Abstract
We reported previously that vascular endothelial growth factor (VEGF) was increased in acetaminophen (APAP) toxicity in mice and treatment with a VEGF receptor inhibitor reduced hepatocyte regeneration. The effect of human recombinant VEGF (hrVEGF) on APAP toxicity in the mouse was examined. In early toxicity studies, B6C3F1 mice received hrVEGF (50 μg s.c.) or vehicle 30 min before receiving APAP (200 mg/kg i.p.) and were sacrificed at 2, 4, and 8 h. Toxicity was comparable at 2 and 4 h, but reduced in the APAP/hrVEGF mice at 8 h (p < 0.05) compared with the APAP/vehicle mice. Hepatic glutathione (GSH) and APAP protein adduct levels were comparable between the two groups of mice, with the exception that GSH was higher at 8 h in the hrVEGF-treated mice. Subsequently, mice received two doses (before and 10 h) or three doses (before and 10 and 24 h) of hrVEGF; alanine aminotransferase values and necrosis were reduced at 24 and 36 h, respectively, in the APAP/hrVEGF mice (p < 0.05) compared with the APAP/vehicle mice. Proliferating cell nuclear antigen expression was enhanced, and interleukin-6 expression was reduced in the mice that received hrVEGF (p < 0.05) compared with the APAP/vehicle mice. In addition, treatment with hrVEGF lowered plasma hyaluronic acid levels and neutrophil counts at 36 h. Cumulatively, the data show that treatment with hrVEGF reduced toxicity and increased hepatocyte regeneration in APAP toxicity in the mouse. Attenuation of sinusoidal cell endothelial dysfunction and changes in neutrophil dynamics may be operant mechanisms in the hepatoprotection mediated by hrVEGF in APAP toxicity.
Acetaminophen (APAP; C8H9NO2) overdose is the most common cause of acute liver failure is the United States (Larson et al., 2005). N-acetylcysteine is the only available treatment of APAP overdose, but its efficacy is limited primarily to the initial, early stages of APAP toxicity. Currently, no other therapies exist for the management of APAP-induced liver failure, other than the management of coagulopathy and support of vital organ functions.
Previous studies have shown that numerous proinflammatory and anti-inflammatory cytokines and chemokines are up-regulated in animal models of APAP toxicity, including interleukin (IL) 1β, IL-6, IL-10, IL-13, macrophage inhibitory protein 2, and monocyte chemotactic protein 1 (Jaeschke, 2005). The role of cytokines and inflammation in APAP toxicity has been reviewed (Jaeschke, 2005), and the available data suggest that cytokines may play a role in the aggravation of cellular injury, but may also limit cell injury and initiate cell and organ repair processes (Hogaboam et al., 1999; Bourdi et al., 2002; James et al., 2003a,b, 2005; Masubuchi et al., 2003; Jaeschke, 2005).
We previously demonstrated that vascular endothelial growth factor (VEGF) was significantly elevated in the late stages of APAP toxicity (Donahower et al., 2006). VEGF is a potent endothelial mitogen that is involved in angiogenesis in physiological and pathological conditions (Leung et al., 1989; Ferrara et al., 2003). In addition, VEGF has been shown to have paracrine-mediated promitotic effects on hepatocytes (LeCouter et al., 2003), suggesting that it could facilitate hepatocyte regeneration after liver injury. We reported previously that murine hepatic VEGF levels were significantly increased at 8 h in the APAP-treated mouse, well after the onset of toxicity, and levels of this growth factor continued to rise in the later stages of toxicity (Donahower et al., 2006). Furthermore, treatment with a VEGF receptor (VEGFR) inhibitor reduced hepatocyte proliferation in APAP-treated mice (Donahower et al., 2006). In addition to these data in the mouse model of APAP toxicity (Donahower et al., 2006), VEGF has been shown to be up-regulated in other models of liver injury, such as partial hepatectomy, ischemia reperfusion, and carbon tetrachloride toxicity (Taniguchi et al., 2001; Tsurui et al., 2005). Collectively, these data prompted us to examine the possible role of exogenous human recombinant VEGF (hrVEGF) as a potential treatment for APAP toxicity. Treatment with hrVEGF has been shown previously to have a protective effect in a mouse model of hepatic ischemia reperfusion injury (Tsurui et al., 2005). In addition, several studies have reported beneficial effects of exogenous hrVEGF in murine models of myocardial ischemia and hindlimb ischemia (Banai et al., 1994; Ware and Simons, 1997). In the following study, the effect of hrVEGF on the early, intermediate, and late stages of APAP toxicity was examined to test the hypothesis that hrVEGF would accelerate the liver repair process after APAP-induced hepatotoxicity in the mouse.
Materials and Methods
Reagents.
APAP (paracetamol) was obtained from Sigma-Aldrich (St. Louis, MO). Coomassie Plus Protein Assay Reagent were obtained from Pierce Chemical Co. (Rockford, IL). Gills Hematoxylin II and Permount were acquired from Thermo Fisher Scientific, Inc. (Waltham, MA). hrVEGF (or hrVEGF165) was obtained through a material transfer agreement with the National Cancer Institute (Frederick, MD). The hrVEGF sequence was expressed in Sf21 cells, and the protein was purified by sequential chromatography to more than 97% purity (Leung et al., 1989). Its biological activity has been examined previously in human umbilical vein endothelial cells and been shown to stimulate [3H]thymidine incorporation, with an ED50 of 2 to 6 ng/ml (Conn et al., 1990). The monoclonal anti-proliferating cell nuclear antigen (PCNA) antibody (1:500) was obtained from Dako North America, Inc. (Carpinteria, CA).
Experimental Animals.
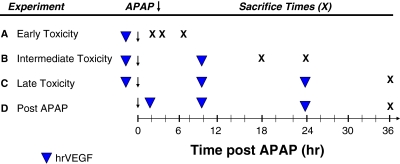
Six-week-old male B6C3F1 mice (mean weight, 24.4 g) were obtained from Harlan (Indianapolis, IN). All animal experimentation was in accordance with the criteria of the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences. Protocols for animal experimentation were approved by the University of Arkansas for Medical Sciences Animal Care and Use Committee. Mice were acclimatized 1 week before the planned experiments. Mice were fed ad libitum and housed in individual cages on a 12-h light/dark cycle. On the day before experiments, mice were fasted overnight, and dosing studies began at 8 AM the next morning. In one experiment (Fig. 1), mice were dosed with hrVEGF (50 μg s.c. in PBS; Tsurui et al., 2005) 30 min before APAP dosing (200 mg/kg i.p.) and sacrificed at 2, 4, and 8 h (n = 5 per time point and treatment group). Treatment control mice received PBS vehicle (veh) 30 min before receiving APAP. Control mice received PBS. The same dose of APAP and hrVEGF was used in all subsequent studies. In the intermediate study (Fig. 1B), mice received hrVEGF 30 min before and 10 h after receiving APAP and were sacrificed at 18 and 24 h (n = 6 per time point). In a third experiment (Fig. 1C), mice were dosed with hrVEGF 30 min before and 10 and 24 h after APAP dosing and sacrificed at 36 h (n = 11 per time point). In the final experiment, (Fig. 1D) mice received hrVEGF at 2, 10 and 24 h after APAP and were sacrificed at 36 h (n = 12 per treatment group).
Fig. 1.
Summary of study designs. Four experimental designs were used in the study. A–C, hrVEGF was administered as 50 μg s.c. (denoted by ▼), and mice were sacrificed at various times (denoted by X) to characterize the effect of hrVEGF on the early (A), intermediate (B), and late (C) stages of toxicity. D, a rescue design study was performed in which hrVEGF treatment was initiated 2 h after APAP administration.
At the indicated times, animals were anesthetized with CO2. Blood was removed from the retro-orbital plexus, allowed to coagulate at room temperature, then centrifuged, and the serum was removed for measurement of alanine aminotransferase (ALT). Mice were then euthanized with CO2, and the livers were removed. The livers were weighed, and a portion was preserved in formalin for histological sections. The remaining livers were snap-frozen in liquid nitrogen and stored at −80° for additional analyses.
Toxicity, Histology, and Metabolism Assays.
Serum ALT levels were measured by using a Cobas Mira AutoAnalyzer (Roche Diagnostics, Indianapolis, IN). Hematoxylin and eosin (H&E) staining was performed for histological examination of mouse livers. The extent of necrosis in liver sections was quantified by outlining the necrotic areas with the interactive spline measuring tool in the AxioVision 4.6.3 program (Carl Zeiss GmbH, Jena Germany). Three images were obtained from each section at 10× magnification. Quantification of the extent of necrosis was expressed as a percentage of the entire histological field. For examination of neutrophil counts, H&E-stained sections were examined by an investigator who was blinded to the experimental groups. In each mouse, the slide was scanned, three regions of high neutrophil accumulation were identified, and the number of neutrophils within the interstitium was counted [40× magnification; Olympus (Tokyo, Japan) BX50]. Hepatic glutathione (GSH) was measured by a colorimetric method using Ellman's reagent as modified previously by Mitchell et al. (1973). APAP protein adducts in the liver were measured by assaying supernatants of liver homogenates using a high-performance liquid chromatograph-electrochemical detection method developed in our laboratory (Muldrew et al., 2002).
Cytokine, Growth Factor, and Hyaluronic Acid Assays.
Snap-frozen liver samples were thawed, weighed, and homogenized in solutions containing 1 ml of protease inhibitor cocktail (Complete; Roche Diagnostics). The resulting supernatants were analyzed in duplicate and standardized to the weight of the homogenized liver sample. Cytokines, growth factors, and growth factor receptors (hVEGF, murine VEGF, VEGFR1, VEGFR2, IL-6) were measured in the supernatants of liver homogenates by using highly specific ELISA kits per the manufacturer's instructions (R&D Systems, Minneapolis, MN). Very low cross-reactivity (0.2%) for the murine VEGF ELISA and exposure to high (50,000 pg/ml) concentrations of hVEGF have been noted (R&D Systems). Hyaluronic acid (HA) in plasma was measured by using an ELISA kit purchased from Corgenix (Broomfield, CO).
Immunoblotting and Immunohistochemistry.
PCNA and platelet endothelial cell adhesion molecule (PECAM) expression in liver homogenates was measured by Western blot as described previously (Donahower et al., 2006). Band detection was performed by using ECL Plus detection (GE Healthcare, Little Chalfont, Buckinghamshire, UK).
Quantitative Image Analysis.
To quantify the effect of hrVEGF on the proliferation of hepatic nuclei, slides of liver sections were scanned to a digital file by using the Scanscope T2 system (Aperio, Vista, CA). This system produces a navigable high-resolution file of each slide at magnification up to 200×. PCNA-labeled slides were analyzed over the entire slide by using the Aperio Immunohistochemistry Nuclear algorithm. This algorithm is based on optical density of staining by using color deconvolution to analyze only chromagen staining. Nuclei are recognized by shape, size, and staining characteristics. Results were reported as percentage of positive nuclei. Algorithm settings were optimized for PCNA liver staining in our laboratory.
Statistical and Pharmacokinetic Analysis.
Results are expressed as means ± S.E. Comparisons between multiple groups were by one-way analysis of variance followed by the Tukey HSD post hoc test. SPSS version 10.0 (SPSS Inc., Chicago, IL) was used for statistical analyses. hrVEGF concentration data from the initial experiment were standardized to baseline and vehicle values.
Results
Demonstration of hrVEGF Uptake in Mouse Liver and Its Effect on Receptor Expression.
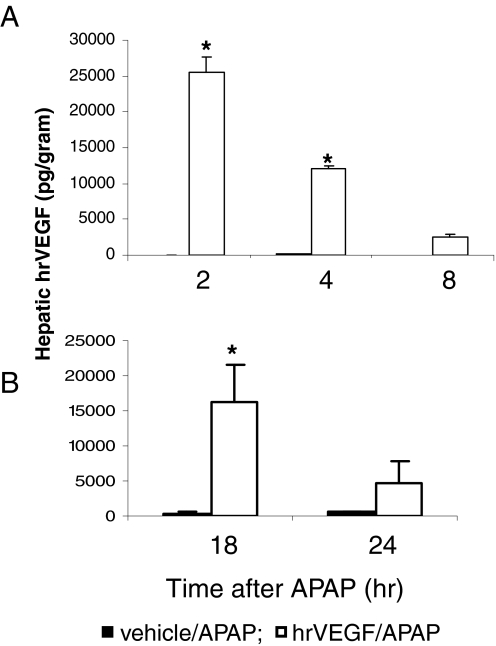
In the early toxicity experiment (Fig. 1A), mice were dosed with APAP (200 mg/kg i.p.) and vehicle (PBS, subcutaneously) or APAP and hrVEGF (50 μg s.c. in PBS). Control mice received only PBS. Mice were sacrificed at 2, 4, and 8 h after APAP dosing. To verify that hrVEGF entered the liver, hVEGF levels were measured in the supernatants of liver homogenates. In the mice that received hrVEGF, hVEGF levels peaked at 2 h and remained significantly elevated at 4 h, compared with vehicle/APAP-treated mice (Fig. 2A). Administration of hrVEGF to mice had no significant effects on murine VEGF levels (data not shown).
Fig. 2.
Demonstration of hrVEGF in mouse liver after treatment with hrVEGF. A, mice were treated with APAP (200 mg/kg i.p.) plus vehicle (PBS) or APAP plus hrVEGF (50 μg, s.c.) and sacrificed at the indicated times. hVEGF levels were significantly elevated at 2 and 4 h in mice treated with hrVEGF. B, mice were treated with APAP and received two doses of hrVEGF or vehicle and were sacrificed at the indicated times. hVEGF levels were significantly elevated at 18 h in mice treated with hrVEGF. hVEGF levels in PBS-treated mice were 0.0 pg/g in both experiments (data not shown). *, significant difference from APAP/veh groups, p < 0.05.
In the intermediate toxicity experiment (Fig. 1B), mice were dosed with hrVEGF as above, followed by a second dose of hrVEGF 10 h after APAP, and sacrificed at 18 or 24 h after APAP dosing. hVEGF was higher in the mice that received hrVEGF at both 18 and 24 h, and this finding was statistically significant at 18 h (Fig. 2B). A late toxicity experiment (Fig. 1C) was also performed in which mice received three doses of hrVEGF or vehicle at 30 min before or 10 and 24 h after APAP and were subsequently sacrificed at 36 h. hVEGF levels were increased by 30% in the hrVEGF-treated mice in this experiment (354.3 ± 153.9) compared with the APAP/veh mice (164.7 ± 93.9), but this change was not at a level of statistical significance.
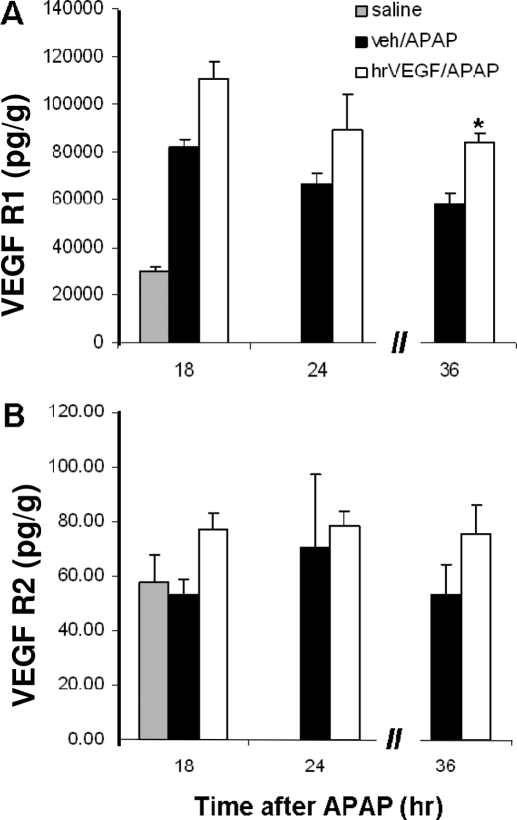
In addition, the effect of hrVEGF on the expression of VEGFR1 and VEGFR2 was examined by using ELISA. Modest increases in VEGFR1 and VEGFR2 expression were observed in the mice that received hrVEGF, compared with the APAP/vehicle mice. These relative changes in receptor expression were significant at the 36-h time point for VEGFR1 (Fig. 3).
Fig. 3.
Effect of hrVEGF on VEGFR1 (A) and VEGFR2 (B) expression. VEGFR1 and VEGFR2 levels were examined in the mice from the early (not shown), intermediate, and late experiments. Mean (± S.E.) hepatic VEGFR1 and VEGFR2 levels were increased in the hrVEGF-treated mice compared with the mice that received vehicle/APAP. This finding was significant for VEGFR1 in the 36-h experiment. *, p < 0.05.
Effect of hrVEGF on APAP Toxicity and Metabolism.
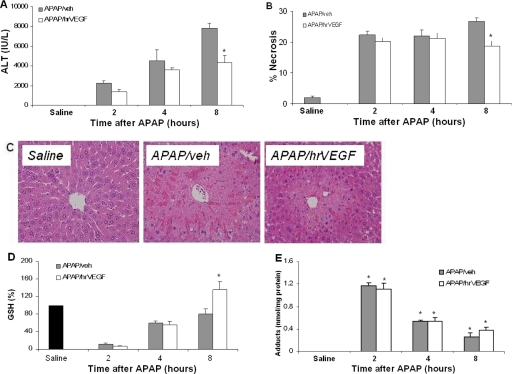
The effect of hrVEGF on APAP toxicity was examined by measurement of serum ALT and histological analysis of liver sections. ALT levels were lower in the APAP/hrVEGF mice compared with the APAP/vehicle mice at 8 h (p < 0.05; Fig. 4A). Area necrosis measures of H&E-stained sections (Fig. 4B) were in agreement with the ALT data. Representative liver sections from the 8-h animals are shown in Fig. 4C. Hepatic GSH was measured to assess the effect of hrVEGF on hepatic metabolic function. GSH levels were significantly reduced to comparable levels in both APAP groups at 2 h. By 8 h, GSH had recovered to baseline in the APAP/veh mice, whereas the APAP/hrVEGF-treated mice had GSH levels that were approximately 40% higher than mice receiving APAP/veh (Fig. 4D). To further examine the effect of hrVEGF on the metabolism of APAP, APAP-cysteine adducts were measured in the supernatants of liver homogenates. Adducts were increased in all APAP-treated mice, and no differences were present between the APAP/veh and the APAP/hrVEGF groups at any time point (Fig. 4E). These data indicated that hrVEGF did not alter the metabolism of APAP in the early stages of APAP toxicity, as reflected by the comparable values for hepatic GSH and hepatic protein adducts at 2 and 4 h.
Fig. 4.
Effect of hrVEGF on ALT, histology, and metabolism. Mice were treated with APAP/veh or APAP/hrVEGF and sacrificed at the indicated times. A, at 8 h, mice receiving hrVEGF plus APAP had significantly reduced ALT levels compared with the mice that received vehicle plus APAP. *, p < 0.05, significant difference from APAP/veh group. B, histological examination of H&E-stained slides revealed decreased necrosis in the hrVEGF-treated mice at 8 h. C, histology of saline-, APAP/veh-, and APAP/hrVEGF-treated mice. D, at 2 and 4 h, APAP/hrVEGF mice and APAP/veh mice had comparable reductions in GSH levels. At 8 h, hrVEGF-treated mice had significantly higher GSH levels than the APAP/veh mice. *, p < 0.05, significant difference from APAP/veh group. E, APAP protein adducts in liver homogenates of mice treated with APAP/veh or APAP/hrVEGF. Adducts were increased at 2, 4, and 8 h in all treatment groups. No differences were present between the APAP/veh and the APAP/hrVEGF groups at any time point.
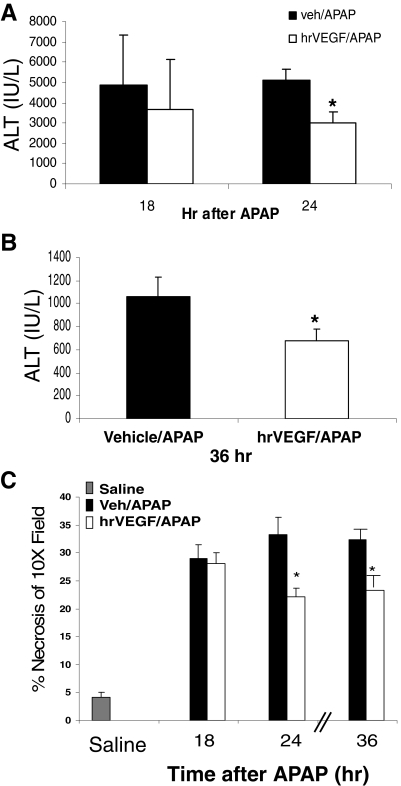
In the intermediate experiment (Fig. 1B), mice that received hrVEGF had ALT levels that were 25 and 43% lower at 18 and 24 h, respectively, compared with APAP/veh mice. This difference was statistically significant at 24 h (Fig. 5A). In the late experiment (Fig. 1C), mice received three doses of hrVEGF or vehicle control and were sacrificed at 36 h after APAP. Mice treated with hrVEGF had significantly lower ALT levels compared with the APAP/veh mice at 36 h (Fig. 5B). Histological necrosis measurements of the H&E-stained sections in these two experiments were in agreement with the ALT data (Fig. 5C). Thus, in summary, the toxicity data showed that mice pretreated with hrVEGF had reduced hepatic injury at 8, 24, and 36 h.
Fig. 5.
Effect of hrVEGF on ALT levels in the intermediate and late stages of toxicity. Mice were treated with APAP/veh or APAP/hrVEGF and sacrificed at the indicated times. A, at 18 and 24 h, mice receiving hrVEGF had reduced ALT levels compared with the APAP/veh mice. Mean (± S.E.) ALT values in PBS mice were 28.9 ± 5.3 IU/liter (data not shown). B, mice treated with hrVEGF had significantly lower ALT levels than vehicle-treated mice at 36 h. Mean ALT (± S.E.) values in control mice were 16.2 ± 2.1 IU/liter (data not shown). *, p < 0.05, significant difference from veh/APAP group. C, necrosis scores of mice sacrificed at 18, 24, and 36 h. Necrosis was reduced (*, p < 0.05) in the hrVEGF-treated mice at 24 and 36 h compared with the APAP/veh-treated mice.
No differences in hepatic GSH levels were detected between the treatment groups in the intermediate and late toxicity experiments, in contrast to the 8-h data (Fig. 4C).
VEGF, initially known as vascular permeability factor, can increase vascular extravasation (Zhang et al., 2000) and microvascular permeability (Senger et al., 1983). To examine for any potential adverse events related to these known mechanisms, hemoglobin and hematocrit values of the mice were examined in the 18- and 24-h experiments. No differences were found at these time points for these parameters between the APAP/hrVEGF, APAP/veh, and vehicle-treated mice (data not shown).
Effect of hrVEGF on Hepatocyte Regeneration.
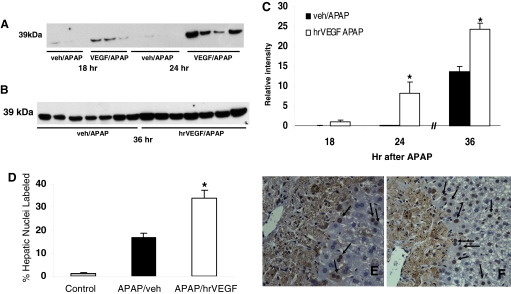
In a previous study, we showed that treatment with an inhibitor of VEGF-mediated signaling reduced hepatocyte proliferation in APAP toxicity. Therefore, the effect of administration of hrVEGF on PCNA expression was examined. As demonstrated in Fig. 6, mice dosed with hrVEGF and sacrificed at 18 or 24 h had increased up-regulation of PCNA compared with APAP/veh-treated mice (Fig. 6A). Likewise, mice that received three doses of hrVEGF had noticeable up-regulation of PCNA compared with vehicle-treated mice (Fig. 6B). Densitometric analysis indicated that PCNA expression was significantly higher at 24 and 36 h in the hrVEGF-treated mice (Fig. 6C; p < 0.05), compared with the APAP/veh-treated mice. To further examine PCNA expression between the two groups, quantitative image analysis of PCNA-immunostained slides was performed and showed an increased percentage of nuclear staining for PCNA in the APAP/hrVEGF mice compared with the APAP/veh mice (Fig. 6D).
Fig. 6.
Effect of hrVEGF on PCNA expression. A, mice were treated with APAP/veh or APAP/hrVEGF and sacrificed at 18 or 24 h. hrVEGF treatment increased PCNA expression by Western blot at 18 and at 24 h compared with APAP/veh-treated mice. B, mice were treated with APAP/veh or APAP/hrVEGF and sacrificed at 36 h. Mice treated with hrVEGF had increased expression of PCNA by Western blot compared with vehicle-treated mice. C, densitometric analysis indicated a significant difference in PCNA expression, represented as mean (±S.E.), at 24 and 36 h in the hrVEGF-treated mice (p < 0.05) compared with the APAP/veh mice at the same time points. D, image analysis of PCNA-stained slides from the 36-h experiment indicated increased PCNA labeling in the hepatic nuclei of the mice treated with hrVEGF compared with the vehicle-treated mice. *, p < 0.05. E, representative mouse liver section of PCNA staining in an APAP/veh-treated mouse. F, representative mouse liver section of PCNA staining from an APAP/hrVEGF-treated mouse.
Effect of hrVEGF on IL-6 Production.
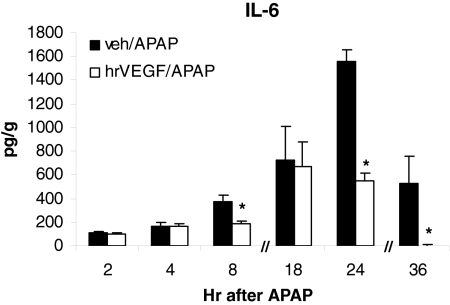
We previously showed that IL-6 was unregulated in the early stages of APAP toxicity and IL-6 was important to hepatocyte regeneration in APAP toxicity in the mouse (James et al., 2003a). Specifically, we found that IL-6 knockout mice had reduced PCNA expression in the late stages of APAP toxicity compared with comparable wild-type mice (James et al., 2003a). To determine whether administration of hrVEGF altered the endogenous production of IL-6 in APAP toxicity, IL-6 levels were examined in the early, intermediate, and late stages of toxicity. As demonstrated in Fig. 7, significant differences in hepatic IL-6 levels between the treatment groups were detected at 8, 24, and 36 h. Although lower levels of IL-6 were found in the mice receiving hrVEGF at other time points, those values were not significantly different.
Fig. 7.
Effect of hrVEGF on hepatic IL-6 expression. IL-6 levels were examined in the mice from the early, intermediate, and late experiments. Mean (± S.E.) hepatic IL-6 levels were reduced in mice that received hrVEGF compared with vehicle-treated mice. *, p < 0.05.
Effect of hrVEGF on Platelet Endothelial Cell Adhesion Molecule Expression.
In a previous study, we showed that inhibition of VEGFR2 signaling reduced PECAM expression (Donahower et al., 2006). PECAM is a commonly used marker of neovascularization (Yoshiji et al., 2004). PECAM expression in whole liver homogenates was increased in both groups of APAP-treated mice, compared with the saline group, but no differences were noted between the treatment groups (data not shown).
Effect of hrVEGF Given After APAP Administration.
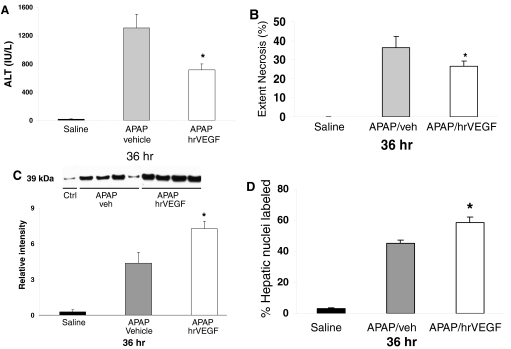
In the final experiment, the effect of hrVEGF given after APAP administration was examined (Fig. 1D). Mice were dosed with APAP as described above and received hrVEGF or vehicle at 2, 10, and 24 h. The mice were sacrificed at 36 h. As with the late toxicity experiment above, ALT values were significantly lower (Fig. 8A), and the extent of necrosis (Fig. 8B) was reduced in the mice that received hrVEGF compared with the treatment control mice. In addition, PCNA expression by Western blot analysis (Fig. 8C) and image analysis of PCNA staining of hepatocyte nuclei (Fig. 8D) was greater in the hrVEGF-treated mice than in the PBS-treated mice. Thus, hrVEGF delivered after APAP had hepatoprotective effects, including reduced toxicity and the augmentation of hepatocyte regeneration.
Fig. 8.
Effect of treatment with hrVEGF on ALT levels, necrosis, and PCNA expression at 36 h. Mice were treated with APAP followed by hrVEGF or vehicle at 2, 10, or 24 h and sacrificed at 36 h. A, at 36 h, mice receiving hrVEGF had reduced ALT levels compared with the APAP/veh mice. B, mice treated with hrVEGF had significantly reduced necrosis scores compared with the vehicle-treated mice. C, PCNA expression by immunoblot. Mice treated with hrVEGF had increased expression of PCNA by immunoblot analysis. Lanes signify representative mice, and densitometry represents summary of all mice (n = 12 per treatment group). D, summary of image analysis for PCNA-labeled nuclei. *, p < 0.05 for all comparisons of APAP/veh versus APAP/hrVEGF.
Effect of hrVEGF on Hyaluronic Acid Expression.
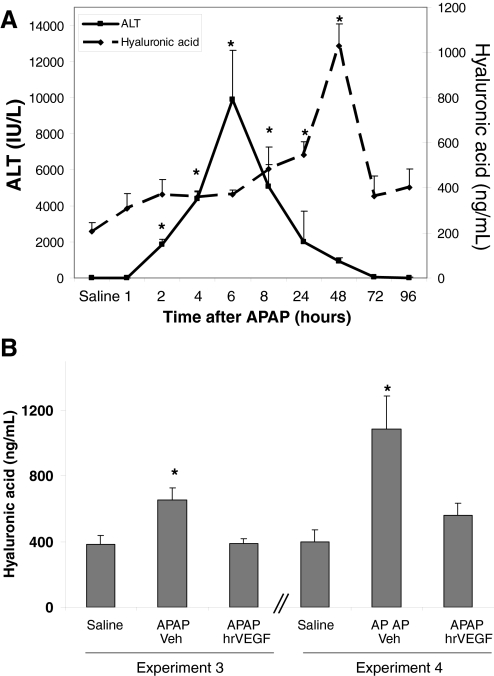
To understand the potential mechanisms of the effect of hrVEGF as a hepatoprotectant in APAP toxicity, plasma levels of hyaluronic acid were examined. We postulated that hrVEGF may play a role in improving sinusoidal endothelial cell dysfunction because VEGF has mitotic effects on endothelial cells, the cells that line the hepatic sinusoid. In addition, VEGF alters vascular permeability (Senger et al., 1983), which could affect hepatic microcirculation. Previous data have shown that hepatic microvascular injury, including sinusoidal cell injury, occurs early in APAP toxicity (Walker et al., 1985; McCuskey, 2006, 2008). Because HA is cleared by normal endothelial cells, increased levels of plasma HA represent a functional marker of sinusoidal cell injury. Figure 9A demonstrates the time course of plasma HA levels in mice treated with APAP (200 mg/kg i.p.) and sacrificed at 1, 2, 4, 6, 8, 24, 48, 72, and 96 h after APAP treatment. HA levels in plasma gradually increased from baseline and were significantly increased at 4, 8, 24, and 48 h after APAP treatment and thereafter abruptly returned to baseline levels (Fig. 9A). In addition, elevation of plasma HA followed the onset of ALT elevation in the APAP-treated mice. To assess the role of treatment with hrVEGF on sinusoidal endothelial cell dysfunction, plasma levels of HA were determined in the mice treated with hrVEGF from experiments 3 and 4 (Fig. 1). HA levels were markedly increased in the APAP/veh-treated mice (Fig. 9B; experiment 3) in comparison with saline-treated mice (p < 0.05). In comparison, mice that received hrVEGF before APAP had significantly reduced plasma HA levels that were comparable with the saline-treated mice. Furthermore, mice that received hrVEGF 2 h (Fig. 9B; experiment 4) after APAP had reduced HA levels as well. Thus, the data suggest that one mechanism for the hepatoprotective effects of hrVEGF on APAP toxicity in the mouse is alleviation of sinusoidal endothelial cell dysfunction.
Fig. 9.
Effect of hrVEGF on plasma levels of HA. A, mice were treated with APAP (200 mg/kg i.p.) and sacrificed at the indicated times. Plasma HA levels were significantly increased at 4, 8, 24, and 48 h (p < 0.05), and the onset of plasma HA elevation occurred before the ALT response. B, plasma HA levels were measured in mice that received hrVEGF before APAP and 2 h after APAP. Additional doses of hrVEGF were administered at 10 and 24 h, and the mice were sacrificed at 36 h. Plasma HA levels were reduced at 36 h in both groups of mice treated with hrVEGF. *, p < 0.05.
Effect of hrVEGF on Neutrophil Influx in APAP Toxicity.
Neutrophil influx to the liver in APAP toxicity has been reported by a number of investigators (Lawson et al., 2000). Liu et al. (2004, 2006) have postulated that neutrophils play a role in the propagation and severity of liver injury. The summary data (mean ± S.E.) for the neutrophil counts are presented in Table 1. The data showed that neutrophils were increased above control mice in the APAP/veh mice and the APAP/hrVEGF mice at 8 h. Modest changes in neutrophil counts between the two treatment groups were noted at some of the time points, but they were not statistically significant. However, at 36 h, mice that received hrVEGF had lower neutrophil counts than the APAP/veh-treated mice. This finding was observed in the mice that were pretreated with hrVEGF (experiment 3) and those that received hrVEGF at 2 h (experiment 4). The data thus suggest that hrVEGF altered the dynamics of neutrophil accumulation in APAP toxicity.
TABLE 1.
Neutrophil counts
Neutrophil counts (mean ± S.E.) in liver in mice treated with APAP (200 mg/kg i.p.) and hrVEGF (50 μg s.c.)
| Experiment | Time Point | Treatment |
p Value | |
|---|---|---|---|---|
| APAP/veh | APAP/hrVEGF | |||
| h | ||||
| 1 | 2 | 0.66 ± 0.31 | 1.53 ± 0.79 | 0.992 |
| 4 | 1.86 ± 0.83 | 1.0 ± 0.20 | 0.988 | |
| 8 | 6.58 ± 1.35a | 5.4 ± 1.04a | 0.946 | |
| 2 | 18 | 3.67 ± 2.11 | 10.48 ± 1.56a | 0.173 |
| 24 | 7.87 ± 0.66 | 8.98 ± 2.51a | 0.994 | |
| 3 | 36 | 7.7 ± 1.96a | 2.88 ± 0.49 | 0.018b |
| 4 | 36 | 6.19 ± 1.00a | 2.83 ± 0.62 | 0.027b |
Comparison with saline group (mean = 0.33 ± 0.24 neutrophils per high-powered field; 40× magnification).
Comparison between APAP/veh and APAP/hrVEGF groups.
Discussion
The cytokine VEGF is the only known mitogen to act specifically on endothelial cells. It has proangiogenic effects in the developing vasculature and has been implicated in the pathogenesis of diabetes, tumors, retinopathy, and numerous other conditions (Ray et al., 2004; Schoeffner et al., 2005). We previously reported that endogenous murine VEGF levels were markedly increased in the late stages of APAP toxicity and inhibition of VEGF signaling significantly reduced hepatocyte proliferation (PCNA expression) in the late stages of toxicity (Donahower et al., 2006). In light of these previous data, we examined the effects of administration of exogenous VEGF on various phases of APAP toxicity in the mouse (Fig. 1).
Treatment of APAP-treated mice with hrVEGF resulted in significant uptake of hrVEGF into the liver (Fig. 2). Although calculation of the elimination half-life for hrVEGF in the mice was not performed because of the limited number of samples obtained after the administration of hrVEGF, the relatively higher values of hrVEGF obtained at 24 h (4556 ± 3128) in the intermediate toxicity experiment compared with the values at 8 h in the intermediate toxicity experiment (2510 ± 379) suggest that clearance of hrVEGF may have been slower in the later stage of toxicity. It is possible that this observed change in clearance was secondary to hepatic congestion and the relative increase in hepatic plasma volume that has been reported to occur in APAP toxicity (Walker et al., 1985).
Administration of hrVEGF also affected the expression of VEGF receptors, particularly VEGFR1 expression (Fig. 3B), which was statistically increased at 36 h. VEGFR1 and VEGFR2 have distinct functions and unique binding affinities for VEGF under in vitro conditions. VEGFR1 has a binding affinity for VEGF that is 10-fold greater than that of VEGFR2 (Shibuya, 2006). However, the tyrosine kinase activity of VEGR1 is approximately 10-fold less than that of VEGFR2 (Shibuya, 2006). Thus, the increased expression of VEGFR1 at 36 h may have been a reflection of enhanced binding affinity of VEGFR1 in vivo. Of interest, LeCouter et al. (2003) showed previously that treatment of liver sinusoidal endothelial cells with a selective VEGFR1 mutant increased the expression of hepatocyte promitotic factors (IL-6 and hepatocyte growth factor) in vitro. In addition, in vivo studies showed that the VEGFR1 mutant was protective in carbon tetrachloride toxicity in the mouse (LeCouter et al., 2003). The exact role of VEGFR1 is controversial (Ferrara et al., 2003), and some laboratories have reported that it may serve as a decoy, making VEGF unavailable to VEGFR2, which is known to mediate the angiogenic effects of VEGF (Ferrara et al., 2003). We did not note significant changes in VEGFR2 or PECAM expression in whole liver homogenates in this study, endpoints that would suggest enhancement of the angiogenic effects of VEGF.
The primary goals of the present study were to examine the effect of hrVEGF on APAP toxicity and hepatocyte regeneration. Pretreatment of APAP-treated mice with hrVEGF resulted in a significant decrease of serum ALT values and a corresponding reduction in necrosis that was statistically significant at 8 h (Fig. 4). Of interest, Tsurui et al. (2005) examined the effect of hrVEGR in a mouse model of hepatic ischemia reperfusion and observed a hepatoprotective response that was similar in magnitude to the data of the present study. In previous time course studies of APAP toxicity in the mouse (James et al., 2003b), we noted the elevation of serum ALT at 2 and 4 h (Fig. 9). In addition, hepatic GSH depletion occurs by 1 h with restoration of hepatic GSH at 8 h. The data from the present study demonstrated that pretreatment with hrVEGF did not affect the early metabolism stages of toxicity as reflected by comparable values for serum ALT, histology, hepatic protein adduct formation, and hepatic GSH at 2 and 4 h. However, ALT values and histology were improved at 8 h, suggesting that the mechanisms for hepatoprotection involved events downstream or independent of metabolism. The significance of higher values of hepatic GSH at 8 h in the present study is unclear but may indicate that there was an improvement in the metabolic capacity of the cells. In an in vitro study, VEGF treatment of endothelial cells was shown to increase intracellular levels of GSH and serve an antioxidant role in a cell-based model (Kuzuya et al., 2001).
Likewise, hrVEGF treatment also reduced ALT values and the extent of necrosis at later time points in the toxicity (18, 24, and 36 h; Figs. 3 and 4). These effects were associated with enhanced regeneration of hepatocytes in the late stages of toxicity. As described above, our previous studies have supported a role for endogenous VEGF in hepatocyte regeneration (Donahower et al., 2006), and similar data were reported recently in a rat model of APAP toxicity (Papastefanou et al., 2007). In the present study, hrVEGF enhanced PCNA expression by Western blot assays performed on whole liver homogenates. At 18 h, this effect was slight, and by 24 and 36 h, more pronounced expression of PCNA was apparent in the hrVEGF-treated mice (Fig. 5), compared with the vehicle/APAP mice. Quantitative image analysis of immunohistochemical assays for PCNA expression in hepatocyte nuclei supported the findings of the Western blot assays. Thus, cumulatively, the data support the hypothesis that hrVEGF enhances hepatocyte regeneration in APAP toxicity in the mouse. To further test this effect, studies were performed in which hrVEGF was administered 2 h after APAP. As demonstrated in Fig. 8, hrVEGF given at 2, 10, and 24 h resulted in lower ALT values, reduced histologic necrosis, and enhanced expression of PCNA. Thus, hrVEGF administered to mice after APAP was hepatoprotective, and the data suggest that future study of hepatoregenerative therapies are warranted in the preclinical model of APAP toxicity.
Treatment with hrVEGF also reduced the expression of IL-6 (Fig. 6). IL-6 is known to be an important factor in hepatocyte regeneration in several models of liver injury, including partial hepatectomy and carbon tetrachloride-mediated hepatotoxicity (Cressman et al., 1996). We and others have shown that IL-6 expression is increased in APAP toxicity and IL-6 KO mice have increased toxicity compared with wild-type mice (James et al., 2003a; Masubuchi et al., 2003). We also found that IL-6 knockout mice had reduced PCNA expression compared with wild-type mice (James et al., 2003a). However, treatment with murine IL-6 did not alter toxicity or hepatocyte regeneration after APAP toxicity (Bajt et al., 2003; James et al., 2003a). Despite the reduction in the IL-6 endogenous response in the present study, treatment with hrVEGF enhanced recovery. One possibility is that hrVEGF accelerated recovery, and as a secondary effect, the endogenous IL-6 response was suppressed; this interpretation of the data would be consistent with previous data showing the redundancy of repair networks in the regeneration response (Bajt et al., 2003; James et al., 2003a).
Administration of hrVEGF also resulted in alleviation of sinusoidal endothelial cell dysfunction as measured by reduced plasma HA levels in mice treated with hrVEGF (Fig. 9B). Changes in the cellular integrity of the hepatic sinusoid have been described in APAP toxicity (Walker et al., 1985; McCuskey, 2006, 2008) and include the development of large pores in the sinusoidal endothelial cells and the filling of the space of Disse with red blood cells (Walker et al., 1985). HA is cleared by normal sinusoidal endothelial cells, and with the onset of sinusoidal endothelial cell injury HA levels in plasma rise. The significant reduction of plasma HA in hrVEGF-treated mice suggests that hrVEGF may have hastened the recovery of cells in the hepatic sinusoid, but further experiments are needed to examine the potential mechanisms of hrVEGF on the hepatic sinusoid in APAP toxicity. Endogenous VEGF and other angiogenic growth factors have been shown to be important in the reconstitution of the hepatic sinusoid after hepatic injury (Enomoto et al., 2004). Another related possibility is that hrVEGF altered microvascular perfusion. In a model of cerebral stroke, treatment of rats with hrVEGF led to improvements in cerebral microvascular perfusion (Zhang et al., 2000).
Treatment with hrVEGF also resulted in significant reductions in neutrophil counts at 36 h. Neutrophils represent the greatest fraction of inflammatory cells present in livers of APAP-treated mice (Liu et al., 2004), although their role in the mediation of APAP toxicity is controversial (Jaeschke et al., 2006). Lawson et al. (2000) showed that treatment with an antibody against B2 integrins did not protect against toxicity. However, other data suggest that neutrophils are mechanistically important in the propagation of APAP toxicity (Liu et al., 2004, 2006). Two laboratories (Ishida et al., 2006; Liu et al., 2006) independently showed that induction of neutropenia by administration of the anti-Gr-1 antibody to APAP-treated mice reduced toxicity at 6 and 24 h. To our knowledge, the role of neutrophils in the very late stages of APAP toxicity (beyond 24 h) has not been previously examined. The mechanism for the reduction of neutrophil counts in the hrVEGF-treated mice is unclear. One possibility is that treatment with hrVEGF may have dampened other endogenous repair responses, such as chemokine up-regulation, which has been reported to be important in the process of hepatocyte regeneration (Hogaboam et al., 1999) but also may enhance neutrophil infiltration. Alternatively, VEGF is well known to enhance vascular permeability, and it is possible that this effect may have altered neutrophil dynamics in the hrVEGF-treated mice. Further studies are warranted to examine the mechanisms responsible for neutrophil reduction in the hrVEGF mice.
It has been proposed that acute liver injury occurs in two phases: phase 1 or initiation and phase 2 or progression (Limaye et al., 2006). Liver regeneration is thought to limit the progression of phase 2 injury (Limaye et al., 2006). Cumulatively, the data in the present study suggest that hrVEGF had dual effects: mitigation of toxicity and enhancement of hepatocyte regeneration. Differential, time-dependent effects of hrVEGF in a model of brain injury have recently been reported (Zhang et al., 2000). The effect of hrVEGF on APAP toxicity was not apparent until a relatively late time point in the toxicity (i.e., 8 h) and did not alter the metabolism of APAP at earlier time points. It is possible that the hepatoprotective effects of hrVEGF were secondary to the alleviation of sinusoidal endothelial cell injury in hrVEGF-treated mice and this process is mechanistically important in both attenuating toxicity and facilitating the onset of hepatocyte regeneration. Further study should be focused on defining the potential treatment window for which reversal of sinusoidal endothelial cell dysfunction is possible and examining the direct effects of hrVEGF on the cellular architecture within the hepatic sinusoid and the resulting effects on toxicity and hepatocyte regeneration. Because the clinical antidote N-acetylcysteine targets primarily the metabolic phase of toxicity, its efficacy is limited by the time it is administered relative to an APAP dose. Novel therapies focused on new molecular targets that may mitigate the progression of toxicity and/or enhance the regeneration response could represent mechanistically relevant advancements to the currently available treatment approaches for humans with APAP poisoning. Based on the present data, further studies of hrVEGF as a potential treatment for APAP toxicity seem warranted.
This work was supported in part by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant 075936]; the National Institutes of Health National Institute of Environmental Health Sciences [Grant ES10952]; the University of Arkansas for Medical Sciences College of Medicine Children's University Medical Group Fund Grant Program; and the University of Arkansas for Medical Sciences Graduate Student Research Fund.
A portion of this work was presented in part at the annual meeting of Experimental Biology 2007: Donahower BC, McCullough S, Simpson P, Lamps L, Hinson J, and James L, (2007) Effect of exogenous VEGF on acetaminophen toxicity; 2007 April 28–May 2; Washington, DC.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.163840.
- VEGF
- vascular endothelial growth factor
- VEGFR
- VEGF receptor
- hrVEGF
- human recombinant VEGF
- APAP
- acetaminophen
- ALT
- alanine aminotransferase
- GSH
- glutathione
- HA
- hyaluronic acid
- IL
- interleukin
- PCNA
- proliferating cell nuclear antigen
- PBS
- phosphate-buffered saline
- PECAM
- platelet endothelial cell adhesion molecule
- veh
- vehicle
- H&E
- hematoxylin and eosin
- ELISA
- enzyme-linked immunosorbent assay.
References
- Bajt ML, Knight TR, Farhood A, Jaeschke H. (2003) Scavenging peroxynitrite with glutathione promotes regeneration and enhances survival during acetaminophen-induced liver injury in mice. J Pharmacol Exp Ther 307:67–73 [DOI] [PubMed] [Google Scholar]
- Banai S, Jaklitsch MT, Shou M, Lazarous DF, Scheinowitz M, Biro S, Epstein SE, Unger EF. (1994) Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation 89:2183–2189 [DOI] [PubMed] [Google Scholar]
- Bourdi M, Masubuchi Y, Reilly TP, Amouzadeh HR, Martin JL, George JW, Shah AG, Pohl LR. (2002) Protection against acetaminophen-induced liver injury and lethality by interleukin 10: role of inducible nitric oxide synthase. Hepatology 35:289–298 [DOI] [PubMed] [Google Scholar]
- Conn G, Soderman DD, Schaeffer MT, Wile M, Hatcher VB, Thomas KA. (1990) Purification of a glycoprotein vascular endothelial cell mitogen from a rat glioma-derived cell line. Proc Natl Acad Sci USA 87:1323–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. (1996) Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274:1379–1383 [DOI] [PubMed] [Google Scholar]
- Donahower B, McCullough SS, Kurten R, Lamps LW, Simpson P, Hinson JA, James LP. (2006) Vascular endothelial growth factor and hepatocyte regeneration in acetaminophen toxicity. Am J Physiol Gastrointest Liver Physiol 291:G102–G109 [DOI] [PubMed] [Google Scholar]
- Enomoto K, Nishikawa Y, Omori Y, Tokairin T, Yoshida M, Ohi N, Nishimura T, Yamamoto Y, Li Q. (2004) Cell biology and pathology of liver sinusoidal endothelial cells. Med Electron Microsc 37:208–215 [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. (2003) The biology of VEGF and its receptors. Nat Med 9:669–676 [DOI] [PubMed] [Google Scholar]
- Hogaboam CM, Simpson KJ, Chensue SW, Steinhauser ML, Lukacs NW, Gauldie J, Strieter RM, Kunkel SL. (1999) Macrophage inflammatory protein-2 gene therapy attenuates adenovirus- and acetaminophen-mediated hepatic injury. Gene Ther 6:573–584 [DOI] [PubMed] [Google Scholar]
- Ishida Y, Kondo T, Kimura A, Tsuneyama K, Takayasu T, Mukaida N. (2006) Opposite roles of neutrophils and macrophages in the pathogenesis of acetaminophen-induced acute liver injury. Eur J Immunol 36:1028–1038 [DOI] [PubMed] [Google Scholar]
- Jaeschke H. (2005) Role of inflammation in the mechanism of acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol 1:389–397 [DOI] [PubMed] [Google Scholar]
- Jaeschke H. (2006) How relevant are neutrophils for acetaminophen hepatotoxicity? Hepatology 43:1191–1194 [DOI] [PubMed] [Google Scholar]
- James LP, Lamps LW, McCullough S, Hinson JA. (2003a) Interleukin 6 and hepatocyte regeneration in acetaminophen toxicity in the mouse. Biochem Biophys Res Commun 309:857–863 [DOI] [PubMed] [Google Scholar]
- James LP, McCullough SS, Lamps LW, Hinson JA. (2003b) Effect of N-acetylcysteine on acetaminophen toxicity in mice: relationship to reactive nitrogen and cytokine formation. Toxicol Sci 75:458–467 [DOI] [PubMed] [Google Scholar]
- James LP, Kurten RC, Lamps LW, McCullough S, Hinson JA. (2005) Tumour necrosis factor receptor 1 and hepatocyte regeneration in acetaminophen toxicity: a kinetic study of proliferating cell nuclear antigen and cytokine expression. Basic Clin Pharmacol Toxicol 97:8–14 [DOI] [PubMed] [Google Scholar]
- Kuzuya M, Ramos MA, Kanda S, Koike T, Asai T, Maeda K, Shitara K, Shibuya M, Iguchi A. (2001) VEGF protects against oxidized LDL toxicity to endothelial cells by an intracellular glutathione-dependent mechanism through the KDR receptor. Arterioscler Thromb Vasc Biol 21:765–770 [DOI] [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, et al. (2005) Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 42:1364–1372 [DOI] [PubMed] [Google Scholar]
- Lawson JA, Farhood A, Hopper RD, Bajt ML, Jaeschke H. (2000) The hepatic inflammatory response after acetaminophen overdose: role of neutrophils. Toxicol Sci 54:509–516 [DOI] [PubMed] [Google Scholar]
- LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, Ferrara N. (2003) Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science 299:890–893 [DOI] [PubMed] [Google Scholar]
- Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246:1306–1309 [DOI] [PubMed] [Google Scholar]
- Limaye PB, Bhave VS, Palkar PS, Apte UM, Sawant SP, Yu S, Latendresse JR, Reddy JK, Mehendale HM. (2006) Up-regulation of calpastatin in regenerating and developing rat liver: role in resistance against hepatotoxicity. Hepatology 44:379–388 [DOI] [PubMed] [Google Scholar]
- Liu ZX, Govindarajan S, Kaplowitz N. (2004) Innate immune system plays a critical role in determining the progression and severity of acetaminophen hepatotoxicity. Gastroenterology 127:1760–1774 [DOI] [PubMed] [Google Scholar]
- Liu ZX, Han D, Gunawan B, Kaplowitz N. (2006) Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology 43:1220–1230 [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Bourdi M, Reilly TP, Graf ML, George JW, Pohl LR. (2003) Role of interleukin-6 in hepatic heat shock protein expression and protection against acetaminophen-induced liver disease. Biochem Biophys Res Commun 304:207–212 [DOI] [PubMed] [Google Scholar]
- McCuskey RS. (2006) Sinusoidal endothelial cells as an early target for hepatic toxicants. Clin Hemorheol Microcirc 34:5–10 [PubMed] [Google Scholar]
- McCuskey RS. (2008) The hepatic microvascular system in health and its response to toxicants. Anat Rec (Hoboken) 291:661–671 [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. (1973) Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther 187:211–217 [PubMed] [Google Scholar]
- Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. (2002) Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab Dispos 30:446–451 [DOI] [PubMed] [Google Scholar]
- Papastefanou VP, Bozas E, Mykoniatis MG, Grypioti A, Garyfallidis S, Bartsocas CS, Nicolopoulou-Stamati P. (2007) VEGF isoforms and receptors expression throughout acute acetaminophen-induced liver injury and regeneration. Arch Toxicol 81:729–741 [DOI] [PubMed] [Google Scholar]
- Ray D, Mishra M, Ralph S, Read I, Davies R, Brenchley P. (2004) Association of the VEGF gene with proliferative diabetic retinopathy but not proteinuria in diabetes. Diabetes 53:861–864 [DOI] [PubMed] [Google Scholar]
- Schoeffner DJ, Matheny SL, Akahane T, Factor V, Berry A, Merlino G, Thorgeirsson UP. (2005) VEGF contributes to mammary tumor growth in transgenic mice through paracrine and autocrine mechanisms. Lab Invest 85:608–623 [DOI] [PubMed] [Google Scholar]
- Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. (1983) Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219:983–985 [DOI] [PubMed] [Google Scholar]
- Shibuya M. (2006) Vascular endothelial growth factor receptor-1 (VEGF-1/Flt-1): a dual regulator of angiogenesis. Angiogenesis 9:225–230; discussion 231 [DOI] [PubMed] [Google Scholar]
- Taniguchi E, Sakisaka S, Matsuo K, Tanikawa K, Sata M. (2001) Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J Histochem Cytochem 49:121–130 [DOI] [PubMed] [Google Scholar]
- Tsurui Y, Sho M, Kuzumoto Y, Hamada K, Akashi S, Kashizuka H, Ikeda N, Nomi T, Mizuno T, Kanehiro H, et al. (2005) Dual role of vascular endothelial growth factor in hepatic ischemia-reperfusion injury. Transplantation 79:1110–1115 [DOI] [PubMed] [Google Scholar]
- Walker RM, Racz WJ, McElligott TF. (1985) Acetaminophen-induced hepatotoxic congestion in mice. Hepatology 5:233–240 [DOI] [PubMed] [Google Scholar]
- Ware JA, Simons M. (1997) (1997) Angiogenesis in ischemic heart disease. Nat Med 3:158–164 [DOI] [PubMed] [Google Scholar]
- Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, Wu Y, Yanase K, Namisaki T, Kitade M, et al. (2004) Halting the interaction between vascular endothelial growth factor and its receptors attenuates liver carcinogenesis in mice. Hepatology 39:1517–1524 [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. (2000) VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 106:829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]