Abstract
Although ethanol has been considered to be an anxiolytic agent, consumption of ethanol has also been shown to increase plasma adrenocorticotropin and glucocorticoids. The corticotrophin-releasing factor (CRF) receptor 1α (CRF-R1) is a G protein-coupled receptor that activates adenylyl cyclase (AC), leading to adrenocorticotropin (and subsequently glucocorticoid) release into the circulation. There are nine members of the membrane-bound AC family, and the type 7 AC (AC7) is most sensitive to ethanol, which enhances the responsiveness of AC7 to G protein-coupled receptor activation. We determined the time course of ethanol's effect on plasma adrenocorticotropin and corticosterone levels in male and female AC7 transgenic (Adcy7huTG) mice (in which AC7 is overexpressed in neural tissue) and AC7 heterozygous knockdown [Adcy7(+/−)] mice (in which AC7 is underexpressed in neural tissue), and their respective littermate controls [wild type (WT)]. CRF-R1 mRNA and mRNA and protein for different forms of ACs were measured by using gene expression arrays, quantitative reverse transcription-polymerase chain reaction, and immunoblotting in pituitaries of all animals. Our results demonstrated increased levels of AC7 in pituitary of Adcy7huTG mice and decreased levels in pituitary of Adcy7(+/−) mice compared with WT animals. Male and female Adcy7huTG mice displayed higher plasma adrenocorticotropin and corticosterone levels than WT and/or Adcy7(+/−) mice after ethanol injection. Female mice displayed higher adrenocorticotropin and corticosterone levels after ethanol injection than males, regardless of genotype. The data provide evidence for an integral role of AC7 in the increase of plasma adrenocorticotropin and corticosterone levels during alcohol intoxication.
A well established, but sometimes overlooked, fact about the physiologic actions of ethanol is that even modest doses of ethanol in humans or other animals can increase circulating levels of adrenocorticotropin and cortisol or corticosterone (Tabakoff et al., 1978; Rivier et al., 1984; Thiagarajan et al., 1988; Ogilvie et al., 1997).
The mechanism by which ethanol increases circulating glucocorticoid levels has been traced to increases in adrenocorticotropin release (Rivier et al., 1984, 1996; Lee et al., 2004), and corticotrophin-releasing factor (CRF) has also been shown to be important for ethanol's actions. Thus, it was found that administration of anti-CRF serum totally abolished ethanol-induced release of adrenocorticotropin (Rivier et al., 1984). Whether ethanol was injected intraperitoneally or intracerebroventricularly, there was no increase in circulating adrenocorticotropin if hypothalamic CRF was absorbed by preinjection of CRF antibodies (Lee et al., 2004).
A significant increase in CRF mRNA and CRF peptide release in primary hypothalamic cell cultures did occur from 30 min to 4 h after application of alcohol (25 mM) (Li et al., 2005). However, significant increases in adrenocorticotropin release in vivo have been reported within minutes and reached a peak as early as 15 min after systemic ethanol injection (Thiagarajan et al., 1988; Ogilvie et al., 1997; Lee et al., 2004). These data suggest that the early in vivo effect of ethanol on adrenocorticotropin release may not depend on an increase in CRF release. Taking these findings into account, one can hypothesize that ethanol can enhance CRF-stimulated adrenocorticotropin release by acting at the level of the CRF receptor, i.e., ethanol can increase signal transduction occurring in the presence of the activated CRF receptor.
It is well accepted that CRF receptor 1α (CRF-R1) is a GS-coupled receptor, and CRF binding to this receptor initiates signaling by activating adenylyl cyclase (AC) through GSα (Wynn et al., 1985; Aguilera et al., 1986; Grigoriadis et al., 1993; De Souza, 1995). What was not investigated, until the recent work of Antoni et al. (2003), was the identity of the isoforms of AC to which CRF-R1 couples. Using pituitary corticotrophs, Antoni et al. (2003) demonstrated coupling of CRF-R1 to AC9, but they also hypothesized that, during stress, CRF-R1 coupling to AC9 in the corticotroph was switched to coupling to AC7. This switch was proposed to be a result of the actions of arginine vasopressin (AVP), acting through the AVP1b receptor (V1b receptor) to produce changes in receptor/effector (CRF-R1/AC) coupling through a PKC-mediated phosphorylation. We have previously shown that PKCδ-mediated phosphorylation of AC7 increases AC7 responsiveness to GSα (Nelson et al., 2003), whereas GSα-stimulated AC9 activity is significantly attenuated by PKC-induced phosphorylation (Cumbay and Watts, 2004). Further attenuation of AC9 activity can be produced by the AVP/Gi/phospholipase C/inositol trisphosphate-mediated release of intracellular Ca2+ (Antoni et al., 1998), whereas AC7 is Ca2+-insensitive (Antoni et al., 1998; Mons et al., 1998). Because ethanol exerts its effects on GSα-AC7 coupling via ethanol-induced phosphorylation of AC7 by PKCδ (Nelson et al., 2003), one can propose that ethanol would be able to increase CRF-R1 and GSα-mediated cAMP signaling by promoting the phosphorylation of AC7, in a manner similar to what was suggested for vasopressin.
One way in which the role of AC7 can be better defined with regard to ethanol's actions on adrenocorticotropin and corticosterone release in animals is to genetically manipulate the expression of AC7 in pituitary of mice by creating transgenic (Adcy7huTG) and knockdown [Adcy7(+/−)] mice. We have created and used such mice for these studies of ethanol's actions.
Materials and Methods
Animals.
Experiments were performed on 70- to 100-day-old mice. AC7 transgenic (Adcy7huTG) mice overexpress human AC7 in the brain (Yoshimura et al., 2000) and had been backcrossed to C57BL/6 mice for 13 generations at the time of these experiments. AC7 heterozygous “knockdown” mice, in which one copy of the AC7 gene was disrupted [Adcy7(+/−)] (Hines et al., 2006), were backcrossed to C57BL/6 mice for 12 generations. (Disruption of both copies of the AC7 gene produced death of the fetus.) The respective littermates were used as controls (WT). The animals were housed in a colony room with a 12-h light/12-h dark cycle with controlled temperature (23°C) and humidity (40%). Access to food and water was ad libitum. All efforts were made to minimize any potential suffering, and all experimental procedures were approved by the University of Colorado Denver Institutional Animal Care and Use Committee. To desensitize mice to handling, animals were handled once a day for a week before experiments. Animals were singly housed 1 day before the experiment. Ethanol was administered, and blood was collected starting at approximately 8 AM.
Microarray Analysis.
Naive male and female Adcy7(+/−), Adcy7huTG, and WT animals were used for microarray analysis. Animals were rapidly anesthetized with CO2 and decapitated, and whole pituitary glands were quickly removed and placed in RNAlater RNA Stabilization Reagent (QIAGEN, Valencia, CA). Total RNA from two pooled whole pituitaries (Adcy7huTG and WT animals), or single pituitaries [Adcy7(+/−) and WT animals], was extracted by using the RNeasy Micro Kit (QIAGEN) following the manufacturer's instructions. Gene expression data were obtained by using the Affymetrix (Santa Clara, CA) GeneChip Mouse Genome 430 version 2.0 arrays as described previously (Tabakoff et al., 2008) and stored on the PhenoGen Informatics web site (http://phenogen.ucdenver.edu). Data were subjected to quality control (Saba et al., 2006) and normalization by using the Robust Multichip Average/Robust Multiarray Analysis method (Irizarry et al., 2003) after probes of poor sequence integrity were removed. The expression levels (mRNA) of corticotrophin-releasing hormone receptor 1 (Crhr1), Gsα protein (Gnas), proopiomelanocortin (Pomc), and AC1–AC9 (Adcy1–Adcy9) genes in the pituitary glands of male and female Adcy7(+/−), Adcy7huTG, and corresponding WT animals were analyzed by one-way ANOVA with pairwise contrasts for the four comparisons of interest: female Adcy7(+/−) versus WT, female Adcy7huTG versus WT, male Adcy7(+/−) versus WT, and male Adcy7huTG versus WT. A false discovery rate (FDR) (Benjamini and Hochberg, 1995) was used to adjust for multiple comparisons within each pairwise contrast.
Quantitative Reverse Transcription Real-Time Polymerase Chain Reaction.
Quantitative real-time polymerase chain reaction (qRT-PCR) (Prism 7900 Sequence Detection System; Applied Biosystems, Foster City, CA) was used, in addition to microarray analysis, to quantitate the expression of the transgene (human ADCY7) mRNA and endogenous (mouse Adcy7) mRNA in anterior pituitary from male and female Adcy7huTG and Adcy7(+/−) mice and their wild-type littermates.
Sequence-specific TaqMan probes and primer sets for human ADCY7 were designed by using PrimerExpress software (Applied Biosystems); probes and primers for mouse Adcy7 were designed by Applied Biosystems. The probes and primers are described elsewhere (Hines et al., 2006).
Anterior pituitaries from three to four animals were pooled, and total RNA was extracted by using an RNeasy Plus Mini-Kit (QIAGEN). Double-stranded cDNA was synthesized from total RNA and used to obtain biotin-labeled cRNA by an in vitro transcription reaction. All samples were assayed in triplicate on a single plate for human ADCY7 and mouse Adcy7. After correction for the endogenous control, mouse glyceraldehyde-3-phosphate dehydrogenase (Gadph) mRNA, the relative quantities of each transcript were calculated as described by Livak and Schmittgen (2001). Data were analyzed by two-way ANOVA (sex and genotype) and post-hoc contrasts when appropriate.
Immunoblotting for AC7 Protein.
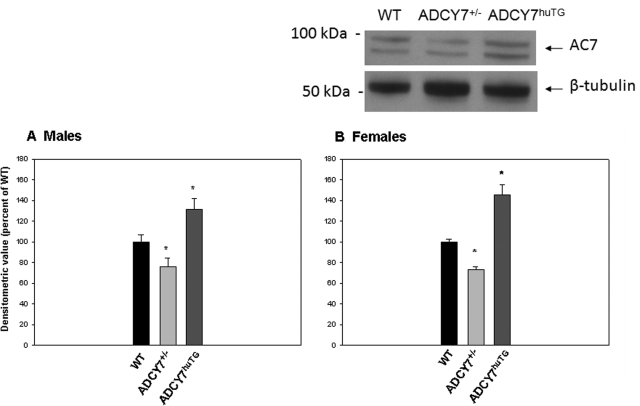
AC7 protein was measured as described elsewhere (Hines et al., 2006) with modifications. In brief, antiserum was generated in rabbits against a peptide sequence within the C1b region of AC7 (ETHVPNGRRPKSVPQRHRRTC), and the antibodies recognized both human and mouse AC7 protein. Mice were killed with CO2, and the whole pituitary gland was dissected. Glands were homogenized by using a Polytron (KINEMATICA AG, Lucerne, Switzerland) in 10 mM Tris-HCl buffer, pH 7.4, containing 1 mM EDTA, 0.25 M sucrose, and protease and phosphatase inhibitor cocktails (Sigma-Aldrich, St. Louis, MO) and centrifuged at 1600g (10 min, 4°C). The protein concentration of the particulate-containing supernatant was determined by the BCA method (Pierce Chemical, Rockford, IL). Protein (45 μg per lane) was mixed with NuPAGE LDS sample buffer and NuPAGE reducing agent (Invitrogen, Carlsbad, CA), separated on 4 to 12% Bis-Tris polyacrylamide gels (NuPAGE gels; Invitrogen) and transferred to nitrocellulose membranes. The blots were blocked with StartingBlock (phosphate-buffered saline) blocking buffer (Thermo Fisher Scientific, Waltham, MA) and incubated with primary antibody to AC7 (1:3000) and monoclonal antibody to β-tubulin (1:10,000; BD Biosciences PharMingen, San Diego, CA), followed by goat anti-rabbit IgG (1:10,000) and goat anti-mouse IgG (1:30,000) coupled to horseradish peroxidase. Protein bands were visualized with enhanced chemiluminescence reagent (PerkinElmer Life and Analytical Sciences, Waltham, MA). After exposure to X-ray film, protein bands were quantitated by image analysis using Quantity One software (version 4.5.1; Bio-Rad Laboratories, Hercules, CA). In many instances (but not all), AC7 appears as a doublet (molecular mass 100 and ∼80 kDa; see Fig. 2). We had previously noted a less prominent doublet in hippocampal tissue (Hines et al., 2006). Where a doublet was noted, both bands were included in the densitometric analysis. Results were calculated as densitometric volume of the appropriate bands, and levels of AC7 protein were normalized based on β-tubulin levels. Every gel contained protein from WT, Adcy7huTG, and Adcy7(+/−) mice. On each blot, the densitometric ratio values (AC7/β-tubulin) for the Adcy7huTG and Adcy7(+/−) mice were converted to percentage of WT ratios on that blot (data from male and female animals were analyzed separately). These percentage values were averaged across blots for male and female mice. Data were analyzed by two-way ANOVA (sex and genotype) with a random effect of subject, followed by post-hoc contrasts when appropriate.
Fig. 2.
Time course of blood ethanol levels after ethanol injection. Male (A) and female (B) WT (○), Adcy7huTG (▼), and Adcy7(+/−) (●) mice were injected intraperitoneally with 2.25 g/kg ethanol, and blood ethanol levels were measured by head-space gas chromatography at the indicated times after injection. Values represent mean ± S.E.M. from four to nine mice per group at each time point.
Effect of Ethanol on Plasma Adrenocorticotropin and Corticosterone Levels.
All experiments were performed between 8:00 and 9:30 AM. Ethanol was injected intraperitoneally at a dose of 2.25 g/kg as a 15% solution (diluted in saline). At 0, 15, 30, 45, and 75 min after ethanol injection or 15 min after saline injection (as a control for the effect of injection per se) mice were anesthetized by exposure to CO2 for 10 s and decapitated, and trunk blood was collected in ice-chilled Eppendorf tubes containing 20 μl of 5% EDTA. Blood was centrifuged to separate plasma. The separated plasma was stored at −80°C until analysis. Corticosterone concentrations were determined in 25 μl of plasma by using a commercially available enzyme immunoassay kit (Diagnostic Systems Laboratories, Inc., Webster, TX) following the manufacturer's directions. Adrenocorticotropin was measured in 100 μl of plasma by using an adrenocorticotropin enzyme-linked immunosorbent assay kit (ALPCO Diagnostics, Windham, NH). Blood ethanol levels were determined (in 20-μl samples from the same mice used for the endocrine assays) by head-space gas chromatography, using a Varian, Inc. (Palo Alto, CA) 3800 gas chromatograph as described previously (Tabakoff et al., 1978).
Statistical Analysis of Injection Stress on Adrenocorticotropin Levels.
A three-way ANOVA was used to analyze the effect of injection stress on adrenocorticotropin levels across sexes and genotypes (sex × genotype × time). Data were included from all three genotypes and both sexes at baseline, i.e., immediately after injection with ethanol (0 time) and 15 min after injection with saline. Sex effects and sex-specific genotype effects were examined at baseline and after saline injection. In addition, the injection effect (adrenocorticotropin levels 15 min after saline injection minus adrenocorticotropin levels at baseline) was examined separately by sex and genotype. Effect P values were adjusted for multiple comparisons by using the Bonferroni multiple comparison procedure.
Statistical Analyses of Ethanol Effects on Adrenocorticotropin and Corticosterone Levels.
Before analysis, the data were tested for equality of variances across groups created by the combination of sex, genotype, and time, using Levene's test for homogeneity (Levene, 1960). This test indicated a significant difference among group variances (F = 3.25, P < 0.0001 for adrenocorticotropin; F = 3.92, P < 0.0001 for corticosterone) that was not resolved by commonly used transformations such as log or square root. Therefore, data were analyzed by using a three-way ANOVA model that allows heterogeneous variances, as executed by using the MIXED procedure in SAS version 9.2 (SAS Institute, Cary, NC). General significance (differences associated with genotype and/or sex) within each time point was then tested for each outcome (adrenocorticotropin or corticosterone levels). For time points that indicated a significant effect, genotype, sex, and other specific effects were tested by using appropriate linear contrasts, and specific group differences of interest were estimated. P values for the general significance at each time point were adjusted for multiple comparisons by using the Bonferroni multiple comparison procedure.
Areas under the curve (AUC) were calculated from 0 to 75 min by using the mean values for each genotype and sex combination. The variance of the AUC was calculated by using the serial sacrifice method described by Nedelman et al. (1995). To determine the effect of ethanol on AUC, each AUC was calculated assuming no change in baseline levels over time. For this calculation, the mean variance and sample size from time 0 were used as baseline data to correct values at 15, 30, 45, and 75 min after injection. AUC values were compared pairwise among genotypes within sexes and between sexes within genotypes, using t tests with the degrees of freedom estimated by using the Satterthwaite approximation assuming unequal variances outlined in Nedelman et al. (1995).
Data are presented as mean and standard errors calculated by using the least-squares estimation procedure within the ANOVA. P values ≤0.05 were considered statistically significant throughout.
Results
Microarray Analysis of Whole Pituitary Gland.
Analysis of microarray data (Table 1) revealed no significant differences in pituitary expression (mRNA levels) of corticotrophin-releasing hormone receptor 1 (Crhr1), Gsα protein (Gnas), or proopiomelanocortin (Pomc) between Adcy7huTG and WT, or Adcy7(+/−) and WT, animals of either sex, after correction for multiple comparisons. Although probe sets for mouse Adcy1–Adcy9 were present on arrays, only mRNAs for Adcy2, Adcy3, Adcy6, and Adcy7 were found to be present in pituitary glands, based on the criterion that at least one probe set for the transcript was called “present” on all arrays by the Affymetrix MAS 5.0 algorithm (Affymetrix, 2001). There were no significant differences in expression levels of Adcy2 or Adcy6 between Adcy7huTG or Adcy7(+/−) and WT animals of either sex. Adcy3 mRNA was significantly lower (by approximately 20%) in both male Adcy7(+/−) and Adcy7huTG animals compared with WT littermates. As far as Adcy7 mRNA was concerned, Adcy7(+/−) male and female animals displayed lower expression of the mouse Adcy7 gene (mRNA) in the pituitary gland compared with their WT littermates. In male Adcy7(+/−) mice, Adcy7 expression was approximately 20% lower than WT, whereas in female Adcy7(+/−) mice, Adcy7 expression was 14 to 35% lower than WT, depending on the probe set. The results we obtained by measuring Adcy7 gene expression by microarray technology were noted to demonstrate a large amount of biological variability, which precluded statistical significance, and thus we pursued further experiments to measure Adcy7 mRNA by qRT-PCR.
TABLE 1.
Measures of pituitary levels of mRNA for adenylyl cyclase isoforms, CRF-1, Gsα protein, and proopiomelanocortin by microarray analysis
The percentage change in all cases represents the amount of difference in the quantity of mRNA for a particular transcript found in whole pituitary of the genetically modified strains versus the respective WT mice. All measurements were accomplished by using Affymetrics 430 v0.2 arrays, and data are included only if the probeset produced a “present” response (i.e., hybridization above background) on all the arrays (23) used in this experiment. P values were calculated by contrasting group means within a one-way ANOVA and were adjusted for multiple testing within gender by using a FDR.
| Gene Symbol | Affymetrix Probe ID | Female Mice |
Male Mice |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adcy7(+/−) |
Adcy7huTG |
Adcy7(+/−) |
Adcy7huTG |
||||||||||
| Percentage Change | Unadjusted P Value | FDR | Percentage Change | Unadjusted P Value | FDR | Percentage Change | Unadjusted P Value | FDR | Percentage Change | Unadjusted P Value | FDR | ||
| % | % | % | % | ||||||||||
| Adcy2 | 1455462_at | −5 | 0.451 | 0.650 | −5 | 0.520 | 0.817 | 7 | 0.271 | 0.541 | −1 | 0.828 | 0.876 |
| Adcy3 | 1421960_at | 14 | 0.027 | 0.293 | 10 | 0.135 | 0.744 | −19 | 0.001 | 0.010 | −23 | <0.001 | 0.002 |
| Adcy6 | 1418128_at | 2 | 0.866 | 0.866 | 7 | 0.514 | 0.817 | 3 | 0.773 | 0.817 | −2 | 0.810 | 0.876 |
| Adcy7 | 1450065_at | −14 | 0.473 | 0.296 | −17 | 0.438 | 0.916 | −23 | 0.229 | 0.916 | 10 | 0.643 | 0.876 |
| Adcy7 | 1456307_s_at | −35 | 0.054 | 0.650 | −9 | 0.670 | 0.817 | −20 | 0.290 | 0.817 | 3 | 0.876 | 0.876 |
| Crhr1 | 1418810_at | 2 | 0.788 | 0.866 | −1 | 0.933 | 0.933 | −16 | 0.060 | 0.933 | 5 | 0.555 | 0.876 |
| Gnas | 1450186_s_at | 3 | 0.179 | 0.536 | −3 | 0.337 | 0.780 | 0 | 0.921 | 0.921 | −1 | 0.766 | 0.876 |
| Gnas | 1427789_s_at | −13 | 0.351 | 0.608 | −30 | 0.042 | 0.374 | −4 | 0.777 | 0.874 | 22 | 0.188 | 0.847 |
| Pomc | 1433800_a_at | 3 | 0.411 | 0.650 | 1 | 0.775 | 0.916 | −4 | 0.314 | 0.916 | 3 | 0.411 | 0.876 |
Expression of the Human ADCY7 and Mouse Adcy7 mRNA in the Anterior Pituitary Gland Assessed by qRT-PCR.
By qRT-PCR, mouse Adcy7 mRNA levels in anterior pituitary gland of Adcy7(+/−) mice were approximately half of those in WT animals (male, relative quantity 0.44, P < 0.0001; female, relative quantity 0.48, P < 0.0001). Mouse Adcy7 mRNA levels in male Adcy7huTG mice were similar to those in WT mice (relative quantity 1.14, P < 0.128), whereas mouse Adcy7 mRNA levels were lower in female Adcy7huTG mice compared with WT (relative quantity 0.77, P = 0.007). However, as measured by qRT-PCR with primers specific for human ADCY7 mRNA, the human ADCY7 mRNA was expressed only in Adcy7huTG male and female mice and added measurably to the total ADCY7 mRNA in anterior pituitary of the transgenic mice.
Expression of AC7 Protein in Whole Pituitary Gland.
To analyze AC7 protein content in the mouse pituitary, we used an antibody designed to recognize both human (transgene) and mouse AC7 protein. There was a significant reduction (male, 24% lower, P = 0.030; female, 27% lower, P < 0.0001) in AC7 protein in the Adcy7(+/−) animals compared with WT (Fig. 2). The expression of the human transgene in the Adcy7huTG mice resulted in a significant increase in the total AC7 protein content in anterior pituitary tissue. AC7 protein levels in Adcy7huTG mice were 31% higher (P = 0.013) than WT levels in males and 45% higher (P = 0.0007) than WT levels in females (Fig. 1).
Fig. 1.
Protein levels of AC7 in pituitary gland. Levels of AC7 in whole pituitary glands of male (A) and female (B) Adcy7huTG, Adcy7(+/−), and WT mice were determined by immunoblotting as described under Materials and Methods. Each blot included tissue from male or female WT, Adcy7huTG, and/or Adcy7(+/−) mice, and the level of AC7 protein, quantitated as described in the text, was normalized to β-tubulin and expressed as percentage of WT on each blot. Percentage values were averaged across blots. Data are expressed as the mean ± S.E.M. from three to nine mice per group. *, P < 0.02 versus WT (two-way ANOVA and post-hoc contrasts). Representative blots are shown.
Blood Alcohol Levels.
There was no difference in blood alcohol levels among WT, Adcy7huTG, and Adcy7(+/−) groups of animals, of either sex, after the injection of 2.25 g/kg of ethanol at any time point. There was also no difference in blood alcohol levels between male and female animals at any of the time points measured [three-way ANOVA (genotype, sex, and time)] (Fig. 2).
Injection Effect and Baseline Adrenocorticotropin Levels.
Table 2 displays the mean adrenocorticotropin levels at baseline (0 time after ethanol injection) and 15 min after saline injection. The 10-s exposure to CO2 before decapitation has previously been shown to have no effect on plasma adrenocorticotropin levels (Reed et al., 2009). At baseline, there were no significant differences among genotypes in either males or females. However, there was a significant difference between adrenocorticotropin levels in male and female mice, per se, at baseline (F3,56 = 6.25, P = 0.012), with adrenocorticotropin levels being higher in females. The biggest sex effect was in the wild-type mice (difference between females and males, 15.5 ± 4.4 pg/ml plasma), and the smallest sex effect was in the transgenic mice (7.1 ± 4.8 pg/ml plasma). When adrenocorticotropin levels were measured at 15 min after saline injection, there were no significant differences among genotypes in males or females, but the rank order of adrenocorticotropin levels in males was Adcy7huTG > WT > Adcy7(+/−), whereas in females, the rank order was WT > Adcy7huTG > Adcy7(+/−). There was no significant difference in adrenocorticotropin levels between males and females at this time.
TABLE 2.
Adrenocorticotropin levels at baseline and 15 min after saline injection
Units of adrenocorticotropin are pg/ml plasma. Means and S.E. were calculated by using the least-squares method in a three-way ANOVA model. There is a significant difference between males and females at baseline (P = 0.012) in all genotypes. There was also a significant effect of saline injection in males (P < 0.001) and females (P = 0.001) of all genotypes.
| Genotype | Female Mice |
Male Mice |
||||||
|---|---|---|---|---|---|---|---|---|
| Baseline |
After Saline Injection |
Baseline |
After Saline Injection |
|||||
| n | Mean (S.E.) | n | Mean (S.E.) | n | Mean (S.E.) | n | Mean (S.E.) | |
| Adcy7(+/−) | 4 | 21.7 (4.2) | 4 | 45.1 (4.2) | 7 | 11.3 (3.1) | 4 | 28.8 (4.2) |
| Wild type | 6 | 27.0 (3.4) | 8 | 34.5 (2.9) | 9 | 11.5 (2.8) | 6 | 33.0 (3.4) |
| Adcy7huTG | 6 | 23.9 (3.4) | 5 | 36.0 (3.7) | 6 | 16.8 (3.4) | 3 | 37.4 (4.8) |
There was, however, a significant injection effect (i.e., adrenocorticotropin levels 15 min after saline injection minus adrenocorticotropin levels at baseline) in both males (F3,56 = 15.90, P < 0.001) and females (F3,56 = 8.14, P = 0.001). The injection effect did not differ among genotypes or between males and females.
Baseline Corticosterone Levels.
Baseline corticosterone levels did not differ significantly among genotypes. Also there were no significant differences between sexes in the Adcy7(+/−) or Adcy7huTG mice. However, WT female mice had significantly higher corticosterone levels than WT male mice (t = 3.65, P = 0.0004).
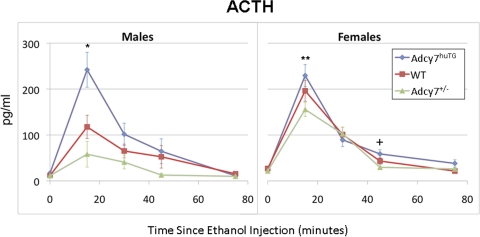
Adrenocorticotropin Levels after Ethanol Injection: Genotype Effects.
Figure 3 shows adrenocorticotropin levels in the three genotypes at baseline (0 time) and four time points after ethanol injection for male and female mice. Ethanol treatment increased adrenocorticotropin levels in all groups of mice (F24,153 = 15.50, P < 0.001) at 15, 30, and 45 min after injection, with a peak at 15 min after injection. The ANOVA model indicated a significant three-way interaction between effects of genotype, sex, and time on adrenocorticotropin levels. The analyses within time points indicated significant effects of genotype and/or sex on adrenocorticotropin levels at 15 min (F5,153 = 6.31, P < 0.0001), 45 min (F5,153 = 7.35, P < 0.0001), and 75 min after ethanol injection (F5,153 = 9.52, P < 0.0001).
Fig. 3.
Effect of ethanol on plasma adrenocorticotropin levels. Male (left) and female (right) WT (red ■), Adcy7huTG (blue ♦), and Adcy7(+/−) (green ▲) mice were injected intraperitoneally with 2.25 g/kg ethanol and killed at the time points indicated. Adrenocorticotropin was measured by using an enzyme immunoassay kit as described in the text. Data are expressed as mean ± S.E.M. from four to eleven mice per group. *, P = 0.007, male Adcy7huTG compared with WT mice, and P < 0.001 compared with Adcy7(+/−) mice; **, P < 0.01, female Adcy7huTG compared with female Adcy7(+/−) mice; †, P < 0.002, female Adcy7huTG compared with Adcy7(+/−) mice. At 15, 45, and 75 min after ethanol injection, some groups of females had significantly higher adrenocorticotropin levels than males (see text).
At 15 min after ethanol injection, there was a significant difference in adrenocorticotropin levels among genotypes in males (F2,153 = 7.59, P = 0.001). Male Adcy7huTG mice had higher adrenocorticotropin levels than wild type (t = 2.72, P = 0.007) and Adcy7(+/−) male mice (t = 3.89, P < 0.001). There was also a significant difference among genotypes in female mice at 15 min after ethanol injection (F2,153 = 3.66, P = 0.03). Female Adcy7huTG mice had higher adrenocorticotropin levels than Adcy7(+/−) mice (t = 2.60, P = 0.01).
At 45 min after ethanol injection, there was a significant difference in adrenocorticotropin levels among genotypes in females (F2,153 = 6.22, P = 0.003). The female Adcy7huTG mice had higher adrenocorticotropin levels than Adcy7(+/−) mice (t = 3.12, P = 0.002). The genotype effect in males at this time only marginally missed the significance threshold (F2,153 = 3.00, P = 0.053).
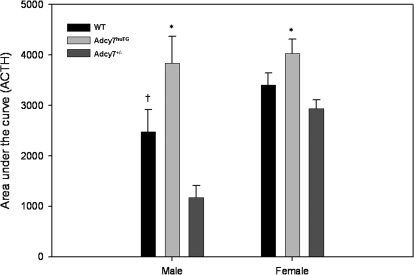
To obtain a more integrated assessment of the effect of ethanol on adrenocorticotropin levels, we calculated AUC for adrenocorticotropin levels over time after injection (Fig. 4). Ethanol treatment significantly increased the AUC for all groups of mice (p < 0.05). The AUC was significantly higher in the Adcy7huTG mice than in the Adcy7(+/−) mice of both sexes (males: t = 4.56, df = 17.84, P < 0.001; females: t = 3.32, df = 24.05, P = 0.003). In addition, the wild-type male mice had a significantly higher AUC than the Adcy7(+/−) male mice (t = 2.61, df = 21.54, P = 0.016).
Fig. 4.
Area under the adrenocorticotropin level × time curve after ethanol injection. Data shown in Fig. 3 were used to calculate AUC for male and female Adcy7huTG, WT, and Adcy7(+/−) mice. Data are expressed as mean ± S.E.M. *, P < 001 (males), P < 0.003 (females), Adcy7huTG compared with Adcy7(+/−) mice. †, P = 0.016, male WT compared with male Adcy7(+/−) mice. The AUC for female Adcy7(+/−) mice is significantly higher than that for male Adcy7(+/−) mice (see text).
Adrenocorticotropin Levels after Ethanol Injection: Effect of Sex.
There were differences in adrenocorticotropin levels between males and females after ethanol injection at 15 min (F3,153 = 4.90, P = 0.003), 45 min (F3,153 = 5.42, P = 0.001), and 75 min (F3,153 = 11.15, P < 0.001). At 15 min after ethanol injection, male Adcy7(+/−) and WT mice had lower adrenocorticotropin levels than their female counterparts (t = 3.06, P = 0.003; t = 2.30, P = 0.023, respectively). Likewise, at 45 min after injection, male Adcy7(+/−) mice had lower adrenocorticotropin levels than female Adcy7(+/−) mice (t = 4.01, P < 0.001). At 75 min after ethanol injection, male Adcy7huTG and Adcy7(+/−) mice had lower adrenocorticotropin levels than females of their respective genotype (t = 3.40, P < 0.001; t = 4.52, P < 0.001, respectively).
In terms of AUC measures after ethanol injection, with regard to sex differences, male mice of all genotypes had a lower AUC for adrenocorticotropin than females of the same respective genotype, but this difference reached statistical significance only in the Adcy7(+/−) mice (t = 5.89, df = 15.58, P < 0.001).
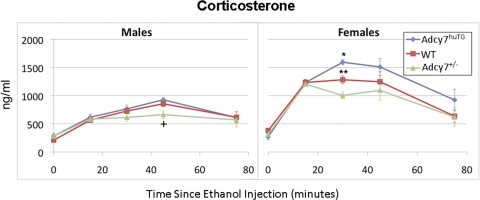
Corticosterone Levels after Ethanol Injection: Genotype Effects.
Figure 5 shows the corticosterone levels in the three genotypes at baseline (0 min) and the four time points after ethanol injection in male and female mice. Ethanol treatment resulted in increased corticosterone levels in all groups of mice (F24,141 = 67.78, P < 0.001) at all time points after injection, with peak levels seen at 30 to 45 min after injection. The ANOVA model indicated a significant three-way interaction between genotype, sex, and time on circulating corticosterone levels. A significant effect of genotype and/or sex was seen after ethanol injection at 15 min (F5,141 = 138.81, P < 0.001), 30 min (F5,141 = 51.71, P < 0.001), and 45 min (F5,141 = 7.02, P < 0.001).
Fig. 5.
Effect of ethanol on plasma corticosterone levels. Male (left) and female (right) WT (red ○), Adcy7huTG (blue ♦), and Adcy7(+/−) (green ▲) mice were injected intraperitoneally with 2.25 g/kg ethanol and killed at the indicated times after injection. Plasma corticosterone levels were measured by using an enzyme-linked immunosorbent assay kit, as described in the text. Data are presented as mean ± S.E.M. from four to nine mice per group. *, P = 0.001 compared with female WT and Adcy7(+/−) mice; **, P < 0.003 compared with female Adcy7(+/−) mice. †, P = 0.024 compared with male WT mice, P < 0.001 compared with male Adcy7huTG mice. For all three genotypes, females had significantly higher corticosterone levels than males at 15, 30, and 45 min after ethanol injection (see text).
At 30 min after ethanol injection, there was a significant genotype-dependent effect on corticosterone levels in females (F2,141 = 31.86, P < 0.001). The Adcy7huTG female mice had higher corticosterone levels than the WT mice (t = 3.72, P < 0.001) and the Adcy7(+/−) mice (t = 7.87, P < 0.001). In addition, the female WT mice had significantly higher corticosterone levels than the Adcy7(+/−) mice (t = 2.98, P = 0.003). Although the genotype effect was not significant in the male mice at 30 min after injection, the rank order of corticosterone levels in male mice of the different genotypes was similar to that in females at this time. At 45 min after ethanol injection, there was a significant difference among genotypes in male mice (F2,141 = 3.19, P = 0.003). The Adcy7(+/−) male mice had lower corticosterone levels than the WT mice (t = 2.28, P = 0.024) and the Adcy7huTG mice (t = 3.51, P < 0.001). The rank order of corticosterone levels across genotypes in females was similar to that in males at this time point.
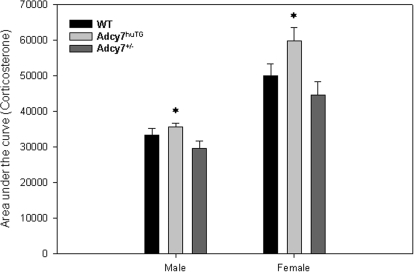
We also calculated the AUC for corticosterone levels over time after ethanol injection (Fig. 6). Results similar to those seen in the AUC analysis of adrenocorticotropin were evident, i.e., ethanol treatment significantly increased the AUC in all groups of mice (p < 0.0002), and the Adcy7huTG mice had a significantly higher AUC than the Adcy7(+/−) mice in both males and females (t = 2.46, df = 9.12, P = 0.036 and t = 2.93, df = 14.62, P = 0.011, respectively).
Fig. 6.
Area under the corticosterone level × time curve after ethanol injection. Data shown in Fig. 5 were used to calculate AUC for male and female Adcy7huTG, WT, and Adcy7(+/−) mice. Data are expressed as mean ± S.E.M. *, P = 0.036 (males) and 0.011 (females), compared with Adcy7(+/−) mice. Females of all genotypes had significantly higher AUCs than males (see text).
Corticosterone Levels after Ethanol Injection: Effect of Sex.
There was a significant difference in corticosterone levels between males and females after ethanol injection at 15 min (F3,141 = 116.77, P < 0.001), 30 min (F3,141 = 56.74, P < 0.001), and 45 min (F3,141 = 7.98, P < 0.001). At 15 min, levels in females were higher than levels in males in all three genotypes [Adcy7(+/−): t = 10.18, P < 0.001; Adcy7huTG: t = 7.57, P < 0.001; WT: t = 13.76, P < 0.001]. Again at 30 min, in all three genotypes, females had higher corticosterone levels than males [Adcy7(+/−): t = 4.03, P < 0.001; Adcy7huTG: t = 10.59, P < 0.0001; WT: t = 6.47, P < 0.001]. At this time, there was also a significant difference in sex effects among the genotypes (F2,141 = 6.61, P = 0.002), i.e., the greatest difference in corticosterone levels between males and females occurred in the Adcy7huTG mice, and the smallest difference occurred in the Adcy7(+/−) mice. Finally, at 45 min after ethanol injection, corticosterone levels were again higher in females than males within all three genotypes [Adcy7(+/−): t = 2.33, P = 0.022; Adcy7huTG: t = 3.76, P < 0.001; WT t = 2.10, P = 0.038].
Within all three genotypes, females had a significantly higher AUC for corticosterone than males [Adcy7huTG: t = 6.31, df = 9.92, P < 0.001; wild type: t = 4.51, df = 12.76, P<0.001; Adcy7(+/−): t = 3.58, df = 10.34, P = 0.005] after injection of ethanol.
Discussion
The critical tools in our demonstration of the involvement of AC7 in pituitary adrenocorticotropin release were the Adcy7(+/−) and Adcy7huTG mice created to underexpress and overexpress AC7 in brain. Although our previous work generated information on brain gene expression in these mice (Hines et al., 2006), we felt it necessary to examine pituitary gene expression in the animals being used in the current studies. The microarray data that we obtained can be accessed on http://phenogen.ucdenver.edu. Using the criteria described in Results, we found that mRNA for Adcy2, Adcy3, Adcy6, and Adcy7 is expressed in pituitary. One would assume that Adcy9 mRNA is expressed in rodent corticotrophs, from the work of Antoni et al. (2003), although, based on our microarray data, the level of expression (mRNA) may be low in mouse. In the microarray experiment, only Adcy3 mRNA was significantly different in the genetically modified mice, i.e., this mRNA was lower in both the Adcy7(+/−) and the Adcy7huTG male mice compared with their WT littermates. With regard to our work, however, the differences in Adcy3 mRNA, even if they are reflected by differences in AC3 protein localized in corticotrophs, would not translate into ethanol-induced changes in cAMP production. We have shown that under several conditions AC3 is unresponsive to ethanol, even at concentrations as high as 200 mM (Yoshimura and Tabakoff, 1995).
The results of the qRT-PCR analysis in the Adcy7(+/−) mice mirrored qualitatively the results of the microarray analysis, but were more consistent and demonstrated a statistically significant, approximately 50% decrease in the mouse Adcy7 mRNA in both male and female Adcy7(+/−) mice compared with WT. The qRT-PCR for mouse Adcy7 mRNA, and the gene array analyses, did not quantitate the product of the human AC7 transgene that was introduced into the oocyte during the creation of the Adcy7huTG strains. The additional expression of the human AC7 in the pituitary of the Adcy7huTG mice was, however, demonstrated by using primers specific for the human ADCY7 mRNA. The results obtained with an antibody that did not distinguish between the mouse and human AC7 substantiated lower AC7 protein levels in anterior pituitary of the Adcy7(+/−) male and female mice and increased levels in Adcy7huTG male and female mice compared with the WT littermates.
Evidence has been presented that in GS-coupled systems the AC component may be rate-limiting in the receptor·GS·AC signal transduction event (i.e., generation of cAMP) (MacEwan et al., 1996). Thus, if signaling through AC7 is important in a physiologic event, one would expect that increasing or decreasing the levels of AC7 may produce related changes in the measured physiologic event. The baseline levels of adrenocorticotropin in the circulation of our male and female mice are comparable with levels reported by others (Lolait et al., 2007; Carter et al., 2009). Fifteen minutes after a “mild” stress of saline injection, adrenocorticotropin levels in plasma increased in both male and female mice. The rank order of increase in male mice [Adcy7(+/−) < WT < Adcy7huTG] followed the pattern expected from the measured levels of AC7 in male pituitaries. The female mice, however, did not illustrate a rank order expected from the genetic modification of their pituitary AC7 levels. One explanation of this observation is that the saline injection increased the adrenocorticotropin levels 2-fold or less in all groups of females, whereas in males the increase was more than 2-fold in all groups. The saline injection stimulus may not have been sufficient to fully engage the AC7 signaling in females.
As already noted, ethanol can potentiate the activation of AC7 by Gs-coupled receptors (e.g., CRF-R1), without the intervention of other stress-related receptor systems (e.g., AVP-R1b) (Tabakoff et al., 2001; Nelson et al., 2003). The Adcy7huTG, Adcy7(+/−), and WT animals (male or female) in our studies did not differ significantly either in CRF-R1 mRNA or GSα mRNA in pituitary. As noted in the Introduction, although ethanol can increase CRF release from the paraventricular nucleus of the hypothalamus both in vivo (Lee et al., 2004) and in vitro (Li et al., 2005), the increases do not correspond, in time, with the increase in adrenocorticotropin after ethanol treatment witnessed in our studies. A parsimonious explanation of our results would be that ethanol rapidly increases adrenocorticotropin levels in the circulation by promoting the response of AC7 to CRF receptor activation, even at constant levels of CRF. The increased cAMP then activates the mechanisms for adrenocorticotropin release (Aguilera et al., 1983; Sobel, 1985; Wynn et al., 1985). Our previous work using cultured cell systems indicates that significant increases in AC7 activity in response to GSα-coupled receptor stimulation occur at concentrations of 20 mM ethanol, and the 2.25 g/kg dose we administered produced blood ethanol concentrations of above 40 mM.
In both males and females, the increase in adrenocorticotropin after ethanol injection was proportional to the pituitary levels of AC7, which was particularly notable when the AUC of the time/response curves for adrenocorticotropin were compared among the Adcy7(+/−), WT, and Adcy7huTG mice. Examination of the blood ethanol concentrations over the time of measurement of adrenocorticotropin indicated that whereas adrenocorticotropin dropped to basal levels within 60 to 80 min after ethanol injection, the ethanol levels in plasma diminished little over this time period. Our previous studies with AC7 in human erythroleukemia cells demonstrated that the ethanol stimulation of cAMP production “desensitized” significantly after a 30-min preincubation with ethanol (Rabbani et al., 1999), and this phenomenon may well be operational in the mouse pituitary corticotrophs.
There were significant quantitative differences in both peak levels and the AUC of the corticosterone concentration/time curves, which correlated in magnitude with the plasma levels of adrenocorticotropin in the Adcy7(+/−), WT, and Adcy7huTG mice given ethanol. The rank order of the corticosterone responses to ethanol was such that the Adcy7huTG mice (male and female) demonstrated the highest peak and, particularly, greater AUC values, WT mice were intermediate, and the Adcy7(+/−) mice had the lowest peak and AUC values.
What is also noticeable in our results (see AUCs) are the significantly higher levels of corticosterone in the circulation of the female mice in comparison with the male mice, especially in the Adcy7huTG mice, in whom levels of adrenocorticotropin after ethanol administration were not statistically different in males and females. These observations suggest that the adrenal tissue of the females may be more responsive to high levels of adrenocorticotropin than that of males. Nevertheless, our work indicates that by manipulating AC7 levels in the pituitary one can alter the magnitude of an animal's adrenocorticotropin response to ethanol and possibly to stress (e.g., intraperitoneal injection of saline in male mice). The adrenocorticotropin in turn produces a graded response in circulating corticosterone levels in mice that is quantitatively related to the levels of AC7 in the pituitary.
The current work not only helps to establish AC7 as an important part of the signaling cascade between the CRF-R1 and adrenocorticotropin release under conditions of ethanol intoxication and/or stress, but also allows some extrapolation to situations witnessed with humans. Our previous work with AC7 polymorphisms in humans demonstrated the association of a haplotype that includes the 3′-untranslated region of human ADCY7 with familial major depressive disorder. This association was primarily evident in females (odds ratio = 2.0). If the same population was divided into women who drink alcohol at harmful levels (>40 g/day) and those who drink <40 g/day the odds ratio for the association of the relevant haplotype in ADCY7 with “harmful” alcohol consumption, in women who also demonstrate familial depression, increased to 3.0. The polymorphic 3′-untranslated region in the ADCY7 gene in humans contains elements that can alter mRNA transcription/stability/translation, including a tetranucleotide repeat region (Hellevuo et al., 1997) and a nearby polymorphic miRNA binding site. These sequence differences may promote different levels of expression of ADCY7 among individuals, making certain individuals more susceptible to increase their circulating levels of adrenocorticotropin and cortisol after alcohol consumption or even emotional stress. There is certainly a plethora of evidence linking high levels of circulating glucocorticoids to major depressive disorder (Pfohl et al., 1985; Mitchell and O'Keane, 1998; Vreeburg et al., 2009). Thus the demonstration in this article that AC7 levels in the pituitary influence adrenocorticotropin and glucocorticoid levels in the circulation generates a testable mechanistic hypothesis regarding ADCY7 polymorphisms, environmental events (including ethanol consumption), and the etiology of major depressive disorder in humans (particularly in women).
This work was supported in part by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grants AA013162, AA016649, AA016663]; and the Banbury Fund.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.110.166793.
- AC
- adenylyl cyclase
- AC7
- type 7 AC
- AC9
- type 9 AC
- AUC
- area under the curve
- AVP
- arginine vasopressin
- CRF
- corticotrophin-releasing factor
- CRF-R1
- CRF receptor 1α
- PKC
- protein kinase C
- WT
- wild type
- ANOVA
- analysis of variance
- qRT-PCR
- quantitative real-time polymerase chain reaction
- FDR
- false discovery rate.
References
- Affymetrix (2001) Statistical Algorithms Reference Guide, Affymetrix, Inc., Santa Clara, CA [Google Scholar]
- Aguilera G, Harwood JP, Wilson JX, Morell J, Brown JH, Catt KJ. (1983) Mechanisms of action of corticotropin-releasing factor and other regulators of corticotropin release in rat pituitary cells. J Biol Chem 258:8039–8045 [PubMed] [Google Scholar]
- Aguilera G, Wynn PC, Harwood JP, Hauger RL, Millan MA, Grewe C, Catt KJ. (1986) Receptor-mediated actions of corticotropin-releasing factor in pituitary gland and nervous system. Neuroendocrinology 43:79–88 [DOI] [PubMed] [Google Scholar]
- Antoni FA, Palkovits M, Simpson J, Smith SM, Leitch AL, Rosie R, Fink G, Paterson JM. (1998) Ca2+/calcineurin-inhibited adenylyl cyclase, highly abundant in forebrain regions, is important for learning and memory. J Neurosci 18:9650–9661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni FA, Sosunov AA, Haunso A, Paterson JM, Simpson J. (2003) Short-term plasticity of cyclic adenosine 3′,5′-monophosphate signaling in anterior pituitary corticotrope cells: the role of adenylyl cyclase isotypes. Mol Endocrinol 17:692–703 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57:289–300 [Google Scholar]
- Carter RN, Paterson JM, Tworowska U, Stenvers DJ, Mullins JJ, Seckl JR, Holmes MC. (2009) Hypothalamic-pituitary-adrenal axis abnormalities in response to deletion of 11β-HSD1 is strain-dependent. J Neuroendocrinol 21:879–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumbay MG, Watts VJ. (2004) Novel regulatory properties of human type 9 adenylate cyclase. J Pharmacol Exp Ther 310:108–115 [DOI] [PubMed] [Google Scholar]
- De Souza EB. (1995) Corticotropin-releasing factor receptors: physiology, pharmacology, biochemistry and role in central nervous system and immune disorders. Psychoneuroendocrinology 20:789–819 [DOI] [PubMed] [Google Scholar]
- Grigoriadis DE, Heroux JA, De Souza EB. (1993) Characterization and regulation of corticotropin-releasing factor receptors in the central nervous, endocrine and immune systems. Ciba Found Symp 172:85–101, discussion 101–107 [DOI] [PubMed] [Google Scholar]
- Hellevuo K, Welborn R, Menninger JA, Tabakoff B. (1997) Human adenylyl cyclase type 7 contains polymorphic repeats in the 3′ untranslated region: investigations of association with alcoholism. Am J Med Genet 74:95–98 [DOI] [PubMed] [Google Scholar]
- Hines LM, Hoffman PL, Bhave S, Saba L, Kaiser A, Snell L, Goncharov I, LeGault L, Dongier M, Grant B, et al. (2006) A sex-specific role of type VII adenylyl cyclase in depression. J Neurosci 26:12609–12619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264 [DOI] [PubMed] [Google Scholar]
- Lee S, Selvage D, Hansen K, Rivier C. (2004) Site of action of acute alcohol administration in stimulating the rat hypothalamic-pituitary-adrenal axis: comparison between the effect of systemic and intracerebroventricular injection of this drug on pituitary and hypothalamic responses. Endocrinology 145:4470–4479 [DOI] [PubMed] [Google Scholar]
- Levene H. (1960) Robust tests for the equality of variance, in Contributions to Probability and Statistics (Olkin I. ed) pp 278–292, Stanford University Press, Palo Alto, CA [Google Scholar]
- Li Z, Kang SS, Lee S, Rivier C. (2005) Effect of ethanol on the regulation of corticotropin-releasing factor (CRF) gene expression. Mol Cell Neurosci 29:345–354 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- Lolait SJ, Stewart LQ, Jessop DS, Young WS, 3rd, O'Carroll AM. (2007) The hypothalamic-pituitary-adrenal axis response to stress in mice lacking functional vasopressin V1b receptors. Endocrinology 148:849–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEwan DJ, Kim GD, Milligan G. (1996) Agonist regulation of adenylate cyclase activity in neuroblastoma × glioma hybrid NG108-15 cells transfected to co-express adenylate cyclase type II and the β2-adrenoceptor. Evidence that adenylate cyclase is the limiting component for receptor-mediated stimulation of adenylate cyclase activity. Biochem J 318:1033–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A, O'Keane V. (1998) Steroids and depression. BMJ 316:244–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mons N, Decorte L, Jaffard R, Cooper DM. (1998) Ca2+-sensitive adenylyl cyclases, key integrators of cellular signalling. Life Sci 62:1647–1652 [DOI] [PubMed] [Google Scholar]
- Nedelman JR, Gibiansky E, Lau DT. (1995) Applying Bailer's method for AUC confidence intervals to sparse sampling. Pharm Res 12:124–128 [DOI] [PubMed] [Google Scholar]
- Nelson EJ, Hellevuo K, Yoshimura M, Tabakoff B. (2003) Ethanol-induced phosphorylation and potentiation of the activity of type 7 adenylyl cyclase. Involvement of protein kinase Cδ. J Biol Chem 278:4552–4560 [DOI] [PubMed] [Google Scholar]
- Ogilvie K, Lee S, Rivier C. (1997) Effect of three different modes of alcohol administration on the activity of the rat hypothalamic-pituitary-adrenal axis. Alcohol Clin Exp Res 21:467–476 [DOI] [PubMed] [Google Scholar]
- Pfohl B, Sherman B, Schlechte J, Winokur G. (1985) Differences in plasma ACTH and cortisol between depressed patients and normal controls. Biol Psychiatry 20:1055–1072 [DOI] [PubMed] [Google Scholar]
- Rabbani M, Nelson EJ, Hoffman PL, Tabakoff B. (1999) Role of protein kinase C in ethanol-induced activation of adenylyl cyclase. Alcohol Clin Exp Res 23:77–86 [PubMed] [Google Scholar]
- Reed B, Varon J, Chait BT, Kreek MJ. (2009) Carbon dioxide-induced anesthesia results in a rapid increase in plasma levels of vasopressin. Endocrinology 150:2934–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivier C. (1996) Alcohol stimulates ACTH secretion in the rat: mechanisms of action and interactions with other stimuli. Alcohol Clin Exp Res 20:240–254 [DOI] [PubMed] [Google Scholar]
- Rivier C, Bruhn T, Vale W. (1984) Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF). J Pharmacol Exp Ther 229:127–131 [PubMed] [Google Scholar]
- Saba L, Bhave SV, Grahame N, Bice P, Lapadat R, Belknap J, Hoffman PL, Tabakoff B. (2006) Candidate genes and their regulatory elements: alcohol preference and tolerance. Mamm Genome 17:669–688 [DOI] [PubMed] [Google Scholar]
- Sobel DO. (1985) Role of cyclic AMP in corticotropin releasing factor mediated ACTH release. Peptides 6:591–595 [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Jafee RC, Ritzmann RF. (1978) Corticosterone concentrations in mice during ethanol drinking and withdrawal. J Pharm Pharmacol 30:371–374 [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Nelson E, Yoshimura M, Hellevuo K, Hoffman PL. (2001) Phosphorylation cascades control the actions of ethanol on cell cAMP signalling. J Biomed Sci 8:44–51 [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Saba L, Kechris K, Hu W, Bhave SV, Finn DA, Grahame NJ, Hoffman PL. (2008) The genomic determinants of alcohol preference in mice. Mamm Genome 19:352–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan AB, Mefford IN, Eskay RL. (1988) Acute effect of intragastric ethanol administration on plasma levels of stress hormones. Adv Alcohol Subst Abuse 7:227–230 [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJ, van Pelt J, Derijk RH, Verhagen JC, van Dyck R, Smit JH, Zitman FG, Penninx BW. (2009) Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry 66:617–626 [DOI] [PubMed] [Google Scholar]
- Wynn PC, Harwood JP, Catt KJ, Aguilera G. (1985) Regulation of corticotropin-releasing factor (CRF) receptors in the rat pituitary gland: effects of adrenalectomy on CRF receptors and corticotroph responses. Endocrinology 116:1653–1659 [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Tabakoff B. (1995) Selective effects of ethanol on the generation of cAMP by particular members of the adenylyl cyclase family. Alcohol Clin Exp Res 19:1435–1440 [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Wu PH, Hoffman PL, Tabakoff B. (2000) Overexpression of type 7 adenylyl cyclase in the mouse brain enhances acute and chronic actions of morphine. Mol Pharmacol 58:1011–1016 [DOI] [PubMed] [Google Scholar]