002), hippocampus, substantia nigra, interpeduncular nucleus, ventral tegmental area (Wada et al., 1990), and medial habenula (Broide et al., 2002), and also peripherally in sympathetic and parasympathetic ganglia (De Biasi, 2002).
Although the α5 subunit cannot yield functional receptors when expressed alone, or as the sole α subunit expressed with either β2 or β4, incorporation of this subunit into α4β2*, α3β2*, or α3β4* nAChRs (where * denotes the possible inclusion of additional nAChR subunits) increases receptor desensitization rates, calcium permeability, and pharmacological properties of the receptor subtypes in response to nicotine (Ramirez-Latorre et al., 1996; Gerzanich et al., 1998; Tapia et al., 2007). The potency and efficacy of nicotine is increased in expressed α3β2α5 nAChR subtypes, with little effect on the α3β4α5 subtype (Wang et al., 1996; Gerzanich et al., 1998). In contrast to α4β2* nAChRs, which are increased by chronic nicotine exposure, the α4β2α5 nAChR subtype is not up-regulated by nicotine treatment in rat hippocampus, striatum, cerebral cortex, or thalamus, brain areas where virtually all the α5-containing nAChRs are of the α4β2α5 subtype (Mao et al., 2008). Furthermore, α5 mRNA is not up-regulated in the brain after chronic nicotine administration (Marks et al., 1992). From these studies, it is clear that incorporation of the α5 nAChR subunit greatly influences modulation of receptor function by nicotine, and it has the ability to do so without significantly altering the number of receptors expressed (Brown et al., 2007).
Indeed, data are emerging in support of a role for α5 in nicotine's behavioral effects. The α4β2α5 nAChR subtype is involved in nicotine-stimulated dopamine release in the striatum (Salminen et al., 2004), and it is expressed in brain areas implicated in nicotine-mediated behaviors. Recently, it was found that α4β2α5 nAChR subtypes mediate a significant fraction of the α-conotoxin MII-resistant dopamine release in striatal synaptosomes (Grady et al., 2010). Mice null for the α5 nAChR subunit have reduced sensitivity to nicotine-induced seizures and hypolocomotion (Salas et al., 2003; Kedmi et al., 2004). In addition, α5(−/−) mice exhibit reduced somatic signs of nicotine withdrawal compared with wild-type [(+/+)] littermates (Jackson et al., 2008; Salas et al., 2009), suggesting a potential role for α5* nAChRs in nicotine dependence.
Several recent studies have also reported a genetic association between the human CHRNA5/CHRNA3/CHRNB4 locus located on chromosome 15q25, encoding the α5, α3, and β4 nAChR subunits, respectively, and nicotine dependence, as measured by heavy smoking (daily cigarettes smoked), Fagerström Test for Nicotine Dependence scores, and age-dependent severity of nicotine dependence (Saccone et al., 2007; Berrettini et al., 2008; Bierut et al., 2008; Thorgeirsson et al., 2008; Weiss et al., 2008). Of the many polymorphisms studied, one nonsynonymous polymorphism, rs16969968, changes the 398th amino acid from aspartic acid to asparagine (D398N) in the CHRNA5 gene (Saccone et al., 2007). Furthermore, α4β2α5 nAChRs containing this amino acid substitution exhibit reduced responses to nicotinic agonists in vitro, which may contribute to an increased risk for developing nicotine dependence (Bierut et al., 2008). These genetic studies provide compelling evidence that the CHRNA5/CHRNA3/CHRNB4 locus is involved in heavy smoking and nicotine dependence, and, taken together with the aforementioned molecular and in vivo studies, indicate the need to establish the roles of α5-containing nAChR subtypes in nicotine dependence.
The current study investigates the role of the α5 nAChR subunit in various aspects of nicotine dependence by measuring the initial sensitivity to selected pharmacological effects of nicotine (antinociception, hypothermia, and locomotor activity), reward using the conditioned place preference (CPP) model, and discriminative stimulus properties using a two-lever drug discrimination model. Furthermore, 86Rb+ efflux and 125I-epibatidine binding assays were conducted to examine the effect of α5 nAChR subunit deletion on expression and activity of functional nAChRs. Due to the lack of α5-selective antagonists, the present study used α5(−/−) mice to examine the role of α5* nAChRs. Data obtained from this study will further understanding of the α5 nAChR subunit and its role in the development of nicotine dependence.
Materials and Methods
Animals
Male C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and used for morphine sulfate studies. Mice null for the α5 nicotinic receptor subunit (C57BL/6 background) and (+/+) littermates were shipped from Baylor College of Medicine (Houston, TX) (for information regarding initial breeders, see Salas et al., (2003)) and were subsequently bred in an animal care facility at Virginia Commonwealth University (Richmond, VA). Mice null for the α4, α5, β2, and β4 subunits and their wild-type and heterozygotic littermates were bred at the Institute of Behavioral Genetics in Colorado and used for Rb86+ efflux studies. α4 mice were originally described by Ross et al. (2000), the α5 mice were originally described by Salas et al. (2003), the β4 mice were originally described by Xu et al., (1999), and β2 mice were originally described by Picciotto et al. (1998). For all experiments, mice were backcrossed at least 8 to 10 generations. Mutant, heterozygotes, and wild types were obtained from crossing heterozygote mice. This breeding scheme controlled for any irregularities that might occur with crossing solely mutant animals. Animals were 8 to 10 weeks of age at the start of the experiments and were group-housed in a 21°C humidity-controlled Association for Assessment and Accreditation of Laboratory Animal Care-approved animal care facility with ad libitum access to food and water. Experiments were performed during the light cycle and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Drugs
(−)-Nicotine hydrogen tartrate salt [(−)-1-methyl-2-(3-pyridyl) pyrrolidine (+)-bitartrate salt] was purchased from Sigma-Aldrich (St. Louis, MO). Morphine sulfate [morphine hemi(sulfate pentahydrate)] was supplied by the National Institute on Drug Abuse (Bethesda, MD). Metanicotine oxalate [(E)-N-methyl-4-(3-pyridinyl)-3-buten-1-amine oxalate] was synthesized as described by Acheson et al. (1980). (−)-Epibatidine [(±)-exo-2-(6-chloro-3-pyridinyl)-7-azabicyclo[2.2.1.]heptane] was supplied by Dr. S. Fletcher (Merck Sharp and Dohme, Essex, UK). Drugs were dissolved in physiological saline (0.9% sodium chloride) and injected subcutaneously at a volume of 10 ml/kg body weight unless noted otherwise. Doses are expressed as the free base of the drug, with the exception of morphine sulfate.
Acute Nicotine Assessment
Naive male mice were injected subcutaneously with various doses of nicotine. Antinociception using the tail-flick and hot-plate tests, locomotor activity, and body temperature were measured. Groups of 8 to 10 mice were used for each test.
Behavioral Tests.
Tail-flick test.
Mice were tested 5 min after a subcutaneous injection of nicotine, metanicotine, or epibatidine, or 20 min after a subcutaneous injection of morphine. Mice were lightly restrained by hand while a radiant heat light source was shone onto the upper portion of the tail. Latency to remove the tail from the heat source was recorded for each animal. A control response (2–4-s latency) was determined for each mouse before treatment, and test latency was determined after drug administration. To minimize tissue damage, a maximal latency of 10 s was imposed. Antinociceptive response was calculated as percentage maximal possible effect (%MPE), where %MPE = [(test value − baseline)/(cut-off time (10 s) − control value)] × 100, where baseline represents the value before nicotine or other drugs.
Hot-plate test.
Mice were tested 2 h before and 5 min after a subcutaneous injection of nicotine, or 20 min after morphine subcutaneous injection. The animals were placed on a 55°C platform (Harvard Apparatus Inc., Holliston, MA) and were observed until they started to show pain avoidance behavior such as jumping or licking of the paws. Animals that did not respond to the noxious heat stimulus after 40 s were removed from the plate. Latency to pain avoidance measured in seconds was used to calculate %MPE, with the following equation: [(test value − baseline)/(cut-off time (40 s) − baseline)] × 100. Baseline latency that lasted 8 to 12 s was assessed with a saline injection.
Locomotor activity.
Mice were placed into individual Omnitech photocell activity cages (28 × 16.5 cm) 5 min after subcutaneous administration of either saline or nicotine. Interruptions of the photocell beams (two banks of eight cells each) were then recorded for the next 10 min. Data are expressed as number of photocell interruptions.
Body temperature.
Rectal temperature was measured by a thermistor probe (inserted 24 mm) and digital thermometer (YSI Inc., Yellow Springs, OH). Readings were taken just before and at 30 min after the subcutaneous injection of either saline or nicotine. The difference in rectal temperature (Δ°C) before and after treatment was calculated for each mouse. The ambient temperature of the laboratory varied from 21 to 24°C from day to day.
Nicotine CPP Assessment
An unbiased CPP paradigm was used in this study as described in Kota et al. (2007). In brief, place-conditioning chambers consisted of two distinct compartments separated by a smaller intermediate compartment with openings that allowed access to either side of the chamber. On day 1, animals were confined to the intermediate compartment for a 5-min habituation period, and then they were allowed to move freely between compartments for 15 min. Time spent in each compartment was recorded. These data were used to separate the animals into groups of approximately equal bias. Days 2 to 4 were the conditioning days during which the saline group received saline in both compartments and drug groups received nicotine (subcutaneously) in one compartment and saline in the opposite compartment. Drug-paired compartments were randomized among all groups. Activity counts and time spent on each side were recorded via photosensors using interface and software (MED Associates, St. Albans, VT). Data were expressed as time spent on drug-paired side minus time spent on saline-paired side. A positive number indicated a preference for the drug-paired side, whereas a negative number indicated an aversion to the drug-paired side. A number at or near zero indicated no preference for either side.
Nicotine Discrimination
Male α5(−/−) and (+/+) mice (20–25 g) were housed individually in clear plastic cages (18 × 29 × 13 cm) with steel wire fitted tops and wood-chip bedding in a temperature-controlled (20–22°C) vivarium. Water was available ad libitum except while the mice were in the operant chambers. Training and test sessions were conducted at similar times during the light phase of a 12-h light/dark cycle. Mice were maintained at 85 to 90% of free-feeding body weights by restricting daily ration of standard rodent chow.
Apparatus.
Eight standard mice operant conditioning chambers (MED Associates) that were sound- and light-attenuated were used for behavioral training and testing. Each operant conditioning chamber (18 × 18 × 18 cm) was equipped with a house light, two levers (left and right), and a recessed dipper receptacle centered between the levers. A dipper arm delivered sweetened milk in a 0.05-ml cup, which was available for 5 s. Fan motors provided ventilation and masking noise for each chamber. House lights were illuminated during training and testing sessions. A computer with Logic “1” interface and MED-PC software (MED Associates) were used to control schedule contingencies and to record data.
Procedures.
Lever press training.
Each mouse was placed in a standard operant chamber and trained to lever press according to a fixed ratio (FR) 1 schedule of reinforcement. Milk reinforcement was delivered after every lever press. The FR value was gradually increased to the final FR10 schedule of reinforcement in which 10 consecutive responses were required for delivery of milk reinforcement. After mice were trained on one lever, contingency requirements for milk delivery were switched to the other lever. Lever press training at this second lever proceeded identically to that at the first lever. When responding on the second lever under a FR10 schedule was acquired, discrimination training began.
Discrimination training.
Mice were trained to press one lever after administration of 0.8 mg/kg nicotine and to press the other lever after saline administration according to a FR10 schedule of milk reinforcement. Each response on the incorrect lever reset the response requirement on the correct lever. Daily injections were administered on a double alternation sequence of nicotine and saline (e.g., drug, drug, vehicle, vehicle). Daily 15-min training sessions were held Monday–Friday until the mice had met two criteria during 8 of 10 consecutive sessions: 1) the first completed FR10 (e.g., consecutive correct responses ≥10) and 2) ≥80% of the total responding occurred on the correct lever. When these two criteria were met, acquisition of the discrimination was established and substitution testing began. For these studies, nicotine was administered subcutaneously 5 min before the start of the session at a volume of 0.01 ml/g body weight.
Rubidium (86Rb+) Efflux Assay
Materials.
Radioisotopes 86RbCl (4–10 Ci/mg) were purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). Acetylcholine iodide, atropine sulfate, NaCl, KCl, CaCl2, MgSO4, bovine serum albumin, tetrodotoxin, and glucose were obtained from Sigma-Aldrich. Sucrose was obtained from Thermo Fisher Scientific (Waltham, MA). HEPES and HEPES, sodium salt were products of BDH (Poole, Dorset, UK), obtained through VWR (West Chester, PA). CsCl was purchased from Research Products International (Arlington Heights, IL).
Synaptosomal Preparation.
Thalamus; hindbrain; and cervical, thoracic, and lumbar spinal cord were dissected from fresh mouse brains and spinal columns and homogenized in ice-cold isotonic sucrose (0.32 M) buffered with HEPES (5 mM; pH 7.5). The suspension was centrifuged at 12,000g for 20 min, and the pellet resuspended in the uptake buffer and used immediately.
86Rb+ Efflux.
Acetylcholine-stimulated 86Rb+ efflux from synaptosomes was investigated using the published methods of Marks et al. (1999, 2007) and Brown et al. (2007), with minor modifications. In brief, crude synaptosomes prepared from thalamus; hindbrain; and cervical, thoracic and lumbar spinal cord were resuspended in uptake buffer (140 mM NaCl, 1.5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 25 mM HEPES, pH 7.5, and 20 mM glucose) (350 μl/mouse thalamus). Aliquots (25 μl) of the suspension were added to 10 μl of uptake buffer containing 4 μCi of 86Rb+ and incubated at room temperature for 30 min. The whole sample was then collected onto filter paper (Type AE; Gelman Instrument Co., Ann Arbor, MI) and transferred to the perfusion apparatus, perfused with buffer (135 mM NaCl, 5 mM CsCl, 1.5 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 25 mM HEPES, pH 7.5, 20 mM glucose, 50 nM tetrodotoxin, 1 μM atropine, and 0.1% bovine serum albumin) at 2.5 ml/min for 5 min before data collection began. Stimulation by ACh was for 5 s. Effluent was pumped through a 200-μl Cherenkov cell in a β-Ram high-performance liquid chromatography detector (IN/US Systems, Tampa, FL) to continuously monitor radioactivity. Individual samples were exposed to a single concentration of ACh, and samples for each brain region were treated with ACh concentrations of 30 or 1000 μM to measure high-sensitivity and total nAChR-mediated 86Rb+ efflux, respectively. Low-sensitivity 86Rb+ efflux was estimated as the difference between total and high sensitivity 86Rb+ efflux. ACh concentrations used in these studies were determined from preliminary experiments in which complete ACh concentration-effect curves (0.1–1000 μM) were constructed.
125I-Epibatidine Binding Experiment
Tissue Preparation.
Mice were killed by cervical dislocation. Brains were removed and placed on ice, and the thalamus and hindbrain were dissected. The spinal column was isolated and divided into thoracic, cervical, and lumbar regions. Each segment of the spinal cord was removed from the spinal column by gentle flushing with ice-cold, isotonic saline. The dissected tissue was subsequently placed in hypotonic buffer (14 mM NaCl, 0.2 mM KCl, 0.2 mM CaCl2, 0.1 mM MgSO4, and 2 mM HEPES, pH 7.5). Samples were homogenized using a glass-Teflon tissue grinder. Homogenized samples were centrifuged at 10,000g for 20 min, and the supernatant was discarded. The pellet was resuspended in hypotonic buffer and centrifuged again at 10,000g for 20 min. This procedure was repeated two more times. The final washed pellet was resuspended in dilute buffer to a concentration of 1 to 2 mg/ml protein.
125I-Epibatidine Binding.
125I-Epibatidine (specific activity, 2200 Ci/mmol; PerkinElmer Life and Analytical Sciences) binding was conducted essentially as described previously (Marks et al., 1998). Samples were incubated for 3 h in 30 μl of buffer (135 mM NaCl, 2 mM KCl, 2 mM CaCl2, 1 mM MgSO4, and 0.02% bovine serum albumin at 20°C, 25 mM HEPES, pH 7.5) using a final 125I-epibatidine concentration of 400 pM. Cytisine-resistant binding was initially measured by constructing inhibition curves for cytisine (cytisine concentrations from 1 × 10−9 to 5 × 10−5 M) for two mice of each strain. Subsequently, cytisine-resistant sites were calculated from data obtained for binding in the presence of 0, 50, and 150 nM cytisine. Blanks were established using 0.1 mM nicotine. Blanks represented less than 5% of specific binding. After the incubation, samples were filtered using an Inotech Biosystems (Rockville, MD) harvester and two layers of glass fiber filter [top filter, type GC (Advantec MFS, Inc., Dublin, CA); bottom filter, A/E (Gelman Instrument Co.), both of which had been soaked in 0.1% polyethylenimine]. Samples were washed six times with ice-cold buffer without serum albumin. Samples were counted using a Tri-Carb liquid scintillation spectrometer (PerkinElmer Life and Analytical Sciences) at 45% efficiency after the addition of 1.5 ml of Budget Solve scintillation mixture (Sigma/RBI, Natick, MA).
Statistical Analyses
ED50 values with 95% confidence limits for acute tests were calculated by unweighted least-squares linear regression. For the body temperature studies, ED6°, the dose required to lower body temperature by 6°C,was calculated. If confidence limit values did not overlap, then the shift in the dose-response curve was considered significant. Statistical analyses for the nicotine CPP study were conducted using a two-way analysis of variance (ANOVA) with the StatView program (SAS Institute, Cary, NC). Treatment and genotype were used as between subject factors and Newman-Keuls post hoc test was used to further analyze significant effects. For drug discrimination studies, acquisition indices were the percentage of animals that pressed the first FR on the correct lever and achieved ≥80% of the total responding on the correct lever during the course of the session. For each test session, percentage of responses on the drug lever and response rate (responses per second) were calculated. ED50 values were calculated for percentage of responses on the nicotine lever using least-squares linear regression analysis followed by calculation of 95% confidence limits. Because mice that responded less than 10 times during a test session did not press either lever a sufficient number of times to earn a reinforcer, their data were excluded from analysis of nicotine lever selection, but their response rate data were included. Response-rate suppression (relative to rates after vehicle administration) was determined by separate ANOVAs using GB-STAT software (Dynamic Microsystems, Inc., Silver Spring, MD). Significant ANOVAs were further analyzed with Dunnett's post hoc tests (α = 0.05) to specify differences between means.
Results
Antinociceptive Activity of nAChR Agonists Is Absent in α5(−/−) Mice.
The antinociceptive effects of acute nicotine were measured in α5(+/+), α5 ±, and α5(−/−) mice using the tail-flick and hot plate tests. Results of the assessment are shown in Fig. 1. There was a dose-dependent increase in tail-flick (Fig. 1A) and hot plate latency (Fig. 1B) in both α5(+/+) and (−/+) mice after nicotine exposure; however, α5(−/−) mice were less sensitive to the effects, as observed by a significant shift to the right in the dose response curves for both tail-flick and hot-plate tests (Table 1). Confidence limits for α5(−/−) mice could not be determined, because the response in either test failed to reach 50% effect at the highest dose tested (4 mg/kg). Although there was a slight dose-dependent response in α5(−/−) mice in the hot-plate test, the effect was significantly less than that observed in α5(+/+) counterparts (Fig. 1B). Control responses were recorded and did not differ between α5(+/+) and (−/−) mice in either the tail-flick [2 ± 1% MPE [(+/+)] and 3 ± 1% MPE [(−/−)]] or hot-plate [10 ± 4% MPE [(+/+)] and 11 ± 6% MPE [(−/−])] tests. Antinociceptive activity was also measured in naive α5(+/+) and (−/−) mice using the tail-flick test after treatment with the nicotinic agonists metanicotine and epibatidine. Results are presented in Fig. 2. Although metanicotine (70 mg/kg s.c.) and epibatidine (10 mg/kg s.c.) produced significant antinociception in α5(+/+) (F5,32 = 56.05; p < 0.0001), this effect was absent in α5(−/−) mice. Baseline responses did not differ between α5(+/+) and (−/−) mice [tail-flick: 2.3 ± 0.1 [(+/+)] versus 2.3 ± 0.1 [(−/−)]; hot-plate: 11.4 ± 1.5 [(+/+)] versus 10.7 ± 0.7 [(−/−)]].
Fig. 1.
Nicotine antinociceptive activity α5(−/−) mice. Antinociception was measured in α5(+/+), (−/+), and (+/+) mice using the tail-flick and hot-plate tests. Acute nicotine induced a significant antinociceptive response in the tail-flick (A) and hot-plate (B) measures in both α5(+/+) and (−/+) mice; however, there was a gene-dosage effect, because α5(−/+) and (−/−) mice were progressively less sensitive to both behavioral measures compared with (+/+) counterparts, suggesting that α5(−/−) mice are less sensitive to the acute antinociceptive effects of nicotine. *, p > 0.05 versus the corresponding (+/+) group. Each point represents the mean ± S.E.M. of six to eight mice per group.
TABLE 1.
Summary of the potency of the effects of nicotine in the tail-flick and hot-plate tests after acute administration in α5(+/+) and (−/+) mice
Potency is expressed as ED50 ± CL (milligrams per kilogram). Each group contained 8 to 10 mice.
p < 0.05 denotes significance vs. α5 (+/+) mice.
Fig. 2.
Antinociceptive activity of nicotinic agonists in α5(−/−) mice using the tail-flick test. The nicotinic receptor agonists metanicotine (70 mg/kg s.c.) and epibatidine (10 mg/kg s.c.) significantly induced antinociception in the tail-flick test in α5(+/+) mice. This response was absent in α5(−/−) mice after treatment with both agonists. Control responses did not differ between genotypes. *, p > 0.05 versus the corresponding (+/+) group. Each point represents the mean ± S.E.M. of six to eight mice per group.
The specificity of this lack of antinociceptive effect in α5(−/−) mice was examined by measuring antinociceptive effects after acute morphine administration using the tail-flick and hot-plate tests. Results are shown in Fig. 3. Nicotine (2.5 mg/kg s.c.) and morphine sulfate (8 mg/kg s.c.) induced a significant antinociceptive response in both the tail-flick (F3,24 = 26.5; p < 0.0001) and hot-plate measures (F3,24 = 15.76; p < 0.0001) in α5(+/+) mice. Consistent with the results observed in Fig. 1, there was a lack of nicotine-induced antinociceptive effect in α5(−/−) mice in both tests. Alternatively, treatment with morphine produced a significant antinociceptive response in α5(−/−) mice that did not differ from α5(+/+) counterparts in either the tail-flick or hot-plate tests, suggesting that lack of an antinociceptive response in α5(−/−) mice is specific to nicotinic receptor agonists.
Fig. 3.
Antinociceptive effects of morphine in α5(−/−) mice. Antinociception was measured using the tail-flick and hot-plate tests in α5(+/+) and (−/−) mice after treatment with morphine sulfate (8 mg/kg s.c.) or nicotine (2.5 mg/kg s.c.). Acute nicotine-induced antinociception was observed in α5(+/+) mice, but absent in α5(−/−) mice in both behavioral measures. Conversely, morphine produced significant antinociception in both behavioral tests in α5(+/+) and α5(−/−) mice, suggesting that the loss of antinociception in α5(−/−) mice is specific to nicotine.*, p > 0.05 versus the corresponding (+/+) group. Each point represents the mean ± S.E.M. of six to eight mice per group.
α5(−/−) Mice Are Less Sensitive to the Locomotor and Body Temperature Effects of Acute Nicotine.
Nicotine's acute effects on locomotor activity and body temperature were measured in α5(+/+), α5(−/+), and α5(−/−) mice. Results show that both α5(+/+) and α5(−/−) mice exhibit dose-dependent decreases in locomotor activity (Fig. 4A); however, determination of ED50 values revealed that α5(+/+) mice were more sensitive to the locomotor depressing effects of nicotine than were α5(−/−) mice (Table 2). In the body temperature assessment, acute nicotine dose-dependently induced hypothermia in α5(+/+), (−/+), and (−/−) mice (Fig. 4B); however, there was a significant shift to the right for change in degrees Celsius in α5(−/−) mice, indicating that α5(+/+) and α5(−/+) mice are more sensitive to the hypothermic effects of nicotine than α5(−/−) mice (Table 2). Saline baseline responses did not differ between groups for any test [locomotor activity: 503 ± 24 [(+/+)] versus 492 ± 25 [(−/−)]; and body temperature: 36.8 ± 0.2 [(−/−)] versus 37.0 ± 0.1 [(+/+)]].
Fig. 4.
Acute nicotine effects on locomotor activity and body temperature in α5(−/−) mice. Nicotine dose-dependently reduced locomotor activity in α5(+/+) and (−/−) mice (A) and produced hypothermia in α5(+/+), (−/+), and (−/−) mice (B); however, observed effects were more potent in α5(+/+) compared with α5(−/+) and (−/−) mice, suggesting that α5(−/−) mice are less sensitive to the acute locomotor and hypothermia effects of nicotine. *, p > 0.05 versus the corresponding (+/+) group. Each point represents the mean ± S.E.M. of six to eight mice per group.
TABLE 2.
Summary of the potency of effects of nicotine in the locomotor activity and body temperature tests after acute administration in α5(+/+) and (−/+) mice
Potency is expressed as ED50 ± CL (milligrams per kilogram) for locomotor activity or ED6° ± CL (milligrams per kilogram) for body temperature. Each group contained 8 to 10 mice.
| Test | α5(+/+) | α5(−/+) | α5(−/−) |
|---|---|---|---|
| Locomotor activity | 0.44 (0.37–0.5) | N.A. | 1.2 (1.1–1.4)* |
| Body temperature | 1.3 (1.1–1.6) | 1.8 (1.5–2.0) | 5.1 (4.3–5.7)* |
N.A., not applicable.
p < 0.05 denotes significance vs. α5(+/+) and α5(−/+) mice.
α5(−/−) Mice Are Sensitive to the Rewarding Effects of Nicotine Measured by the CPP Model.
Evaluation of the α5 subunit in nicotine reward is shown in Fig. 5. There were significant main effects of treatment (F6,63 = 6.845; p < 0.0001), genotype (F6,63 = 6.236; p < 0.05), and a significant treatment × genotype interaction (F6,63 = 4.253; p < 0.05) in the CPP assessment. Nicotine produced a significant CPP in both α5(−/−) and α5(+/+) mice at 0.3 mg/kg (p < 0.001), 0.5 mg/kg (p < 0.001), and 1 mg/kg (p < 0.5). At higher doses of nicotine (2 and 3 mg/kg), CPP was not maintained in α5(+/+) mice; however, a significant CPP was present in α5(−/−) mice at these doses (Fig. 5). Overall, the α5(−/−) mice did not differ from their (+/+) counterparts at any nicotine doses tested, except at the highest doses, 2 and 3 mg/kg. Baseline responses and locomotor activity did not differ between genotypes.
Fig. 5.
Nicotine reward in α5(−/−) mice using the CPP model. Nicotine dose dependently produced a significant CPP in both α5(+/+) and (−/−) mice at 0.3, 0.5, and 1 mg/kg nicotine; however, preference for nicotine is maintained at high doses (2 and 3 mg/kg) in α5(−/−) mice but not in (+/+) littermates. Control responses did not differ between groups. *, p > 0.05 versus the corresponding saline group. Each point represents the mean ± S.E.M. of 10 mice per group. Sal, saline; nic, nicotine.
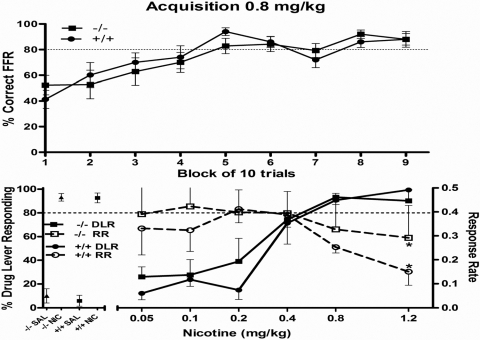
α5(−/−) Mice Do Not Differ from (+/+) Littermates in Sensitivity to Nicotine in a Discrimination Model.
Results of the nicotine discrimination assessment are shown in Fig. 6. Acquisition to criteria of the nicotine discrimination required an average of 67.2 (range, 41–103) for the wild-type mice and 60.8 training sessions (range, 41–95) for the α5(−/−) mice (Fig. 6A). Nicotine fully and dose-dependently substituted for itself with similar patterns of generalization in both (+/+) and α5(−/−) mice and with nearly identical ED50 values of 0.24 mg/kg (95% CL, 0.19–0.32) and 0.19 mg/kg (95% CL, 0.14–0.26), respectively (Fig. 6B). Repeated measures ANOVA conducted on the response rate data from the nicotine dose-effect curves resulted in significant differences as a function of dose for the (+/+) mice and (F5,25 = 5.7; p < 0.05) and α5(−/−) mice (F5,25 = 2.8; p < 0.05). Compared with responding after vehicle injections, response rates were significantly decreased by 1.2 mg/kg nicotine (p < 0.05) in both groups. No other significant changes in response rates for nicotine-treated mice were observed.
Fig. 6.
Nicotine discrimination in α5(−/−) mice. A, percentage of correct final fixed ratios calculated for (+/+) and α5(−/−) mice averaged in blocks of 10 trials. B, effects of nicotine on percentage of nicotine-lever responding (left axis) and response rates (right axis) in α5(−/−) and (+/+) mice trained to discriminate 0.8 mg/kg nicotine from vehicle. Left section of x-axis lists control tests with vehicle and 0.8 mg/kg nicotine conducted before the dose-effect determination. *, p < 0.05, significant decreases or increases in rates of responding compared with vehicle. For each dose-effect curve determination, values represent the mean ± S.E.M. of six mice.
86Rb+ Efflux and Epibatidine Binding in Mice Null for the α5, β2, α4, or β4 Subunits.
The effect of deletion of the α5 subunit on 86Rb+ efflux sensitivity to stimulation by low and high ACh concentrations and on cytisine-sensitive and cytisine-resistant 125I-epibatidine binding is shown in Fig. 7. 86Rb+ efflux stimulated by 30 μM ACh (high sensitivity) was significantly lower in the thalamus (62% reduction) and hindbrain (27% reduction) of α5(−/−) mice than in these regions of α5 (+/+) mice. Although high-sensitivity ACh-stimulated 86Rb+ efflux of α5(−/−) mice tended to be somewhat lower (approximately 15%) than that of α5(+/+) mice in the three regions of the spinal cord, these apparent differences were not statistically significant. Deletion of the α5 subunit had no significant effect on 86Rb+ efflux requiring higher ACh concentrations for activation. Furthermore, deletion of the α5 subunit had no significant effect on either cytisine-sensitive or cytisine-resistant 125I-epibatidine binding in any of the preparations.
Fig. 7.
86Rb+ efflux and epibatidine binding in mice null for the α5 nAChR subunit. 86Rb+ efflux stimulated by 30 μM ACh (top, black bars) was significantly lower in the thalamus and hindbrain of α5(−/−) mice than in these regions of α5(+/+) mice. Deletion of the α5 nAChR subunit had no significant effect on 86Rb+ efflux requiring higher ACh concentrations (1000 μM; top, gray bars) for activation. Furthermore, deletion of the α5 nAChR subunit had no significant effect on either cytisine-sensitive (bottom, black bars) or cytisine-resistant 125I-epibatidine binding (bottom, gray bars) in any region analyzed. *, p > 0.05 versus the corresponding (+/+) group. Each point represents the mean ± S.E.M. of three to five mice per group.
We have demonstrated previously (Marubio et al., 1999) that deletion of either the β2 or α4 subunit significantly reduced the effectiveness of nicotine's potency as an antinociceptive. To provide a comparison to the effects of α5 gene deletion on ACh-stimulated 86Rb+ efflux and 125I-epibatidine binding these parameters were measured in thalamus, hindbrain, cervical spinal cord, thoracic spinal cord, and lumbar spinal cord. Results for the β2 gene are shown in Fig. 8 and those for the α4 gene in Fig. 9.
Fig. 8.
86Rb+ efflux and epibatidine binding in mice null for the β2 nAChR subunit. 86Rb+ efflux stimulated by either low (30 μM; top, black bars) or high (1000 μM; top, gray bars) concentrations of ACh was eliminated in β2(−/−) mice in all five tissues examined. Likewise, deletion of β2 virtually eliminated cytisine-sensitive, high affinity 125I-epibatidine binding (bottom, black bars) in every sample and reduced the number of cytisine-resistant 125I-epibatidine binding sites (bottom, gray bars); however, cytisine-resistant 125I-epibatidine binding sites persisted in the cervical and lumbar spinal cord of β2(−/−) mice. *, p > 0.05 versus the corresponding (+/+) group. Each point represents the mean ± S.E.M. of three to five mice per group.
Fig. 9.
86Rb+ efflux and epibatidine binding in mice null for the α4 nAChR subunit. Deletion of the α4 nAChR subunit induced significant reductions in both high (1000 μM; top, gray bars) and low (30 μM; top, black bars) ACh-sensitive 86Rb+ efflux stimulation. Cytisine-sensitive 125I-epibatidine binding sites (bottom, black bars) were virtually eliminated in α4(−/−) mice compared with (+/+) counterparts, with significant, partial reductions in the cytisine-resistant 125I-epibatidine binding sites (bottom, gray bars). *, p > 0.05 versus the corresponding (+/+) group. Each point represents the mean ± S.E.M. of three to five mice per group.
Deletion of the β2-nAChR gene significantly reduced 86Rb+ efflux stimulated by either low (30 μM) or high (1000 μM) concentrations of ACh in all five tissues examined (Fig. 8) The effect of β2 gene deletion on the high-sensitivity component ranged from 95% in thalamus to 78% in thoracic spinal cord. Likewise, deletion of β2 significantly reduced the low-sensitivity component in all five tissues. However, it should be noted that significant activity remained in every sample, except thalamus, indicating that some of the ACh-stimulated 86Rb+ efflux was not mediated by β2* nAChR. Deletion of β2 virtually eliminated cytisine-sensitive, high-affinity 125I-epibatidine binding in every sample and reduced the number of cytisine-resistant 125I-epibatidine binding sites as well. However, cytisine-resistant 125I-epibatidine binding sites persisted in the cervical and lumber spinal cord of β2(−/−) mice.
The effects of deletion of the α4 nAChR gene were very similar to those of β2 gene deletion: significant reductions in both high and low ACh-sensitive 86Rb+ efflux and virtual elimination of cytisine-sensitive 125I-epibatidine binding sites and with significant, partial reductions in the cytisine-resistant 125I-epibatidine binding sites (Fig. 9).
Because α5 nAChRs can coassemble with α3β4* nAChR subtypes, we evaluated the effect of β4 nAChR subunit deletion on receptor function and binding (Supplemental Fig. 1). There was no statistically significant change in either high or low ACh-sensitive 86Rb+ efflux after deletion of the β4 nAChR subunit. However, there was a trend toward a decrease in the low-sensitivity component in hindbrain and the three spinal cord regions. Cytisine-sensitive125I-epibatidine binding sites were unaltered in all areas tested; however, a significant reduction in cytisine-resistant 125I-epibatidine binding sites in the hindbrain and cervical and thoracic spinal cord was observed.
Discussion
The goal of this study was investigate the role of the α5 nAChR subunit in various aspects of nicotine dependence. The results of this study suggest that α5 nAChRs are involved in the initial pharmacological effects of nicotine and contribute to nicotine reward but are not involved in sensitivity to the discriminative stimulus effects of nicotine.
Assessment of the initial behavioral effects of nicotine in α5(−/−) mice revealed a reduction in nicotine-induced spinal and supraspinal antinociceptive tests as measured by the tail-flick and hot-plate tests, respectively. There was a gene-dosage effect for the antinociceptive response, because α5(−/+) mice were less sensitive to the antinociceptive effects of nicotine than (+/+) counterparts, and the response was less than 20% in α5(−/−) mice, significantly less than both α5(−/+) and (+/+) counterparts. Likewise, antinociception induced by the nicotinic agonists metanicotine and epibatidine was lost in α5(−/−) mice. Morphine, however, induced antinociception in both α5(−/−) and (+/+) mice, suggesting that opiate antinociception does not involve α5-containing nAChRs and that the effect is specific to the nicotinic system. α5(−/−) mice were also found to be less sensitive to the acute nicotine-induced locomotor depression and hypothermia than (+/+) littermates. There was no difference in baseline latencies for antinociception, hypomotility, or hypothermia, indicating that α5 nAChRs are not tonically involved in these effects. Taken together, our results suggest that the α5 nAChR subunit mediates the initial behavioral effects of nicotine. Our data also compliment previous studies showing that α5(−/−) mice are resistant to acute nicotine-induced seizures (Salas et al., 2003; Kedmi et al., 2004) and hypolocomotion (Salas et al., 2003). The current study extends these findings to evaluate the role of the α5 nAChR in nicotine's initial effects on antinociception and hypothermia. It is noted in our results that the most pronounced differences were observed in the antinociceptive tests compared with locomotor activity and body temperature tests. Although epibatidine is a nonselective nAChR agonist, metanicotine has been reported to be α4β2*-selective, supporting a role for the α4β2α5 nAChR subtype in nicotine's acute antinociceptive effects. Indeed, previous studies suggest a role for the α4β2* nAChR subtype in nicotine-induced antinociception (Marubio et al., 1999), as well as in antinociception induced by the nicotinic agonists metanicotine (Damaj et al., 1999) and epibatidine (Damaj et al., 1998). The α5 subunit coassembles with α4β2 nAChRs in the brain to form functional receptors (Mao et al., 2008). These results may have some implication for α5-containing nAChRs, specifically the α4β2α5 subtype, as a therapeutic target for nicotinic analgesic agents.
Rb86+ efflux and epibatidine binding were also conducted in brain and spinal samples prepared from mice null for the α5, α4, β2, or β4 nAChR subunits. Receptor number and function were altered after deletion of the α4 and β2 subunits, an observation consistent with the expression of α4β2* nAChRs in the thalamus, hindbrain, and throughout the spinal cord. These results are also consistent with a role for α4β2* nAChRs in nicotinic modulation of pain, as demonstrated previously by Damaj et al., (2007). Furthermore, consistent with previous results from Brown et al. (2007), deletion of the α5 nAChR subunit reduced function in the thalamus and hindbrain in our studies, yet it had no significant effect on binding. Although the α4 and β2 Rb86+ efflux and binding data provide a clear explanation for the effects on nicotine-induced antinociception (Damaj et al., 2007), functional data for the α5 deletion is not as lucid. Function was significantly decreased in the thalamus for the α4, α5, and β2 deletions, which may provide some explanation of the decreased antinociception in α5(−/−) mice in the hot-plate test, and also may support our hypothesis concerning α4β2α5 nAChR subtype involvement in nicotine supraspinal antinociception. However, surprisingly, there was no statistically significant change in function in the spinal cord with the α5 deletion, although decreased antinociception was also observed in α5(−/−) mice in the tail-flick test. It is noted that all three spinal regions showed an approximate 15% decrease in α5(−/−) mice. Although this decrease may be important, it did not reach statistical significance. Thus, in contrast to α4 and β2, in vitro functional data for the α5 deletion does not correlate with its effects on antinociception. Furthermore, although the α5 subunit was found to be exclusively expressed with α4β2 nAChRs in the thalamus (Mao et al., 2008), the possibility of a role for α3β4α5 nAChR subtypes in the spinal cord cannot be ruled out because significant residual activity persists in spinal cords of β2(−/−) and α4(−/−) mice. Indeed, data on brain and spinal cord regions of β4(−/−) mice revealed no change in receptor function stimulated at low agonist concentrations in any brain or spinal cord region tested; yet, there was a significant decrease in cytisine-resistant binding sites in the hindbrain and cervical and thoracic spinal cord. Although it is possible that the decrease in receptor expression in these regions may indicate that β4-containing nAChRs are relevant to pain modulation pathways (suggesting a potential role for α3β4α5 nAChR subtypes in nicotine-induced antinociception, particularly at high agonist doses or with selective agonists), future behavioral studies are necessary to confirm this hypothesis.
The results of nicotine reward in the CPP assay are particularly interesting. Indeed, at lower doses, α5(−/−) mice did not differ from (+/+) counterparts in the rewarding effects of nicotine; however, at higher nicotine doses, a significant CPP was maintained in α5(−/−) mice but not in (+/+) littermates, suggesting a role for α5 nAChRs in nicotine reward. These results reflect an overall enhancement of reward in the absence of the α5 nAChR subunit. This increase in nicotine-induced CPP could also reflect a decreased aversive response in α5(−/−) mice; however, we recently reported that α5 nAChR subunits are not involved in the aversion associated with nicotine withdrawal (Jackson et al., 2008) as measured by conditioned place aversion. In addition, it is possible that a decrease in some of the acute effects of nicotine involved in conditioning behavior (such as motor function, anxiety, or toxicity-related effects on behavior) could explain the increase in CPP behavior. Indeed, our results show that α5(−/−) mice are less sensitive to several of the initial effects of nicotine, which may explain the sustained presence of nicotine reward at doses that are ineffective in (+/+) mice. In contrast to the CPP assessment, results show that the α5 nAChR subunit is not involved in the discriminative stimulus effects of nicotine, because α5(−/−) mice did not differ from (+/+) counterparts at any dose tested. The β2 and α4 nAChR are involved in nicotine reward (Tapper et al., 2004; Walters et al., 2006) as well as nicotine discrimination (Shoaib et al., 2002; Smith et al., 2007). Based on our results, the α4β2α5* nAChR subtype does not play a role in the discriminative stimulus effects of nicotine, but it may be a candidate in mediating nicotine reward.
Several lines of evidence support a role for an association between nicotine dependence phenotypes and polymorphisms in the CHRNA5/CHRNA3/CHRNB4 gene cluster. In vitro studies show that α4β2α5 nAChRs containing the CHRNA5 amino acid substitution exhibit reduced responses to nicotinic agonists (Bierut et al., 2008). The current and previous reports show that α5(−/−) mice are less sensitive to the initial effects of nicotine. Furthermore, mice null for the α5 subunit continue to express nicotine reward at doses that are ineffective in (+/+) mice. The α5 nAChR subunit was also found to be involved in the somatic signs associated with nicotine withdrawal (Jackson et al., 2008; Salas et al., 2009). Taken together with the current data, it is possible that individuals with the CHRNA5 polymorphism, which renders α5-containing nAChRs less responsive to nicotinic agonists, may in part contribute to decreased sensitivity to the initial effects of nicotine, a decrease in the aversion associated with high doses of nicotine or enhanced nicotine reward, as well as a decrease in aspects of the nicotine withdrawal syndrome. Any or a combination of all of the above-mentioned possibilities could lead to increased nicotine use to obtain the desired effect, and/or increased tolerance to nicotine, contributing to development of nicotine dependence.
Overall, the results of this study provide insight into the role of α5 nAChRs in nicotine dependence. Further research in this area could lead to the development of better, more specific smoking cessation therapies.
Supplementary Material
Acknowledgments
We thank Tie Shan-Han for technical assistance in the acute nicotine studies and Lisa Merritt and Cindy Evans for technical assistance and maintenance of the breeding colony.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA003194 (to M.J.M.), DA12610, DA05274 (both to M.I.D.), DA019498 (to X.C.), DA015663 (to Al Collins, Institute for Behavioral Genetics, University of Colorado, Boulder, CO)].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.165738.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- ACh
- acetylcholine
- nAChR
- nicotinic acetylcholine receptor
- (+/+)
- wild-type
- (−/+)
- heterozygote
- (−/−)
- knockout
- CPP
- conditioned place preference
- %MPE
- percentage maximal possible effect
- FR
- fixed ratio
- CL
- confidence limit(s)
- ANOVA
- analysis of variance.
References
- Acheson RM, Ferris MJ, Sinclair NM. (1980) Transformations involving the pyrrolidine ring of nicotine. J Chem Soc 2:579–585 [Google Scholar]
- Berrettini W, Yuan X, Tozzi F, Song K, Francks C, Chilcoat H, Waterworth D, Muglia P, Mooser V. (2008) Alpha-5/alpha-3 nicotinic receptor subunit alleles increase risk for heavy smoking. Mol Psychiatry 13:368–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, et al. (2008) Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry 165:1163–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broide RS, Salas R, Ji D, Paylor R, Patrick JW, Dani JA, De Biasi M. (2002) Increased sensitivity to nicotine-induced seizures in mice expressing the L250T alpha 7 nicotinic acetylcholine receptor mutation. Mol Pharmacol 61:695–705 [DOI] [PubMed] [Google Scholar]
- Brown RW, Collins AC, Lindstrom JM, Whiteaker P. (2007) Nicotinic α5 subunit deletion locally reduces high-affinity agonist activation without altering nicotinic receptor numbers. J Neurochem 103:204–215 [DOI] [PubMed] [Google Scholar]
- Damaj MI, Fei-Yin M, Dukat M, Glassco W, Glennon RA, Martin BR. (1998) Antinociceptive responses to nicotinic acetylcholine receptor ligands after systemic and intrathecal administration in mice. J Pharmacol Exp Ther 284:1058–1065 [PubMed] [Google Scholar]
- Damaj MI, Fonck C, Marks MJ, Deshpande P, Labarca C, Lester HA, Collins AC, Martin BR. (2007) Genetic approaches identify differential roles for α4β2* nicotinic receptors in acute models of antinociception in mice. J Pharmacol Exp Ther 321:1161–1169 [DOI] [PubMed] [Google Scholar]
- Damaj MI, Glassco W, Aceto MD, Martin BR. (1999) Antinociceptive and pharmacological effects of metanicotine, a selective nicotinic agonist. J Pharmacol Exp Ther 291:390–398 [PubMed] [Google Scholar]
- De Biasi M. (2002) Nicotinic receptor mutant mice in the study of autonomic function. Curr Drug Targets CNS Neurol Disord 1:331–336 [DOI] [PubMed] [Google Scholar]
- Flora A, Schulz R, Benfante R, Battaglioli E, Terzano S, Clementi F, Fornasari D. (2000) Neuronal and extraneuronal expression and regulation of the human alpha5 nicotinic receptor subunit gene. J Neurochem 75:18–27 [DOI] [PubMed] [Google Scholar]
- Gerzanich V, Wang F, Kuryatov A, Lindstrom J. (1998) α5 Subunit alters desensitization, pharmacology, Ca2+ permeability and Ca2+ modulation of human neuronal α3 nicotinic receptors. J Pharmacol Exp Ther 286:311–320 [PubMed] [Google Scholar]
- Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. (2007) Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol 74:1102–1111 [DOI] [PubMed] [Google Scholar]
- Grady SR, Salminen O, McIntosh JM, Marks MJ, Collins AC. (2010) Mouse striatal dopamine nerve terminals express alpha4alpha5beta2 and two stoichiometric forms of alpha4beta2*-nicotinic acetylcholine receptors. J Mol Neurosci 40:91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI. (2008) Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J Pharmacol Exp Ther 325:302–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedmi M, Beaudet AL, Orr-Urtreger A. (2004) Mice lacking the neuronal nicotinic acetylcholine receptor β4-subunit and mice lacking both the α5- and β4-subunits are highly resistant to nicotine-induced seizures. Physiol Genomics 17:221–229 [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. (2007) Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther 322:399–407 [DOI] [PubMed] [Google Scholar]
- Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. (2008) The α4β2α5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem 104:446–456 [DOI] [PubMed] [Google Scholar]
- Marks MJ, Meinerz NM, Drago J, Collins AC. (2007) Gene targeting demonstrates that alpha4 nicotinic acetylcholine receptor subunits contribute to expression of diverse [3H]epibatidine binding sites and components of biphasic 86Rb+ efflux with high and low sensitivity to stimulation by acetylcholine. Neuropharmacology 53:390–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. (1992) Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci 12:2765–2784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Smith KW, Collins AC. (1998) Differential agonist inhibition identifies multiple epibatidine binding sites in mouse brain. J Pharmacol Exp Ther 285:377–386 [PubMed] [Google Scholar]
- Marks MJ, Whiteaker P, Calcaterra J, Stitzel JA, Bullock AE, Grady SR, Picciotto MR, Changeux JP, Collins AC. (1999) Two pharmacologically distinct components of nicotinic receptor-mediated rubidium efflux in mouse brain require the beta2 subunit. J Pharmacol Exp Ther 289:1090–1103 [PubMed] [Google Scholar]
- Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Léna C, Le Novère N, de Kerchove d'Exaerde A, Huchet M, Damaj MI, Changeux JP. (1999) Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature 398:805–810 [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP. (1998) Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature 391:173–177 [DOI] [PubMed] [Google Scholar]
- Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L. (1996) Functional contributions of α5 subunit to neuronal acetylcholine receptor channels. Nature 380:347–351 [DOI] [PubMed] [Google Scholar]
- Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, Scheffer IE, Kola I, Waddington JL, Berkovic SF, et al. (2000) Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J Neurosci 20:6431–6441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, et al. (2007) Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet 16:36–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Orr-Urtreger A, Broide RS, Beaudet A, Paylor R, De Biasi M. (2003) The nicotinic acetylcholine receptor subunit alpha 5 mediates short-term effects of nicotine in vivo. Mol Pharmacol 63:1059–1066 [DOI] [PubMed] [Google Scholar]
- Salas R, Sturm R, Boulter J, De Biasi M. (2009) Nicotinic receptors in the habenulo-interpeduncular system are necessary for nicotine withdrawal in mice. J Neurosci 29:3014–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. (2004) Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol 65:1526–1535 [DOI] [PubMed] [Google Scholar]
- Shoaib M, Gommans J, Morley A, Stolerman IP, Grailhe R, Changeux JP. (2002) The role of nicotinic receptor beta-2 subunits in nicotine discrimination and conditioned taste aversion. Neuropharmacology 42:530–539 [DOI] [PubMed] [Google Scholar]
- Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P, Tricklebank M. (2007) Ligands selective for alpha4beta2 but not alpha3beta4 or alpha7 nicotinic receptors generalise to the nicotine discriminative stimulus in the rat. Psychopharmacology (Berl) 190:157–170 [DOI] [PubMed] [Google Scholar]
- Tapia L, Kuryatov A, Lindstrom J. (2007) Ca2+ permeability of the (α4)3(β2)2 stoichiometry greatly exceeds that of (α4)2(β2)3 human acetylcholine receptors. Mol Pharmacol 71:769–776 [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. (2004) Nicotine activation of α4* receptors: sufficient for reward, tolerance, and sensitization. Science 306:1029–1032 [DOI] [PubMed] [Google Scholar]
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, Manolescu A, Thorleifsson G, Stefansson H, Ingason A, et al. (2008) A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 452:638–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E, McKinnon D, Heinemann S, Patrick J, Swanson LW. (1990) The distribution of mRNA encoded by a new member of the neuronal nicotinic acetylcholine receptor gene family (alpha 5) in the rat central nervous system. Brain Res 526:45–53 [DOI] [PubMed] [Google Scholar]
- Walters CL, Brown S, Changeux JP, Martin B, Damaj MI. (2006) The β2 but not α7 subunit of the nicotinic acetylcholine receptor is required for nicotine-conditioned place preference in mice. Psychopharmacology 184:339–344 [DOI] [PubMed] [Google Scholar]
- Wang F, Gerzanich V, Wells GB, Anand R, Peng X, Keyser K, Lindstrom J. (1996) Assembly of human neuronal nicotinic receptor α5 subunits with α3, β2, and β4 subunits. J Biol Chem 271:17656–17665 [DOI] [PubMed] [Google Scholar]
- Weiss RB, Baker TB, Cannon DS, von Niederhausern A, Dunn DM, Matsunami N, Singh NA, Baird L, Coon H, McMahon WM, et al. (2008) A candidate gene approach identified the CHRNA5–A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet 4:e1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Orr-Urtreger A, Nigro F, Gelber S, Sutcliffe CB, Armstrong D, Patrick JW, Role LW, Beaudet AL, De Biasi M. (1999) Multiorgan autonomic dysfunction in mice lacking the beta2 and the beta4 subunits of neuronal nicotinic acetylcholine receptors. J Neurosci 19:9298–9305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Moretti M, Zanardi A, McIntosh JM, Clementi F, Gotti C. (2002) Identification of the nicotinic receptor subtypes expressed on dopaminergic terminals in the rat striatum. J Neurosci 22:8785–8789 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.