Abstract
Amphetamine (AMPH) poses a serious hazard to public health. Defining the molecular targets of AMPH is essential to developing treatments for psychostimulant abuse. AMPH elicits its behavioral effects primarily by increasing extracellular dopamine (DA) levels through the reversal of the DA transporter (DAT) cycle and, as a consequence, altering DA signaling. In Caenorhabditis elegans, an excess of synaptic DA results in a loss of motility in water, termed swimming-induced paralysis (SWIP). Here we demonstrate that AMPH produces SWIP in a time- and dose-dependent manner in wild-type (wt) animals but has a reduced ability to generate SWIP in DAT knock out worms (dat-1). To determine whether D1-like and/or D2-like receptors are involved in AMPH-induced SWIP, we performed experiments in DOP-1 and DOP-4, and DOP-2, and DOP-3 receptor knockout animals, respectively. AMPH administration resulted in a reduced ability to induce SWIP in animals lacking DOP-3, DOP-4, and DOP-2 receptors. In contrast, in worms lacking DOP-1 receptors, AMPH-induced SWIP occurred at wt levels. Using microamperometry on C. elegans DA neurons, we determined that in contrast to wt cells, AMPH failed to promote DA efflux in dat-1 DA neurons. These data suggest that DA efflux is critical to sustaining SWIP behavior by signaling through DOP-3, DOP-4, and DOP-2. In a double mutant lacking both DAT-1 and DOP-1 expression, we found no ability of AMPH to induce SWIP or DA efflux. This result supports the paradigm that DA efflux through C. elegans DAT is required for AMPH-induced behaviors and does not require DOP-1 signaling.
Dysfunction in DA signaling has been implicated in multiple pathologies, including schizophrenia, attention-deficit/hyperactivity disorder, Parkinson's disease, and AMPH abuse. Although it is well known that dopaminergic pathways are involved in cocaine and AMPH abuse, a comprehensive understanding of the key players coordinating AMPH behaviors is still missing. Upon exocytotic release of DA into the synapse, DATs coordinate the spatial and temporal action of DA by actively clearing the amine from the extracellular space. Although diffusion and enzymatic degradation may partially govern the synaptic concentration of DA, knockout studies have established DAT function as the primary mechanism controlling extracellular DA levels. DAT is the major molecular target responsible for the reward properties and abuse potential of several psychostimulants, including cocaine, methamphetamine, and AMPH. As a substrate of DAT, AMPH elevates extracellular DA levels by antagonizing DA clearance and stimulating DAT-mediated DA efflux. The subsequent increase in dopaminergic signaling mediated by DA receptors leads to secondary plasticity that mediates addiction. Whereas multiple lines of evidence implicate DA receptors in the mechanism of action of abused psychostimulants such as AMPH (Hiroi and White, 1991; Vallone et al., 2000; Ball et al., 2003), the degree to which different receptors support AMPH-induced behaviors has not yet been completely elucidated.
In Caenorhabditis elegans, DA is a prominent neurotransmitter synthesized in eight sensory neurons: two anterior deirid neurons, four cephalic neurons, and two posterior deirid neurons. In addition, three pairs of gender-specific dopaminergic neurons are present in the male tail (Sulston et al., 1975). The mammalian neuronal proteins involved in DA synthesis, vesicle loading, and reuptake all have C. elegans homologs, and mutants for each of these homologs have been described previously (Wintle and Van Tol, 2001; Nass and Blakely, 2003). Sawin et al. (2000) showed that exogenous DA has inhibitory effects on C. elegans motor behavior. It is noteworthy that both DA biosynthesis and DA receptor signaling have been involved in changes in C. elegans locomotion in response to food exposure (Sawin et al., 2000; Chase et al., 2004). McDonald et al. (2007) identified a behavior mediated by DA termed swimming-induced paralysis (SWIP), which emerges when nematodes are placed in water, and is regulated by the DA transporter DAT-1.
Here, we took advantage of the nematode C. elegans to establish a model to elucidate the mechanism of AMPH action at DA synapses and in in vivo subsequent behaviors. First, we show that AMPH treatment rapidly induces SWIP in wt but not DAT-1 knockout (dat-1) worms. In addition, we provide evidence that AMPH-induced SWIP requires DA synthesis, DA storage, and DA signaling. Finally, we demonstrate that cultured nematode DA neurons, like their mammalian counter parts, exhibit AMPH-induced DA efflux, which is required for SWIP. These results strongly suggest that DA released by AMPH through the transporter accounts for the behavior observed in animals treated with AMPH.
Materials and Methods
C. elegans Transgenic Animals.
In this study, the wild-type strain used was N2 Bristol. All C. elegans strains were cultured on bacteria lawns of NA22 or OP50 and maintained at 12 or 20°C using standard methods (Brenner, 1974). The dat-1(ok157)III strain was a gift from J. Duerr and J. Rand (Oklahoma Medical Research Foundation, Oklahoma City, OK), which is a complete loss-of-function mutation that eliminates the majority of the DAT-1 coding sequence. The dop-1(vs100)X, dop-2(vs105)V, dop-3(vs106)X, dop-4(ok1321)X, cat-1(e1111)X, and cat-2(e1112)II were obtained from the Caenorhabditis Genetic Center (University of Minnesota, Minneapolis, MN). dat-1;dop-1 double mutants were created by crossing dat-1(ok157)III males with dop-1(vs100)X hermaphrodites and screened using single-worm polymerase chain reaction (Barstead et al., 1991; Williams et al., 1992) with oligonucleotides directed at dat-1(ok157)III and then further analyzed using oligonucleotides specific to dop-1(vs100)X. Pdat-1 ::GFP;dop-3 animals were created by injecting 90 ng/μl Pdat-1 ::GFP:DAT-1 plasmid into the dop-3 strain. Animals positive for GFP fluorescence in DA neurons were selected. For amperometry experiments, transgenic animals expressing soluble GFP under the control of the dat-1 promoter were used to identify dopaminergic neurons.
SWIP Assay.
Worms used for SWIP were grown on normal growth medium plates spread with NA22 bacteria. In each test, 8 to 20 young adult worms were placed in 40 μl of water with or without AMPH in a single well of a Pyrex spot plate (Thermo Fisher Scientific, Waltham, MA), and the number of paralyzed worms was reported as a percentage of the total number of animals observed in each test. At least 54 worms were tested per group. The number of paralyzed worms was counted every minute using an inverted microscope (Carl Zeiss, Inc., Thornwood, NY). Data were analyzed using Prism software (ver. 4; GraphPad Software, Inc., San Diego, CA).
Measurement of DA Efflux.
DA neurons were isolated 2 days before recording from C. elegans embryos as described previously (Carvelli et al., 2004) and plated in glass-bottomed culture dishes (MatTech Corporation, Cincinnati, OH) coated previously with peanut lectin. Before recording, plates were washed twice with bath solution containing 145 mM NaCl, 5 mM KCl, 1 mM CaCl2, 5 mM MgCl2, 10 mM HEPES, and 20 mM d-glucose, pH 7.2 (325 osmolarity adjusted with sucrose). DA fluxes were recorded using a carbon fiber electrodes connected to an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA), touching the plasma membrane of DA neurons expressing cytosolic GFP. Amperometric currents were digitized with an Axon Digidata 1320A, collected with pClamp 10.2, filtered with a low-pass Bessel filter set at 5 Hz, and analyzed using pClampfit 10.2 (all from (Molecular Devices) and Origin 6.1 software (OriginLab Corp, Northampton, MA).
Results
AMPH-Induced SWIP Requires DAT-1 Expression.
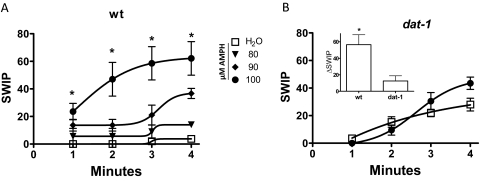
We have shown recently that an increase in endogenous extracellular DA levels triggers SWIP (McDonald et al., 2007). Here, we tested whether the psychostimulant AMPH, which impedes and reverses DAT-1 function and, as a consequence, increases extracellular DA levels, promotes SWIP. Wild-type animals were placed into glass wells containing 40 μl of water (vehicle) with or without AMPH, and the emergence of SWIP was measured as a function of time. Under vehicle treatment, virtually no worms were paralyzed after 2 min, and after 4 min of energetic swimming, only 4 ± 2% of worms tested were paralyzed (Fig. 1A, □). AMPH, in contrast, produced SWIP in a time- and concentration-dependent manner (Fig. 1A , solid symbols). At concentrations greater than 80 μM, AMPH produced significant SWIP at 4 min with respect to water-treated animals. Robust SWIP was achieved at 100 μM AMPH with 62 ± 12% of animals paralyzed after 4 min of AMPH treatment (Fig. 1A, ●). In all subsequent experiments, we exposed animals to 100 μM AMPH for 4 min.
Fig. 1.
AMPH-induced SWIP requires DAT-1. A, AMPH treatment induces a time- and concentration-dependent increase in SWIP (solid symbols). In contrast, vehicle treatment (□) does not induce paralysis (*, p < 0.01, one-way analysis of variance). B, in dat-1 animals, AMPH treatment (100 μM at 4 min; ●) did not induce a significant increase in SWIP with respect to vehicle treated animals (□). Inset, ΔSWIP calculated after treatment for 4 min with 100 μM AMPH for wt (n = 15, 271 animals) and dat-1 animals (n = 8, 136 animals).
To verify whether DAT-1, which is expressed only in DA neurons (Carvelli et al., 2004), is required for AMPH-induced SWIP, we used DAT-1 knockout animals (dat-1) in swimming assays in the presence and absence of AMPH. Figure 1B shows that 100 μM AMPH failed to produce statistically significant SWIP in dat-1 worms (●) with respect to vehicle-treated (□) worms (31 ± 5 versus 24 ± 8% of paralyzed animals, respectively). However, because dat-1 mutants have a higher baseline of SWIP (McDonald et al., 2007) with respect to wt (compare Fig. 1, A, □, with B, □), we focused on the AMPH-induced change in SWIP (ΔSWIP), which is calculated as the number of paralyzed worms upon AMPH treatment minus the number of paralyzed worms upon vehicle treatment. It is noteworthy that we found that AMPH-induced ΔSWIP is much higher in wt animals expressing DAT-1 than in dat-1 animals (Fig. 1B, inset). This supports the hypothesis that ablation of the dat-1 gene results in a decreased propensity of the animals to paralyze in water upon AMPH exposure and strongly supports the role of DAT-1 as a key player in AMPH-induced SWIP behavior.
AMPH-Induced SWIP Is Supported by DA Storage, DA Synthesis, and DA Receptor Signaling.
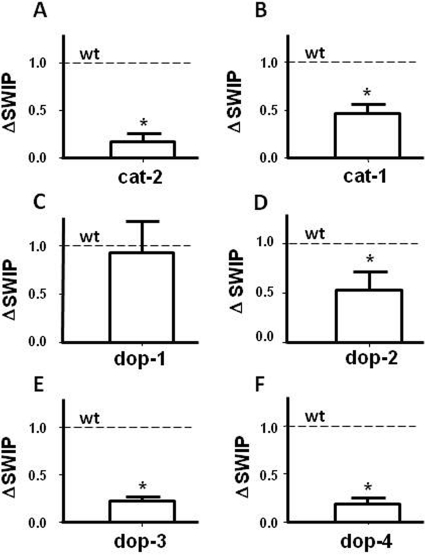
DA signaling plays a central role in the behaviors induced by psychostimulants such as AMPH (Koob and Nestler, 1997). Therefore, to identify the key elements of dopaminergic signaling involved in AMPH-induced behaviors, we measured AMPH-induced SWIP in animals lacking DA biosynthesis (cat-2), DA packaging into vesicles (cat-1), and animals missing each of the four DA receptors (dop-1,2,3,4). Figure 2A shows that cat-2 mutants, which are animals lacking tyrosine hydroxylase and have highly reduced DA levels (Sanyal et al., 2004), displayed significant reduction in AMPH effects compared with wt animals. At 4 min, in the absence of AMPH, these animals did not display basal SWIP (data not shown), possibly because of their highly reduced DA content.
Fig. 2.
AMPH-induced SWIP is supported by CAT-2, CAT-1, DOP-2, DOP-3, and DOP-4 expression. AMPH-induced ΔSWIP was significantly (Student's t test) diminished in A animals lacking tyrosine hydroxylase (A; *, p < 0.01; n = 6, 121 animals), VMAT (B; *, p < 0.05; n = 11, 163 animals), DOP-2 (D; *, p < 0.05; n = 14, 206 animals), DOP-3 (E; *, p < 0.005; n = 8, 151 animals), and DOP-4 (F; *, p < 0.005; n = 4, 106 animals) receptors compared with wt animals (dotted line). In contrast, AMPH-induced SWIP (C) was not significantly reduced in worms lacking DOP-1 (n = 12; 228 animals). All data are expressed as a percentage of wt.
It is well established that AMPH treatment results in a redistribution of DA from synaptic vesicles to the cytosol. Three models have been proposed to explain this phenomenon. First, in the weak base model, AMPH redistributes monoamines to the cytosol by collapsing the proton gradient required for vesicular accumulation of transmitter (Sulzer and Rayport, 1990; Rudnick and Wall, 1992; Sulzer et al., 1993), resulting in a reversal of vesicular monoamine transporters (VMATs). Second, as a substrate of VMAT, AMPH competitively inhibits DA uptake into vesicles, causing a leak of vesicular transmitter (Floor et al., 1995; Pothos et al., 2000). The third model suggests that the binding of nontransported AMPH to the cytosolic face of VMAT induces the release of transmitter from vesicles (Floor and Meng, 1996), although the mechanism of this phenomenon remains obscure. Considering that VMAT seems to play a fundamental role in these three models, we wanted to determine the requirement of VMAT in the mechanism of AMPH-induced SWIP by using cat-1 animals, which do not express VMAT (Sulston et al., 1975; Duerr et al., 1999). cat-1 animals did not show a significant change in basal SWIP with respect to wt animals (4.1 ± 2 versus 4 ± 2%). However, when we treated cat-1 mutants with AMPH, we measured significant reduction in ΔSWIP with respect to wt animals (Fig. 2B). These results suggest that a lack of DA accumulation into vesicles reduces the ability of AMPH to cause its behavioral effects, further supporting the role of VMAT in the AMPH actions.
To date, four DA receptors have been cloned and functionally characterized in C. elegans: the D1-like DA receptors DOP-1 and DOP-4 (Suo et al., 2002; Sugiura et al., 2005); and the D2-like DA receptors DOP-2 and DOP-3 (Suo et al., 2003; Chase et al., 2004; Sugiura et al., 2005). We investigated the involvement of these receptors in AMPH-induced SWIP using knockout lines for each receptor. Animals lacking DOP-1 (dop-1) did not differ from wt in their response to AMPH (Fig. 2C) or basal SWIP (data not shown). However, animals lacking DOP-2 (dop-2) showed a significant increase in basal SWIP compared with wt (34 ± 8% versus 4 ± 2%; p ≤ 0.02 by Student's t test). It is noteworthy that in dop-2 animals, AMPH-induced ΔSWIP was significantly decreased with respect to wt (Fig. 2D). In DOP-3 and DOP-4 knockout animals (dop-3, dop-4), basal SWIP did not differ significantly from wt (data not shown); however, dop-3 and dop-4 animals displayed a robust decrease in AMPH-induced ΔSWIP (Fig. 2, E and F, respectively), achieving ΔSWIP levels similar to those measured in both cat-2 and dat-1 mutants. The 80% reduction in ΔSWIP detected in both dop-3 and dop-4 animals suggests that DOP-3 and DOP-4 receptors play a primary role in the transmission of DA signaling, which ultimately generates paralysis after AMPH treatment.
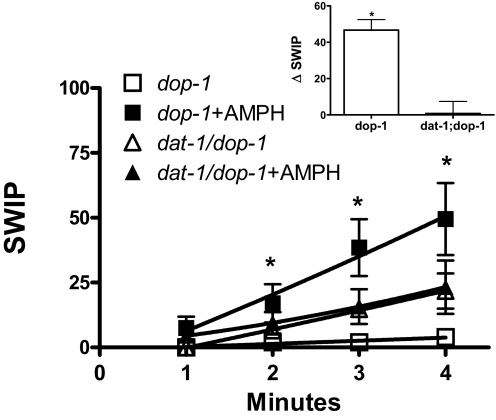
Although dop-1 animals respond to AMPH similarly to wt, it is possible that the decrease in ΔSWIP measured in dat-1 worms is mediated and/or requires DOP-1 signaling. To test this possibility, we generated the transporter/receptor double mutant line, lacking both DAT-1 and DOP-1 expression (dat-1;dop-1). Whereas AMPH-induced ΔSWIP in dop-1 animals showed a magnitude similar to that observed in wt animals (compare ■ in Fig. 3 with ● in Fig. 1A), the dat-1;dop-1 double mutants behaved as dat-1 mutants under vehicle treatment (compare ▵ in Fig. 3 with □ in Fig. 1B). After 4 min in water, 22 ± 7% of dat-1;dop-1 animals were paralyzed, and AMPH treatment did not significantly increase the percentage of paralyzed animals (23 ± 10%). Furthermore, ΔSWIP calculated for dat-1;dop-1 animals (Fig. 3, inset) was robustly reduced with respect to dop-1 animals. These data demonstrate that DOP-1 does not mediate the decrease in AMPH-induced ΔSWIP observed in dat-1 worms.
Fig. 3.
AMPH-induced SWIP is significantly reduced in dat-1/dop-1 double mutants. In dop-1 animals, AMPH induces a significant increase in SWIP (■) compared with vehicle-treated controls (□). A transporter/receptor double mutant, dat-1;dop-1, was generated. AMPH-induced SWIP was not significantly reduced in dat-1;dop-1 animals (▴, n = 4, 54 animals) with respect to vehicle treated controls (▵; n = 4, 59 animals). Inset, the ΔSWIP induced by 100 μM AMPH (4 min) in dop-1 animals was significantly different from that measured in dat-1;dop-1 double mutants (*, p ≤ 0.001, Student's t test, n = 4; 103 animals).
SWIP Correlates with the Ability of AMPH to Induce DA Efflux.
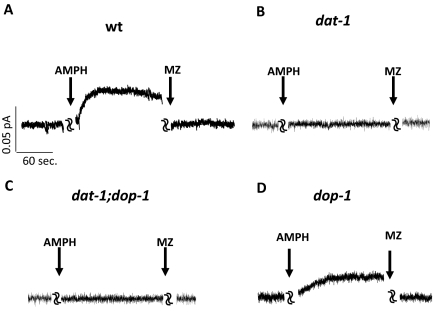
It is known that AMPH treatment induces DA release through DAT, thereby altering the fidelity of DA signaling (Koob and Bloom, 1988; Di Chiara, 1995; Sulzer et al., 2005). Therefore, DAT has been hypothesized to be a key element in the basis of AMPH-induced behaviors (Giros et al., 1996; Sulzer et al., 2005). We sought to determine whether AMPH triggers DA efflux in C. elegans DA neurons and to establish whether efflux requires functional DAT. To this end, we created primary embryonic cultures of C. elegans, isolated dopaminergic neurons using a visual marker, and measured DA efflux by microamperometry. This technique takes advantage of a carbon-fiber electrode held at +700 mV, a voltage greater than the redox potential at which DA is oxidized. Thus, DA efflux stimulated by AMPH can be detected by the carbon fiber electrode as a measure of DA oxidation (Schroeder et al., 1994; Khoshbouei et al., 2003). Primary neurons were obtained from embryonic cells of transgenic C. elegans worms carrying a transcriptional fusion of the dat-1 promoter fused upstream to the GFP gene. DA neurons were easily identified in these mixed cultures by GFP fluorescence. In DA neurons isolated from wt animals (which generate no redox signal on their own), 10 μM AMPH triggered DA efflux (0.07 ± 0.02 pA; n = 6), which was inhibited by 10 μM concentration of the DAT-1 inhibitor mazindol (−0.02 ± 0.003 pA; n = 6; Fig. 4A). AMPH-induced DA efflux was calculated subtracting the control amperometric currents (before AMPH) from the currents recorded in the presence of AMPH. AMPH-induced efflux measured in these cells was also inhibited by cocaine (data not shown). It is noteworthy that in DA neurons isolated from dat-1 animals, 10 μM AMPH failed to generate DA efflux (0.009 ± 0.007 pA, n = 5), and the application of 10 μM mazindol yielded no further effect (Fig. 4B). In DA neurons isolated from dat-1/dop-1 mutants, neither AMPH nor mazindol perfusion produced any effect in DA efflux (Fig. 4C), whereas in DA neurons isolated from dop-1 animals, which display AMPH-induced SWIP, perfusion of 10 μM AMPH induced DA efflux comparable with those measured in wt neurons (0.05 ± 0.03 pA, n = 4; Fig. 4D). Likewise, we measured DA efflux from dop-3 mutants containing the transcriptional fusion Pdat-1::GFP and bearing deletions that prevent AMPH-induced SWIP. No significant difference in AMPH-induced DA efflux was measured between dop-3 mutant (0.16 ± 0.07 pA, n = 3) and wt animals. These data reveal that the ability of AMPH to cause SWIP in C. elegans relies on both reverse transport of DAT and DA receptors signaling.
Fig. 4.
AMPH-induced DA efflux in C. elegans DA neurons. A, in DA neurons isolated from wt animals, 10 μM AMPH induced robust DA efflux (oxidation is represented as a positive current by convention) and inhibited by 10 μM mazindol. B, in contrast, in DA neurons from dat-1 animals, neither AMPH nor mazindol had any effect on DA efflux. C, DA neurons isolated from dat-1;dop-1 double mutants did not show DA effluxes after AMPH treatment. In addition, no changes were caused by mazindol to the basal amperometric current. D, in dop-1 DA neurons, perfusion of 10 μM AMPH induced a mazindol sensitive DA efflux.
Discussion
To understand the long-term consequences induced by drugs of abuse, their initial molecular and cellular targets must be identified. A combination of approaches in animal models and humans has revealed the DA system to be the prime target of addictive drugs. Specifically, psychostimulants such as AMPH and cocaine bind to the DAT and alter its ability to clear DA from the synaptic cleft, thereby terminating the DA signal (Koob and Bloom, 1988; Nestler, 1992; Chen et al., 2006). In addition, AMPH-like drugs induce DAT to operate in reverse (Sulzer et al., 1993), therefore inducing nonvesicular DA efflux. Thus, the final result of AMPH is to elevate the extracellular concentration of DA and, as a consequence, stimulate DA signaling. Yet, the key molecular players involved in the behavioral effects induced by AMPH are still unclear. In particular, which subclass of DA receptors is triggered after AMPH treatment remains in question.
In C. elegans, DA plays at least two distinct roles: 1) it affects learning by mediating the state-dependent olfactory adaptation (Colbert and Bargmann, 1995; Bettinger and McIntire, 2004); and 2) it modulates locomotor behaviors by slowing the locomotion rate when animals encounter bacteria lawns (Sawin et al., 2000). Thus, in C. elegans, DA signaling responds to environmental changes by modulating locomotion, behavior, and learning. These studies relied primarily on the analysis of wt animals exposed to exogenous DA, ablation of DA neurons, and animals mutants for CAT-2, which are deficient in tyrosine hydroxylase activity (Loer and Kenyon, 1993; Liu and Sternberg, 1995; Schafer and Kenyon, 1995; Weinshenker et al., 1999; Sawin et al., 2000; Bettinger and McIntire, 2004; Hills et al., 2004; Sanyal et al., 2004). McDonald et al. (2007) identified SWIP as a DAT-1-mediated behavior in C. elegans. This study took advantage of mutants lacking DAT-1 to show that under stress conditions, such as vigorous swimming, endogenous DA exerts an inhibitory action on motor circuits. However, wt animals, in which DAT-1 clears released DA from the synaptic cleft, do not paralyze. Here, we show that AMPH, which causes an increase in extracellular DA, induces SWIP. In addition, we reasoned that AMPH-induced SWIP might be a useful in vivo tool to determine the key elements of DA signaling sustaining the AMPH actions. Thus, by using C. elegans as a model system and SWIP as a behavioral test, we determined that AMPH specifically targets the DAT to promote DA release, and consequently, engages different classes of DA receptors, including DOP-2, DOP-3, and DOP4.
First, we show that in water, animals treated with different concentrations of AMPH paralyze in a dose- and time-dependent manner. In contrast, DAT-1 knockout animals had almost no response to AMPH treatment. It is noteworthy that DA neurons isolated from wt worms generated DA efflux upon AMPH stimulation, and this effect was blocked by the perfusion of DAT-1 inhibitors such as mazindol and cocaine. Consistent with the SWIP results, we were unable to measure a detectible DA efflux from neurons lacking DAT-1. These results suggest that DAT is a key element supporting the ability of AMPH to increase extracellular DA. Our data also support the hypothesis that increased levels of extracellular DA, released by AMPH and mediated by DAT-1, might be responsible for SWIP. The main role played by DAT-1 in DA transmission is revealed by the loss of AMPH-induced behaviors/efflux recorded in the DAT-1 knockout animals.
It is well known that DA can act on different DA receptor subtypes such as the D1- and D2-like receptors. These subtypes are believed to have different effects on forms of neuronal plasticity and, consequently, on learning and memory, because of their opposite action on the cAMP-dependent protein kinase A pathway (Missale et al., 1998; Cepeda and Levine, 1998; Floresco et al., 2001). Although DA and DA receptors are critically involved in AMPH-induced behaviors, the role of the various DA receptor subtypes has been difficult to delineate. For example, antagonizing D2-like receptors has been shown to attenuate the locomotor and rewarding effects of psychostimulants (Vallone et al., 2000; Ball et al., 2003). However, the role of D2-like receptors in the molecular mechanisms of psychostimulant abuse is not fully understood. In rodents, D2-like receptor activation decreases AMPH-induced locomotor activity and induces no change in AMPH-induced efflux in the ventral tegmental area (Steketee and Kalivas, 1992). Thus, we reasoned that by studying the role of the different DA receptors in AMPH-induced behaviors in a simple organism such as C. elegans, it would allow us to define whether they play a role in the AMPH actions. In this study, we have shown that animals lacking DOP-2, DOP-3, or DOP-4 receptors have a decreased ability to paralyze upon AMPH treatment. In contrast, dop-1 animals still respond to AMPH treatment. Moreover, our data demonstrate that AMPH-induced SWIP requires endogenous DA. Only a very small percentage of animals lacking the tyrosine hydroxylase enzyme are paralyzed after AMPH treatment. Therefore, our results strongly suggest that DA, DAT-1, and the DA receptors DOP-2, DOP-3, and DOP-4 are key players in AMPH-induced behaviors. Our data also support that dat-1 deletion does not require changes in DOP-1 signaling to decrease AMPH-induced SWIP because dat-1;dop-1 animals show robustly reduced ΔSWIP.
These data also identify, for the first time, DOP-4 as an important player in C. elegans motor behavior. DOP-4 receptors have been shown to localize in GABA AVL motoneurons, the PQR chemosensory neurons, and other head neurons (Sugiura et al., 2005). Although we do not know whether the C. elegans DA neurons make synapses with neurons expressing DOP-4 receptors, we cannot exclude the possibility that the release of large amounts of extracellular DA after AMPH treatment may lead to spillover of the neurotransmitter to extrasynaptic sites. Curiously, worms lacking VMAT (cat-1 animals) also showed significant reduction of ΔSWIP compared with wild-type animals. Thus, it seemed that the greater amount of DA released after AMPH application in wt animals is also due, in part, to the action of AMPH on VMAT (Jones et al., 1998; Sulzer et al., 2005). However, although cat-1 animals demonstrated only a 50% reduction of ΔSWIP, dat-1, cat-2, dop-3, and dop-4 animals displayed more dramatic reductions in AMPH response.
Furthermore, we have shown that after AMPH treatment, the increase in extracellular DA targets DOP-2, DOP-3, and DOP-4 signaling to generate SWIP. In addition, SWIP is sustained both by vesicular storage of DA and DA synthesis.
Forward genetic screens are unbiased but extremely costly and time-consuming in mammalian models. Thus, we propose to implement forward genetic screens to study the actions of AMPH using C. elegans. The treatment of intact worms with AMPH leads to a DAT and DA receptor-dependent phenotype termed SWIP, which we propose as a reporter phenotype for a powerful, unbiased screen to identify the genes required for AMPH action. Therefore, the future goals of this research will be to identify suppressors of AMPH-induced SWIP, followed by focused secondary screens that establish a presynaptic locus for identified mutants and their DA and DAT relationship.
Acknowledgments
We thank Dr. Randy Blakely for critical reading of the manuscript.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA13975, DA24797].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.109.062703.
- DA
- dopamine
- DAT
- dopamine transporter
- AMPH
- amphetamine
- DAT-1
- C. elegans dopamine transporter
- SWIP
- swimming-induced paralysis
- wt
- wild type
- GFP
- green fluorescent protein
- VMAT
- vesicular monoamine transporter.
References
- Ball KT, Budreau D, Rebec GV. (2003) Acute effects of 3,4-methylenedioxymethamphetamine on striatal single-unit activity and behavior in freely moving rats: differential involvement of dopamine D(1) and D(2) receptors. Brain Res 994:203–215 [DOI] [PubMed] [Google Scholar]
- Barstead RJ, Kleiman L, Waterston RH. (1991) Cloning, sequencing, and mapping of an alpha-actinin gene from the nematode Caenorhabditis elegans. Cell Motil Cytoskeleton 20:69–78 [DOI] [PubMed] [Google Scholar]
- Bettinger JC, McIntire SL. (2004) State-dependency in C. elegans. Genes Brain Behav 3:266–272 [DOI] [PubMed] [Google Scholar]
- Brenner S. (1974) The genetics of Caenorhabditis elegans. Genetics 77:71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, McDonald PW, Blakely RD, Defelice LJ. (2004) Dopamine transporters depolarize neurons by a channel mechanism. Proc Natl Acad Sci USA 101:16046–16051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Levine MS. (1998) Dopamine and N-methyl-d-aspartate receptor interactions in the neostriatum. Dev Neurosci 20:1–18 [DOI] [PubMed] [Google Scholar]
- Chase DL, Pepper JS, Koelle MR. (2004) Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat Neurosci 7:1096–1103 [DOI] [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. (2006) Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci USA 103:9333–9338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert HA, Bargmann CI. (1995) Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron 14:803–812 [DOI] [PubMed] [Google Scholar]
- Di Chiara G. (1995) The role of dopamine in drug abuse viewed from the perspective of its role in motivation. Drug Alcohol Depend 38:95–137 [DOI] [PubMed] [Google Scholar]
- Duerr JS, Frisby DL, Gaskin J, Duke A, Asermely K, Huddleston D, Eiden LE, Rand JB. (1999) The cat-1 gene of Caenorhabditis elegans encodes a vesicular monoamine transporter required for specific monoamine-dependent behaviors. J Neurosci 19:72–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floor E, Leventhal PS, Wang Y, Meng L, Chen W. (1995) Dynamic storage of dopamine in rat brain synaptic vesicles in vitro. J Neurochem 64:689–699 [DOI] [PubMed] [Google Scholar]
- Floor E, Meng L. (1996) Amphetamine releases dopamine from synaptic vesicles by dual mechanisms. Neurosci Lett 215:53–56 [DOI] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. (2001) Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci 21:2851–2860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. (1996) Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 379:606–612 [DOI] [PubMed] [Google Scholar]
- Hills T, Brockie PJ, Maricq AV. (2004) Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J Neurosci 24:1217–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, White NM. (1991) The amphetamine conditioned place preference: differential involvement of dopamine receptor subtypes and two dopaminergic terminal areas. Brain Res 552:141–152 [DOI] [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Wightman RM, Caron MG. (1998) Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. J Neurosci 18:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshbouei H, Wang H, Lechleiter JD, Javitch JA, Galli A. (2003) Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism. J Biol Chem 278:12070–12077 [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. (1988) Cellular and molecular mechanisms of drug dependence. Science 242:715–723 [DOI] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. (1997) The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci 9:482–497 [DOI] [PubMed] [Google Scholar]
- Liu KS, Sternberg PW. (1995) Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron 14:79–89 [DOI] [PubMed] [Google Scholar]
- Loer CM, Kenyon CJ. (1993) Serotonin-deficient mutants and male mating behavior in the nematode Caenorhabditis elegans. J Neurosci 13:5407–5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald PW, Hardie SL, Jessen TN, Carvelli L, Matthies DS, Blakely RD. (2007) Vigorous motor activity in Caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter DAT-1. J Neurosci 27:14216–14227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. (1998) Dopamine receptors: from structure to function. Physiol Rev 78:189–225 [DOI] [PubMed] [Google Scholar]
- Nass R, Blakely RD. (2003) The Caenorhabditis elegans dopaminergic system: opportunities for insights into dopamine transport and neurodegeneration. Annu Rev Pharmacol Toxicol 43:521–544 [DOI] [PubMed] [Google Scholar]
- Nestler EJ. (1992) Molecular mechanisms of drug addiction. J Neurosci 12:2439–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EN, Larsen KE, Krantz DE, Liu Y, Haycock JW, Setlik W, Gershon MD, Edwards RH, Sulzer D. (2000) Synaptic vesicle transporter expression regulates vesicle phenotype and quantal size. J Neurosci 20:7297–7306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick G, Wall SC. (1992) p-Chloroamphetamine induces serotonin release through serotonin transporters. Biochemistry 31:6710–6718 [DOI] [PubMed] [Google Scholar]
- Sanyal S, Wintle RF, Kindt KS, Nuttley WM, Arvan R, Fitzmaurice P, Bigras E, Merz DC, Hébert TE, van der Kooy D, et al. (2004) Dopamine modulates the plasticity of mechanosensory responses in Caenorhabditis elegans. EMBO J 23:473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR. (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26:619–631 [DOI] [PubMed] [Google Scholar]
- Schafer WR, Kenyon CJ. (1995) A calcium-channel homologue required for adaptation to dopamine and serotonin in Caenorhabditis elegans. Nature 375:73–78 [DOI] [PubMed] [Google Scholar]
- Schroeder TJ, Jankowski JA, Senyshyn J, Holz RW, Wightman RM. (1994) Zones of exocytotic release on bovine adrenal medullary cells in culture. J Biol Chem 269:17215–17220 [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. (1992) Microinjection of the D2 agonist quinpirole into the A10 dopamine region blocks amphetamine-, but not cocaine-stimulated motor activity. J Pharmacol Exp Ther 261:811–818 [PubMed] [Google Scholar]
- Sugiura M, Fuke S, Suo S, Sasagawa N, Van Tol HH, Ishiura S. (2005) Characterization of a novel D2-like dopamine receptor with a truncated splice variant and a D1-like dopamine receptor unique to invertebrates from Caenorhabditis elegans. J Neurochem 94:1146–1157 [DOI] [PubMed] [Google Scholar]
- Sulston J, Dew M, Brenner S. (1975) Dopaminergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol 163:215–226 [DOI] [PubMed] [Google Scholar]
- Sulzer D, Maidment NT, Rayport S. (1993) Amphetamine and other weak bases act to promote reverse transport of dopamine in ventral midbrain neurons. J Neurochem 60:527–535 [DOI] [PubMed] [Google Scholar]
- Sulzer D, Rayport S. (1990) Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: a mechanism of action. Neuron 5:797–808 [DOI] [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. (2005) Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol 75:406–433 [DOI] [PubMed] [Google Scholar]
- Suo S, Sasagawa N, Ishiura S. (2002) Identification of a dopamine receptor from Caenorhabditis elegans. Neurosci Lett 319:13–16 [DOI] [PubMed] [Google Scholar]
- Suo S, Sasagawa N, Ishiura S. (2003) Cloning and characterization of a Caenorhabditis elegans D2-like dopamine receptor. J Neurochem 86:869–878 [DOI] [PubMed] [Google Scholar]
- Vallone D, Picetti R, Borrelli E. (2000) Structure and function of dopamine receptors. Neurosci Biobehav Rev 24:125–132 [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Wei A, Salkoff L, Thomas JH. (1999) Block of an ether-a-go-go-like K+ channel by imipramine rescues egl-2 excitation defects in Caenorhabditis elegans. J Neurosci 19:9831–9840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BD, Schrank B, Huynh C, Shownkeen R, Waterston RH. (1992) A genetic mapping system in Caenorhabditis elegans based on polymorphic sequence-tagged sites. Genetics 131:609–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintle RF, Van Tol HH. (2001) Dopamine signaling in Caenorhabditis elegans-potential for parkinsonism research. Parkinsonism Relat Disord 7:177–183 [DOI] [PubMed] [Google Scholar]