Abstract
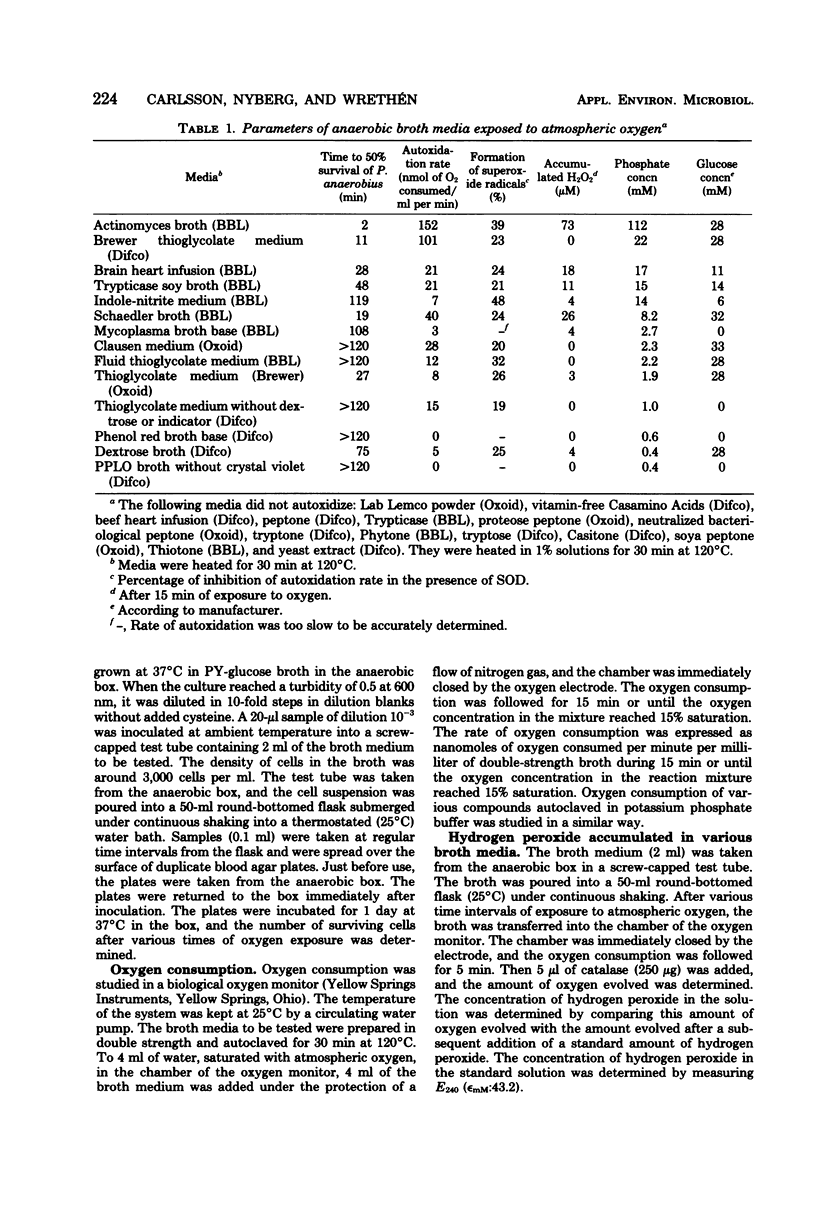
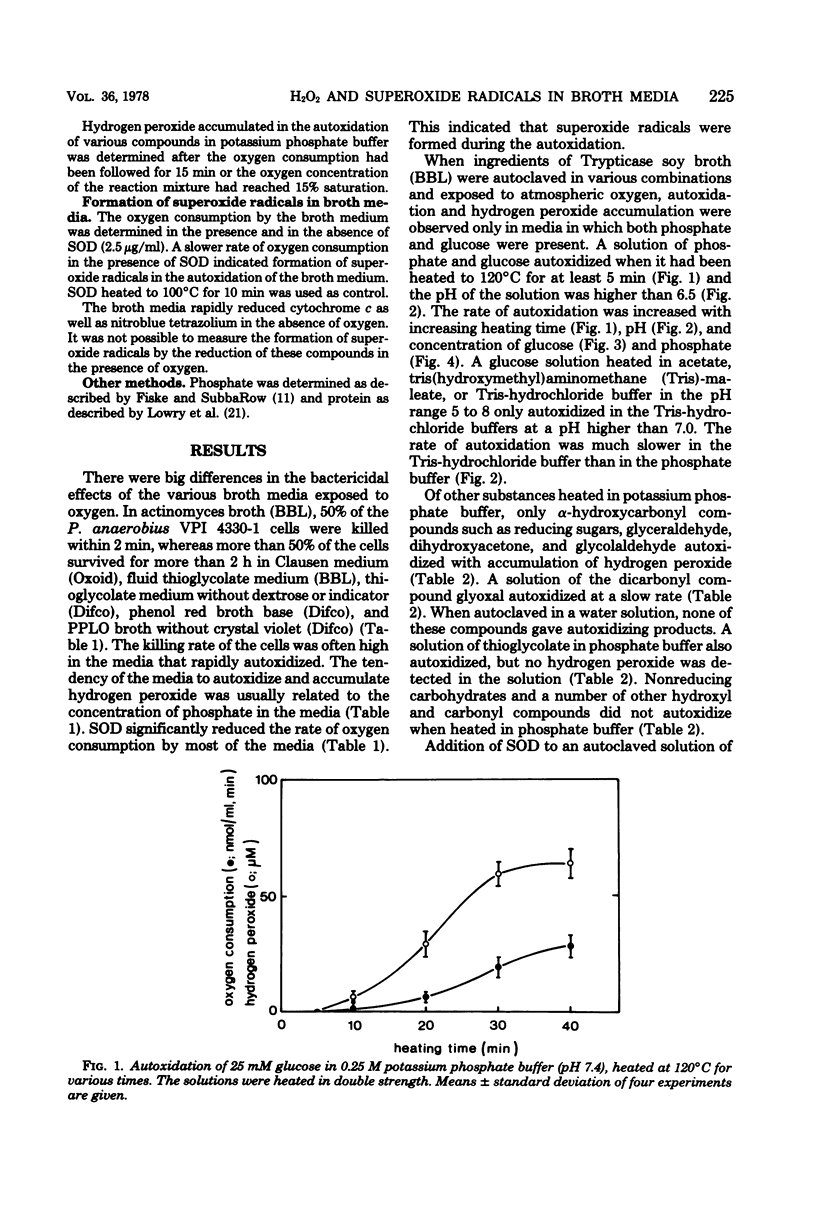
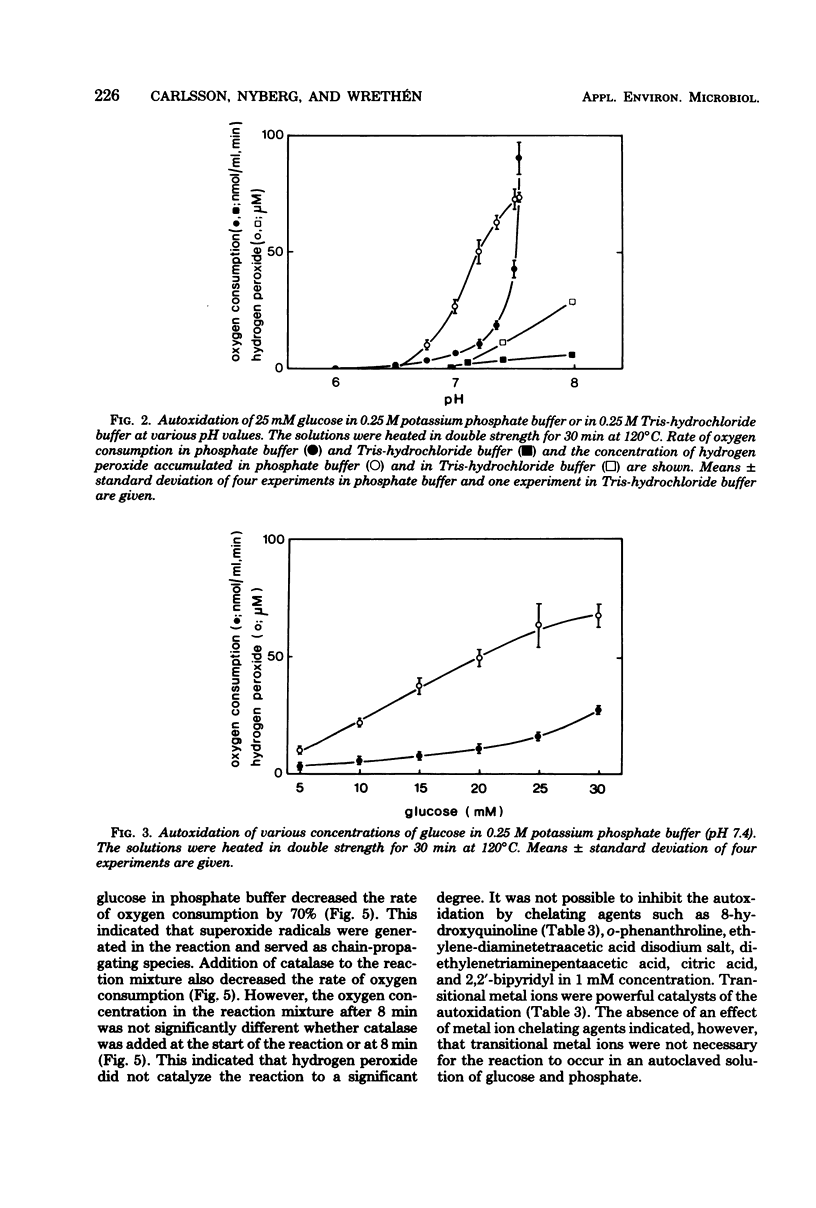
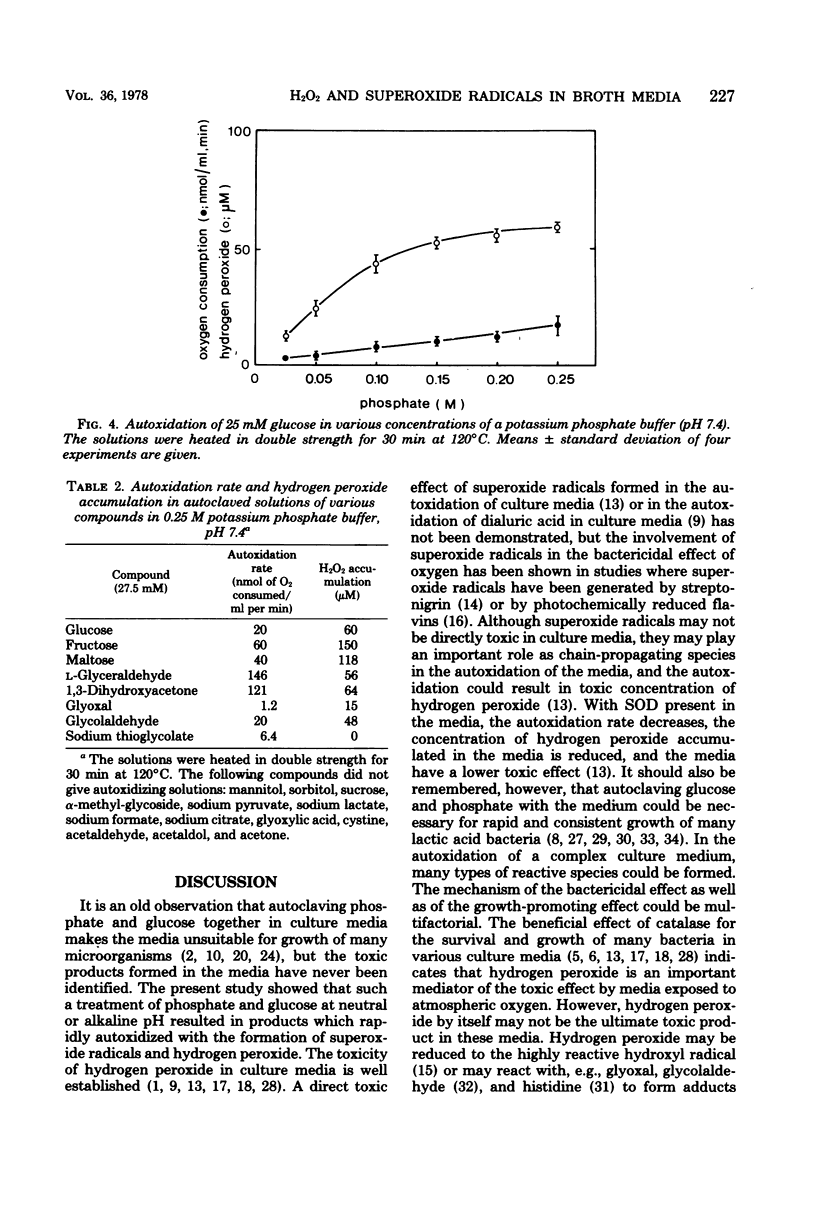
Fourteen different broth media were autoclaved under anaerobic conditions and then exposed to atmospheric oxygen. The hydrogen peroxide and superoxide radical formation as well as the bactericidal effect of the media were studied. The rate of killing of Peptostreptococcus anaerobius VPI 4330-1 was high in media that rapidly autoxidized and accumulated hydrogen peroxide. In actinomyces broth (BBL), 50% of the cells were killed within 2 min, and in Brewer thioglycolate medium (Difco), 50% were killed within 11 min, whereas more than 50% of the cells survived for more than 2 h in Clausen medium (Oxoid), fluid thioglycolate medium (BBL), and thioglycolate medium without dextrose or indicator (Difco). Only media that contained phosphate and glucose had a tendency to accumulate hydrogen peroxide. A solution of phosphate and glucose autoxidized when it had been heated to 120 degrees C for at least 5 min and when the pH of the solution was higher than 6.5. Transitional metal ions catalyzed the autoxidation, but they were not necessary for the reaction to occur. Of the other substances heated in phosphate buffer, only alpha-hydroxycarbonyl compounds autoxidized with accumulation of hydrogen peroxide. Superoxide dismutase decreased the autoxidation rate of most of the broth media. This indicated that superoxide radicals were generated in these media.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRY V. C., CONALTY M. L., DENNENY J. M., WINDER F. Peroxide formation in bacteriological media. Nature. 1956 Sep 15;178(4533):596–597. doi: 10.1038/178596a0. [DOI] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Baumgartner J. G. Heat Sterilised Reducing Sugars and Their Effects on the Thermal Resistance of Bacteria. J Bacteriol. 1938 Oct;36(4):369–382. doi: 10.1128/jb.36.4.369-382.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Frölander F., Sundquist G. Oxygen tolerance of anaerobic bacteria isolated from necrotic dental pulps. Acta Odontol Scand. 1977;35(3):139–145. doi: 10.3109/00016357709056002. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., Goodson P. R., McCall C. E. Bactericidal activity of superoxide anion and of hydrogen peroxide: investigations employing dialuric acid, a superoxide-generating drug. Antimicrob Agents Chemother. 1975 Aug;8(2):146–153. doi: 10.1128/aac.8.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINKELSTEIN R. A., LANKFORD C. E. A bacteriotoxic substance in autoclaved culture media containing glucose and phosphate. Appl Microbiol. 1957 Mar;5(2):74–79. doi: 10.1128/am.5.2.74-79.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frölander F., Carlsson J. Bactericidal effect of anaerobic broth exposed to atmospheric oxygen tested on Peptostreptococcus anaerobius. J Clin Microbiol. 1977 Aug;6(2):117–123. doi: 10.1128/jcm.6.2.117-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen metabolism in Lactobacillus plantarum. J Bacteriol. 1974 Jan;117(1):166–169. doi: 10.1128/jb.117.1.166-169.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen toxicity and the superoxide dismutase. J Bacteriol. 1973 Jun;114(3):1193–1197. doi: 10.1128/jb.114.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Yost F. J., Jr, Fridovich I. Superoxide dismutases of Escherichia coli: intracellular localization and functions. J Bacteriol. 1973 Sep;115(3):987–991. doi: 10.1128/jb.115.3.987-991.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon S. M., Kautter D. A. Beneficial effect of catalase treatment on growth of Clostridium perfringens. Appl Environ Microbiol. 1976 Sep;32(3):409–416. doi: 10.1128/aem.32.3.409-416.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill G. B., Osterhout S. Experimental effects of hyperbaric oxgen on selected clostridial species. I. In-vitro studies. J Infect Dis. 1972 Jan;125(1):17–25. doi: 10.1093/infdis/125.1.17. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis I. M. THE INHIBITION OF PHYTOMONAS MALVACEARA IN CULTURE MEDIA CONTAINING SUGARS. J Bacteriol. 1930 Jun;19(6):423–433. doi: 10.1128/jb.19.6.423-433.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marklund S. Interactions between hydroxymethylhydroperoxide and catalase. Biochim Biophys Acta. 1972 Dec 7;289(2):269–275. doi: 10.1016/0005-2744(72)90077-0. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeen W. E. Interaction Product of Glycine and Dextrose Toxic to Phytophthora fragariae. Science. 1956 Mar 23;123(3195):509–509. doi: 10.1126/science.123.3195.509. [DOI] [PubMed] [Google Scholar]
- PROOM H., WOIWOD A. J., BARNES J. M., ORBELL W. G. A growth-inhibitory effect on Shigella dysenteriae which occurs with some batches of nutrient agar and is associated with the production of peroxide. J Gen Microbiol. 1950 May;4(2):270–276. doi: 10.1099/00221287-4-2-270. [DOI] [PubMed] [Google Scholar]
- ROGERS D., KING T. E., CHELDELIN V. H. Growth stimulation of Lactobacillus gayoni by N-D-glucosylglycine. Proc Soc Exp Biol Med. 1953 Jan;82(1):140–144. doi: 10.3181/00379727-82-20046. [DOI] [PubMed] [Google Scholar]
- Schubert J., Watson J. A., Baecker J. M. Formation of a histidine-peroxide adduct by H2O2 or ionizing radiation on histidine: chemical and microbiological properties. Int J Radiat Biol Relat Stud Phys Chem Med. 1969 Jan 17;14(6):577–583. doi: 10.1080/09553006914551771. [DOI] [PubMed] [Google Scholar]
- Schubert J., Watson J. A., White E. R. Hydroxyalkyl peroxides and the toxicity of irradiated sucrose. Int J Radiat Biol Relat Stud Phys Chem Med. 1967;13(5):485–489. doi: 10.1080/09553006814550511. [DOI] [PubMed] [Google Scholar]
- Smiley K. L., Niven C. F., Sherman J. M. The Nutrition of Streptococcus salivarius. J Bacteriol. 1943 May;45(5):445–454. doi: 10.1128/jb.45.5.445-454.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEITZEL G., BUDDECKE E., SCHNEIDER F. [Cytostatic effect of organic peroxide compounds on ascites tumor cells]. Hoppe Seylers Z Physiol Chem. 1961 May 3;323:211–235. doi: 10.1515/bchm2.1961.323.1.211. [DOI] [PubMed] [Google Scholar]
- Yamada T., Carlsson J. Regulation of lactate dehydrogenase and change of fermentation products in streptococci. J Bacteriol. 1975 Oct;124(1):55–61. doi: 10.1128/jb.124.1.55-61.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



