Abstract
We have shown previously that the vasoactive peptide bradykinin (BK) stimulates proliferation of a cultured murine cell model of the inner medullary collecting duct (mIMCD-3 cells) via transactivation of epidermal growth factor receptor (EGFR) by a mechanism that involves matrix metalloproteinases (collagenase-2 and -3). Because collagenases lack an integral membrane domain, we hypothesized that receptors for extracellular matrix proteins, integrins, may play a role in BK-induced signaling by targeting collagenases to the membrane, thus forming a functional signaling complex. BK-induced phosphorylation of extracellular signal-regulated protein kinase (ERK) in mIMCD-3 cells was reduced by ∼65% by synthetic peptides containing an Arg-Gly-Asp sequence, supporting roles for integrins in BK-induced signaling. Neutralizing antibody against α5β1 integrin partially (∼60%) blocked BK-induced ERK activation but did not affect EGF-induced ERK activation. Silencing of α5 and β1 expression by transfecting cells with small interfering RNAs (siRNA) significantly decreased BK-induced ERK activation (∼80%) and EGFR phosphorylation (∼50%). This effect was even more pronounced in cells that were cotransfected with siRNAs directed against both collagenases and α5β1 integrin. On the basis of our results, we suggested that integrin α5β1 is involved in BK-induced signaling in mIMCD-3 cells. Using immunoprecipitation/Western blotting, we demonstrated association of BK B2 receptor with α5β1 integrin upon BK treatment. Furthermore, BK induced association of α5β1 integrin with EGFR. These data provide the first evidence that specific integrins are involved in BK B2 receptor-induced signaling in kidney cells, and ultimately might lead to development of new strategies for treatment of renal tubulointerstitial fibrosis.
The vasoactive nonapeptide bradykinin (BK) plays important roles in the regulation of kidney functions, such as electrolyte and water excretion (Mukai et al., 1996). Specifically, a role of BK in the control of absorptive function in the kidney collecting ducts is well established (Tomita et al., 1985; Zeidel et al., 1990). BK also acts directly as a potent cellular growth factor for multiple cell types, including kidney cells. We established previously that the BK B2 receptor stimulates early mitogenic signals associated with activation of extracellular signal-regulated protein kinase (ERK) in a murine epithelial cells derived from the inner medullary collecting duct (mIMCD-3 cells), and demonstrated that BK-induced cell proliferation depends on transactivation of the epidermal growth factor receptor (EGFR) (Mukhin et al., 2003). Furthermore, we demonstrated that BK B2 receptor-induced EGFR transactivation involves activation of matrix metalloproteinases (MMPs), namely collagenase-2 and -3 (Mukhin et al., 2006). Because collagenases lack an integral membrane domain, we hypothesized that integrins may play a role in BK-induced signaling by targeting collagenases to the membrane, thus forming a functional signaling complex. Integrins are heterodimeric receptors for cell-surface adhesion molecules and extracellular matrix proteins, which are composed of two subunits, α and β. Each αβ combination has specific signaling properties (for review, see Juliano, 2002). To date, 18 α and 8 β subunits that form at least 24 different αβ integrins have been identified (Humphries et al., 2006).
The first interaction between integrins and MMPs was identified in melanoma cells in which it was demonstrated that the C-terminal domain of gelatinase-A [matrix metalloproteinase (MMP)-2] binds directly to integrin αVβ3, which localizes MMP-2 in a proteolytically active form on the surface of invasive cells (Brooks et al., 1996). Furthermore, the involvement of the αVβ3/MMP-2 complex in tumor growth and angiogenesis has been demonstrated in vivo (Brooks et al., 1998). Integrin αVβ3 also cooperates with gelatinase-B (MMP-9) to regulate migration of breast cancer cells (Rolli et al., 2003). Purified β2 integrin has been shown to bind to the catalytic domain in pro-MMP-9 gelatinase to form complexes of pro-MMP-9 with both the αMβ2 and αLβ2 integrins in leukemic cell lines; these associations probably control the activation of the proenzyme (Stefanidakis et al., 2003). Cell-surface interactions between β2 integrins and gelatinases play roles in normal leukocyte migration and in cancer progression (Stefanidakis and Koivunen, 2006). Interactions with integrins also have been demonstrated for collagenase-1 (MMP-1). Thus, pro-MMP-1 specifically binds to α2β1 integrin on keratinocytes, facilitating the cleavage of type I collagen and keratinocyte migration (Dumin et al., 2001). This binding occurs via the I-domain of the α2 integrin subunit and requires both the linker domain and the hemopexin-like domain of MMP-1 (Stricker et al., 2001). MMP-1 also interacts with α2β1 integrin in human neurons (Conant et al., 2004) and with α1β1 integrin in monocytes (Stricker et al., 2001). However, no interactions between integrins and collagenases 2 or 3 have been described.
Interesting connections between integrins and G protein-coupled receptors (GPCRs) have been described recently. Integrin-mediated cell anchorage affects GPCR signaling to the ERK/mitogen-activated protein kinase (Della Rocca et al., 1999; Short et al., 2000). Muscarinic receptors stimulate tyrosine phosphorylation of focal adhesion kinase via an integrin-dependent mechanism (Slack, 1998). Focal adhesion kinase phosphorylation in response to bombesin and muscarinic signaling depends on the integrity of the cytoskeleton (Hunger-Glaser et al., 2003). Activation of histamine receptors results in translocation of the nonreceptor tyrosine kinase PYK2 to focal adhesions and enhances PYK2 tyrosine phosphorylation leading to ERK activation in HeLa cells (Litvak et al., 2000).
The precise molecular mechanism underlying integrin-mediated GPCR signaling to ERK remains to be defined, but one possibility involves integrin-mediated recruitment of cytoskeletal components to form a scaffold that facilitates efficient assembly of the components of the signaling pathway. Alternatively, some GPCRs interact directly with integrins: P2Y2 nucleotide receptor contains an RGD motif in the first extracellular loop that enables it to interact with αVβ3 and αVβ5 integrins, leading to increased astrocyte migration (Bagchi et al., 2005; Wang et al., 2005). In addition, β1 and β3 integrins colocalize with the μ-opioid receptor in sensory neurons and regulate receptor signaling, probably by altering its coupling to either Gαi or Gαs proteins (Berg et al., 2007).
Possible interactions between the BK B2 receptor and integrins have not been studied. Herein, we provide multiple lines of evidence that suggest the involvement of integrin α5β1 in BK-induced signaling, and we describe physical and functional connections among BK B2 receptor, α5β1 integrin, and collagenase-2 and -3 in mIMCD-3 cells.
Materials and Methods
Materials.
Cell culture supplies were obtained from Invitrogen (Carlsbad, CA), or Corning Life Sciences (Lowell, MA). Bradykinin, EGF, and other reagent-grade chemicals were from Sigma-Aldrich (St. Louis, MO). Cyclic RGD/control RGD peptides and MMP inhibitors were from Calbiochem (La Jolla, CA). Phospho-ERK kit was obtained from Cell Signaling Technology (Danvers, MA). Monoclonal anti-BK B2 receptor antibody was from BD Biosciences Transduction Laboratories (Lexington, KY). EGFR polyclonal antibody; anti-phospho-EGFR (Tyr 1173) monoclonal antibody; monoclonal anti-phosphotyrosine antibody; MMP-8 monoclonal antibody; MMP-13 monoclonal antibody; anti-α5β1 integrin polyclonal antibody; monoclonal blocking antibodies against α2β1, α5β1, αVβ6, and α6 integrins; antibodies against α2, α3, αV, and β1 integrin subunits; and mouse monoclonal glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody were from Millipore (Billerica, MA). Antibodies against integrin α2, α5, α6, β6, and β1 subunits, blocking antibodies against α3, αV, and β3 integrins, MMP-8 siRNA, MMP-13 siRNA, integrin α2 siRNA, integrin α5 siRNA, integrin β1 siRNA, and control siRNA were from Santa Cruz Biotechnology (Santa Cruz, CA). The RNA Stat-60 reagent was from Tel-Test, Inc., (Friendswood, TX). The RT2 Profiler PCR Array System for mouse extracellular matrix and adhesion molecules and the Oligo GEArray kit for mouse extracellular matrix and adhesion molecules were from SuperArray Bioscience Corporation (Frederick, MD).
Cell Culture.
mIMCD-3 cells were obtained from the American Type Culture Collection (Manassas, VA). mIMCD-3 cells were grown in equal mixtures of Dulbecco's modified Eagle's medium and Ham's F12 medium, supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Study of Expression Levels of Integrin Subunits in mIMCD-3 Cells.
To monitor the expression profile of integrins in mIMCD-3 cells, we employed the Oligo GEArray kit for mouse extracellular matrix and adhesion molecules. Using the RNA Stat-60 reagent, we extracted total RNA from mIMCD-3 cells that were grown on plastic plates and converted RNA into biotin-labeled cRNA target probes for Microarray hybridization by using the TrueLabeling-AMP linear RNA amplification kit (SuperArray Bioscience Corporation). The cRNA targets (6 μg of cRNA) next were hybridized with oligonucleotide probes, representing different genes, printed on a nylon membrane. The resulting products on arrayed membranes were detected by a chemiluminescent detection kit, and analyzed by GEArray Analyzer data analysis software. We also used an RT2 Profiler PCR Array System for mouse extracellular matrix and adhesion molecules that validates the expression of 84 relevant genes for cell-cell and cell-matrix interactions, including nine α and four β integrin subunits. Total RNA from mIMCD-3 cells was extracted as above and converted to cDNA using the RT2 Profiler PCR Array first strand kit. Quantitative real-time PCR was performed according to the manufacture's protocol. The presence of various integrin subunits in lysates from mIMCD-3 cells was supported by Western blots using commercially available antibodies.
Transfections of mIMCD-3 Cells: Integrin and MMP Silencing.
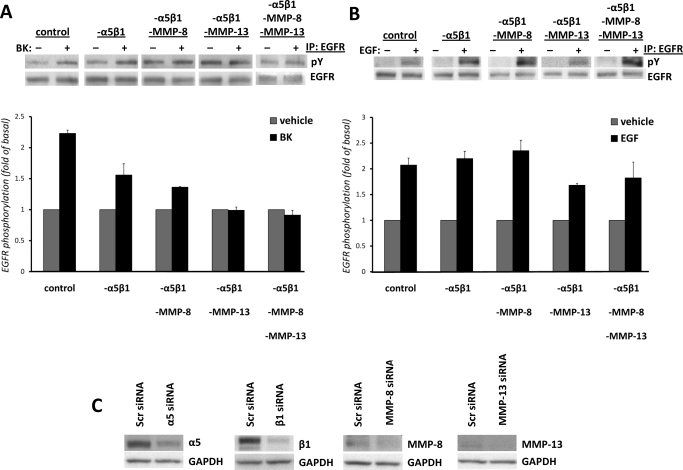
Transfections of mIMCD-3 cells were achieved by nucleofection with an Amaxa Biosystems instrument (Giessen, Germany). Cells (106) were resuspended in 100 μl of Nucleofector solution R and nucleofected with either 100 nM siRNA for MMP-8, MMP-13, or integrin α5β1 siRNA, or with the same amount of control siRNA using manufacturer's protocol T-16. Forty-eight hours after nucleofection, cells were stimulated with BK, EGF, or vehicle; lysed; and analyzed for integrin and MMP expression by Western blotting with anti-MMP-8, anti-MMP-13, or anti-integrin α5 and β1 rabbit polyclonal antibodies and for EGFR phosphorylation and ERK activation. Blots were re-probed with a mouse monoclonal GAPDH antibody to control for protein loading and for silencing specificity and efficiency.
ERK Assay.
ERK phosphorylation was assessed by Western blot using a phosphorylation-state specific antibody that specifically recognizes Thr202- and Tyr204-phosphorylated ERK-1 and ERK-2 as described previously (Mukhin et al., 2003) in mIMCD-3 cells treated for 5 min with BK, EGF, or vehicle. The membranes were stripped using Re-Blot Plus antibody stripping solution (Millipore) and reprobed with the control ERK antibody, which recognizes phosphorylated and nonphosphorylated ERK equally well. Results are presented as intensities of phospho-ERK bands relative to total ERK bands and are expressed as fold of basal phosphorylation (nontreated cells).
EGF Receptor Phosphorylation Assay.
The phosphorylation state of EGFR was assessed by immunoprecipitation/Western blotting studies as described previously (Mukhin et al., 2003). Quiescent mIMCD-3 cells, grown in 100-mm dishes, were pretreated with vehicle or inhibitors for 30 min. Subsequently, cells were treated with 100 nM BK, 1 ng/ml EGF, or vehicle for 5 min and lysed in RIPA buffer (50 mM Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM sodium fluoride, 1 mM Na3VO4, 1 μg/ml each aprotinin, leupeptin, and pepstatin). Cell lysates were precleared by incubating with protein A-agarose bead slurries for 30 min at 4°C. Precleared lysates (1 μg/μl total cell protein) were incubated with 4 μg of anti-EGFR polyclonal IgG overnight at 4°C. The immunocomplexes were captured by the addition of protein A-agarose bead slurries, with incubation for a further 2 h at 4°C. The agarose beads were collected by centrifugation, washed three times with RIPA buffer, resuspended in 2× Laemmli sample buffer, boiled for 5 min, and subjected to SDS-polyacrylamide gel electrophoresis. After wet transfer to polyvinylidene difluoride membranes, the membranes were probed with monoclonal anti-phospho-EGFR (Tyr1173) antibody to assess the phosphorylation state of EGFR, or with EGFR antibody.
Studies of Possible Complex Formation between Integrin α5β1, BK B2 Receptor, and Collagenases.
Quiescent mIMCD-3 cells, grown in 100-mm dishes, were treated with 100 nM BK, 1 ng/ml EGF, or with vehicle for different time periods and lysed in RIPA buffer. Cell lysates were precleared by incubating with protein A-agarose bead slurries for 30 min at 4°C. Precleared lysates (1 μg/μl total cell protein) were incubated with 4 μg of anti-α5β1 integrin polyclonal IgG overnight at 4°C. The immunocomplexes were captured by addition of protein A-agarose bead slurry and incubation for a further 2 h at 4°C. The agarose beads were collected by centrifugation, washed three times with RIPA buffer, resuspended in 2× Laemmli sample buffer, boiled for 5 min, and subjected to SDS-polyacrylamide gel electrophoresis. After wet transfer to polyvinylidene difluoride membranes, the membranes were probed with monoclonal anti-BK B2 receptor, with monoclonal anti-MMP-8 or anti-MMP-13 antibody, and with anti-EGFR antibody to study possible physical associations of these proteins.
Data Analysis.
Data are presented as mean + S.E.M. and were analyzed for repeated measures by Student's t test for unpaired two-tailed analysis and by ANOVA. Differences were considered significant at p < 0.05.
Results
Integrins are Involved in BK-Induced ERK1/2 Activation and EGFR Transactivation in mIMCD-3 Cells.
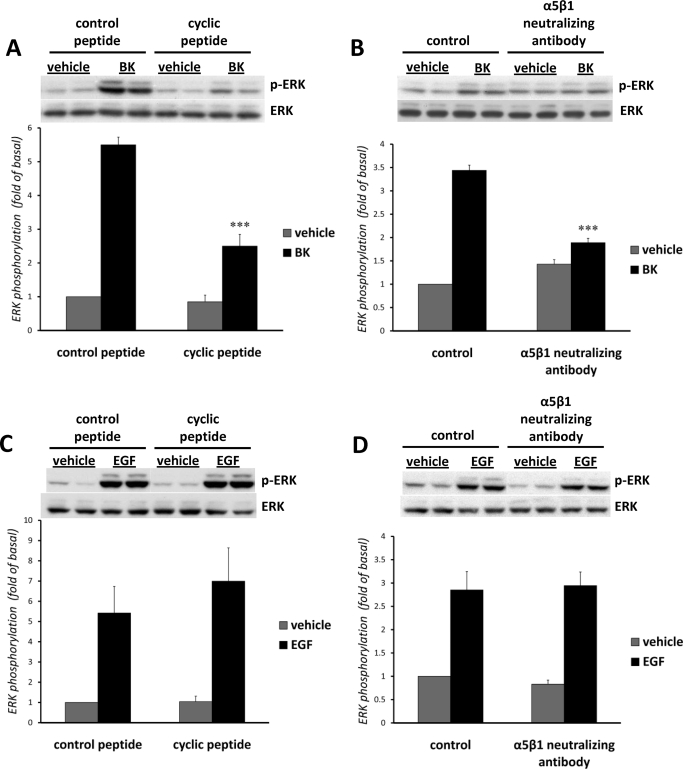
In the first set of experiments, we tested the involvement of integrins in B2 receptor-induced EGFR transactivation and ERK phosphorylation using Arg-Gly-Asp (RGD) peptides. RGD was originally identified as the sequence in fibronectin that is a recognition site for α5β1 integrin, but it also serves as a recognition motif for other integrins, including α3β1, α8β1, αVβ1, αVβ3, αVβ5, αVβ6, and α2bβ3 (Plow et al., 2000). Quiescent mIMCD-3 cells were pretreated for 1 h with 28 μM concentrations of either control or cyclic RGD peptides before stimulation with 100 nM BK or with 1 ng/ml EGF for 5 min. Control RGD peptides were without effect, whereas cyclic RGD peptides reduced the activation of ERK by BK by ∼65%, suggesting that integrins with RGD recognition specificity may be involved in BK-induced ERK phosphorylation (Fig. 1A). At the same time, cyclic RGD peptides did not affect EGF-induced ERK activation (Fig. 1C).
Fig. 1.
Involvement of integrins in BK-induced ERK phosphorylation. Quiescent mIMCD-3 cells were pretreated for 1 h either with 28 μM control or with cyclic RGD peptides before stimulation with 100 nM BK (A) or 1 ng/ml EGF (C) for 5 min. Quiescent mIMCD-3 cells were pretreated for 2 h with 0.1 mg/ml neutralizing anti-integrin antibodies before stimulation with 100 nM BK (B) or 1 ng/ml EGF (D) for 5 min. Control samples were preincubated with 0.1 mg/ml normal rabbit IgG. ERK phosphorylation was measured as described under Materials and Methods. Bars represent intensities of phospho-ERK bands relative to total ERK expressed as fold of basal (cells treated with vehicle). Experiments were performed three times in duplicate. Data are presented as mean + S.E.M. ***, p < 0.001..
Expression Profile of Integrin Subunits in mIMCD-3 Cells.
Having established a probable role for integrins in BK-induced signaling in mIMCD-3 cells, we attempted to examine specific integrins that could mediate this response. To plan more specific experiments, we needed to determine which integrins are present in mIMCD-3 cells, because at least 18 α and 8 β integrin subunits have been identified. First, we employed the Oligo GEArray kit for mouse extracellular matrix and adhesion molecules, which represents 113 genes encoding proteins important for the attachment of cells to their surroundings including various types of cell adhesion molecules (such as the integrins, IgG superfamily members, cadherins and catenins, and selectins) as well as extracellular matrix proteins, proteases (such as the matrix metalloproteinases and the serine and cysteine proteinases), and their inhibitors. This array allowed us to determine simultaneously the expression profile of 13 α and 7 β integrin subunits in mIMCD-3 cells. Table 1 summarizes mRNA expression of integrins in mIMCD-3. Integrin subunits α2b, α3, αV, and β1 seemed to be the most abundant in mIMCD-3 cells. The messages for α2, α6, α7, αX, β6, and β7 integrin subunits also were detectable. To our surprise, we did not detect message for α5 integrin subunit, although we expected to find α5β1 integrin in mIMCD-3 cells based on the ability of RGD peptides to affect BK-induced signaling (Fig. 1A).
TABLE 1.
mIMCD-3 cells express mRNA for α and β integrin subunits
The expression of mRNA was assessed by the Oligo GEArray as described under Materials and Methods. Proteins for which we were able to detect message are shown in bold
| Gene Symbol | Position | Signal Intensity | |
|---|---|---|---|
| 1 | α2 | 44 | + |
| 2 | α2b | 45 | ++++ |
| 3 | α3 | 46 | +++ |
| 4 | α4 | 47 | – |
| 5 | α5 | 48 | – |
| 6 | α6 | 49 | + |
| 7 | α7 | 50 | + |
| 8 | α8 | 51 | – |
| 9 | αE | 52 | – |
| 10 | αL | 53 | – |
| 11 | αM | 54 | – |
| 12 | αV | 55 | +++ |
| 13 | αX | 56 | ++ |
| 14 | β1 | 57 | ++++ |
| 15 | β2 | 58 | – |
| 16 | β3 | 59 | – |
| 17 | β4 | 60 | – |
| 18 | β5 | 61 | – |
| 19 | β6 | 62 | ++ |
| 20 | β7 | 63 | ++ |
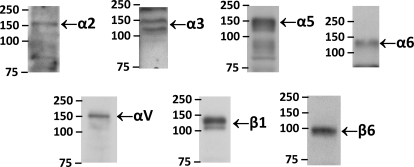
To verify the microarray data, we employed an RT2 Profiler PCR Array System for mouse extracellular matrix and adhesion molecules that measures the expression of 84 relevant genes for cell-cell and cell-matrix interactions, including nine α (α2, α3, α4, α5, αE, αL, αM, αV, and αX) and four β (β1, β2, β3, and β4) integrin subunits. The expression of the GAPDH gene was used as control. Reverse transcription-PCR results confirmed the expression of α2, α3, αX, αV and β1 genes identified by the Oligo microarray (not shown). In addition, an RT2 Profiler PCR Array System allowed us to detect the message for α5 integrin subunit. Our inability to detect the message for α5 integrin subunit by the Oligo GEArray could be caused by a technical issue with this particular Oligo GEArray layout, because the message for α5 integrin (spot 48) is probably masked by a strong message for fibronectin-1 (spot 40), which is highly expressed in mIMCD-3 cells. Furthermore, to confirm the mRNA data and to support the expression of integrins on a protein level, we performed Western blotting on mIMCD-3 lysates using commercially available antibodies against integrins. Results presented in Fig. 2 support the presence of integrins α2, α3, α5, α6, αV, β1, and β6 in mIMCD-3 cells.
Fig. 2.
Western blot analysis of mIMCD-3 lysates. Western blot analyses of mIMCD-3 lysates (40 μg of total protein) were performed with commercially available anti-integrin antibodies to demonstrate the expression of these integrins on a protein level. The bands of α2 (155 kDa), α3 (150 and 130 kDa), α5 (150 kDa), α6 (140 kDa), αV (150 kDa), β1 (130 kDa), and β6 (97 kDa) are indicated.
Roles of Specific Integrins in BK-Induced ERK1/2 Activation in mIMCD-3 Cells.
To examine specific integrins that are involved in BK-induced ERK phosphorylation, we employed commercially available neutralizing antibodies against mouse integrin subunits. Quiescent mIMCD-3 cells were pretreated for 2 h with anti-integrin antibodies or normal rabbit IgG before stimulation with 100 nM BK or with 1 ng/ml EGF for 5 min. Neutralizing antibody against α5β1 integrin, when used in a concentration of 100 ng/ml, significantly (∼60%) blocked BK-induced phosphorylation of ERK in mIMCD-3 cells (Fig. 1B; n = 3, p < 0.001), but did not affect EGF-induced ERK activation (Fig. 1D). Neutralizing antibody against α2β1 integrin in some experiments also partially (∼25%) decreased BK-induced phosphorylation of ERK in mIMCD-3 cells, although these results were not statistically significant (not shown). At the same time, neutralizing antibodies against α3, αV, β6, and αVβ6 were without effect (not shown), suggesting specificity in the involvement of α5β1 integrin in BK-induced ERK phosphorylation.
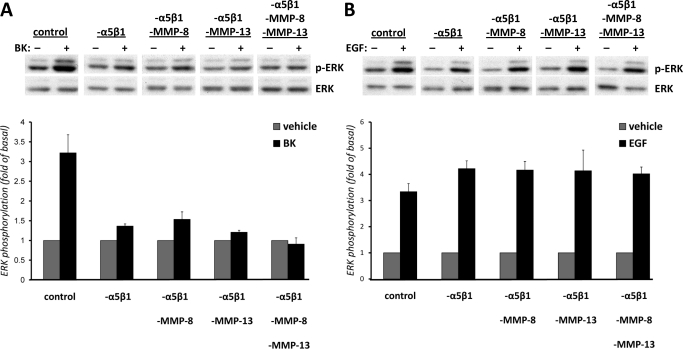
Transfection of mIMCD-3 Cells with Integrin and MMP siRNAs Decreases BK-Induced ERK Activation.
To further support the involvement of integrins in BK-induced ERK activation, we employed RNA-mediated interference to knock down the expression of α5β1 integrin. mIMCD-3 cells were nucleofected with either 100 nM siRNA for integrin α5β1 or with the same amount of control siRNA. Forty-eight hours after nucleofection, cells were stimulated either with vehicle or 100 nM BK or with 1 ng/ml EGF for 5 min, lysed, and analyzed for ERK phosphorylation. Cells transfected with α5β1 integrin siRNAs demonstrated ∼80% less BK-induced ERK activation than cells transfected with control siRNA (Fig. 3A). EGF-induced ERK activation was not affected by α5β1 integrin down-regulation (Fig. 3B). Silencing of α2β1 integrin did not affect either BK-induced or EGF-induced phosphorylation of ERK in mIMCD-3 cells (not shown), suggesting specificity in the involvement of α5β1 integrin in BK-induced ERK phosphorylation.
Fig. 3.
Transfection of mIMCD-3 cells with integrin and MMP siRNAs decreases BK-induced ERK activation. mIMCD-3 cells were nucleofected either with 100 nM siRNA for integrin α5β1 alone (-α5β1) or with combinations of MMP-8 siRNA (-α5β1-MMP-8) and/or MMP-13 siRNA (-α5β1-MMP-13), or with combinations of all siRNAs (-α5β1- MMP-8-MMP-13), or with the same amount of control siRNA (control). Forty-eight hours after nucleofection, cells were stimulated with vehicle or 100 nM BK (A) or with 1 ng/ml EGF (B) for 5 min, lysed, and analyzed for ERK phosphorylation. ERK phosphorylation was measured as described under Materials and Methods. Bars represent intensities of phospho-ERK bands relative to total ERK expressed as fold of basal (cells treated with vehicle). Experiments were performed three times in duplicate. Data are presented as mean + S.E.M. **, p < 0.01 compared with control BK-treated cells. ANOVA -α5β1 compared with -α5β1-MMP-8, -α5β1-MMP-13, or with -α5β1-MMP-8-MMP-13, not significant.
We demonstrated previously the involvement of MMP-8 and MMP-13 in BK-induced ERK activation (Mukhin et al., 2006); therefore, we performed the combined inhibition of MMP-8 or MMP-13 and α5β1 integrins by cotransfecting cells with MMP-8 or MMP-13 siRNA in addition to integrin siRNAs. Cells transfected with a combination of MMP-8 and α5β1 integrin siRNAs demonstrated ∼75% decrease in BK-induced ERK phosphorylation (Fig. 3A). Cells transfected with a combination of MMP-13 and α5β1 integrin siRNAs demonstrated ∼90% decrease in BK-induced ERK phosphorylation. Because in our previous study, we proposed roles for both collagenases in BK-induced ERK phosphorylation (Mukhin et al., 2006), we blocked both MMP-8 and MMP-13 by transfecting cells with a combination of MMP-8 and MMP-13 siRNAs in addition to silencing α5β1 integrin. Each set of transfected cells demonstrated significant decreases in BK-induced ERK phosphorylation compared with the set transfected with scrambled siRNA. Although differences between cells transfected only with α5β1 integrin siRNA and cells cotransfected with MMP-8 and MMP-13 siRNAs in addition to α5β1 integrin siRNA were not statistically significant; in all experiments, there was a trend that down-regulation of both collagenases in addition to α5β1 integrin resulted in a complete inhibition of the signal. Down-regulation of α5β1 integrin either alone or in combination with MMP-8 and/or MMP-13 did not affect EGF-induced ERK activation (Fig. 3B). Effective silencing of integrin and MMP expression in mIMCD-3 cells transfected with siRNAs was supported by immunoblotting (Fig. 4C). These results support the hypothesis that integrin α5β1 very probably mediates BK-induced signaling in mIMCD-3 cells.
Fig. 4.
Transfection of mIMCD-3 cells with integrin α5β1 and MMP siRNAs decreases BK-induced EGFR phosphorylation. Cells were nucleofected with 100 nM α5β1 siRNA (-α5β1) or -α5β1 with a combination of either MMP-8 siRNA (-α5β1-MMP-8) or MMP-13 siRNA (-α5β1-MMP-13); with a combination of all siRNAs (-α5β1- MMP-8-MMP-13); or with the same amount of control siRNA (control), as described under Materials and Methods. Forty-eight hours after nucleofection, cells were stimulated with vehicle or 100 nM BK (A) or with 1 ng/ml EGF (B) for 5 min, lysed, and analyzed for EGFR phosphorylation as described under Materials and Methods. Experiments were performed at least three times. Data are presented as mean + S.E.M. **, p < 0.01 compared with control BK-treated cells. ANOVA (-α5β1) compared with -α5β1-MMP-8, -α5β1-MMP-13, or -α5β1- MMP-8-MMP-13, not significant. C, Western blot analyses of lysates of mIMCD-3 cells transfected with either scrambled siRNA or siRNAs for α5, β1, MMP-8, and MMP-13 (40 μg of total protein) were performed with commercially available antibodies against α5 and β1 integrin subunits, MMP-8 and MMP-13 to demonstrate down-regulation of these proteins. Blots were stripped and re-probed with antibody against GAPDH to control for the specificity of silencing and protein loading.
Transfection of mIMCD-3 Cells with Integrin α5β1 and MMP-13 and MMP-8 siRNAs Decreases BK-Induced EGFR Phosphorylation.
Because BK activates ERK in mIMCD-3 cells via EGFR transactivation (Mukhin et al., 2003), in the next series of experiments, we assessed the involvement of α5β1 integrin in BK-induced EGFR phosphorylation. To test the involvement of integrin α5β1, we transfected mIMCD-3 cells with either 100 nM integrin α5β1 siRNA or with control (scrambled) siRNA. To block the activity of collagenases, we silenced MMP-8 and/or MMP-13 expression by nucleofection with MMP-8 siRNA, MMP-13 siRNA, or with both MMP-8 and MMP-13 siRNAs. Forty-eight hours after nucleofection, cells were stimulated either with vehicle or 100 nM BK or with 1 ng/ml EGF for 5 min, lysed, and analyzed for EGFR phosphorylation as described under Materials and Methods. Silencing of integrin α5β1 significantly (p < 0.01) decreased BK-induced EGFR phosphorylation (Fig. 4A). At the same time, the combined inhibition of MMP-8 and α5β1 integrin significantly blocked BK-induced EGFR phosphorylation by ∼70%, and the combined inhibition of MMP-13 and α5β1 integrin completely abolished the effect of BK (Fig. 4). Similar results were obtained when we blocked the activity of collagenases by pretreatment of mIMCD-3 cells transfected with integrin α5β1 siRNA with chemical inhibitors of MMP-8 and MMP-13 (not shown), suggesting that MMP-8, MMP-13, and α5β1 integrin are involved in BK-induced phosphorylation of EGFR. However, differences in BK-induced EGFR phosphorylation between cells transfected only with α5β1 integrin siRNA and cells cotransfected with MMP-8 and MMP-13 siRNAs in addition to α5β1 integrin siRNA were not statistically significant. At the same time, down-regulation of α5β1 integrin either alone or in combination with MMP-8 and/or MMP-13 did not affect EGF-induced EGFR phosphorylation (Fig. 4B).
BK Induces Complex Formation between α5β1 Integrin, EGFR, MMP-13, MMP-8, and BK B2 Receptor.
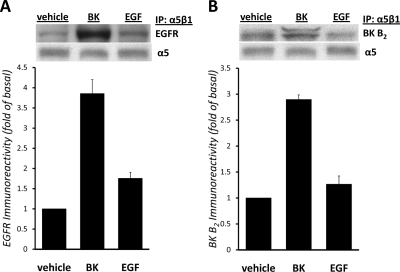
We next proposed that BK stimulates the assembly of a signal transduction complex, which includes molecules involved in EGFR phosphorylation, and examined whether BK could induce a physical interaction between α5β1 integrin, EGFR, and collagenases. To explore this possibility, we used immunoprecipitation of lysates from cells treated with vehicle, BK, or EGF with anti-α5β1 integrin antibody, followed by Western blotting with antibody against EGFR, MMP-8, MMP-13, and BK B2 receptor. Figure 5A shows that α5β1 integrin and EGFR (175 kDa) coimmunoprecipitate and that their association can be increased by stimulation of mIMCD-3 cells with BK. Stimulation with EGF did not significantly increase association between α5β1 integrin and EGFR, supporting the idea that α5β1 integrin is not essential for EGF-induced EGFR activation. Figure 5B shows that immunoprecipitation of α5β1 integrin from mIMCD-3 cells treated with BK, but not with EGF, resulted in coprecipitation of the BK B2 receptor (42 kDa). The BK B2 receptor antibody that we employed for these studies has been used previously used by our group (Kramarenko et al., 2009) and others (Golser et al., 2000; Xie et al., 2000) to demonstrate the presence of the endogenous and/or transfected BK B2 receptor in different cell lines by Western blotting, and also to immunoprecipitate BK B2 receptor (Golser et al., 2000). The bands corresponding to EGFR or BK B2 receptor were not detectable in precipitates obtained from identical samples precipitated in the presence of normal rat immunoglobulins instead of α5β1 integrin antibody (data not shown). These results suggest that BK induces coprecipitation of α5β1 integrin with EGFR and BK B2 receptor. Furthermore, we found that MMP-8 and MMP-13 also are present in BK-induced signaling complex (not shown).
Fig. 5.
BK induces complex formation between EGFR and α5β1 integrin. Lysates from mIMCD-3 cells treated with vehicle, 100 nM BK, or 1 ng/ml EGF were immunoprecipitated with anti-α5β1 integrin antibody as described under Materials and Methods. Immunoblotting was performed with antibodies against EGFR (A) and BK B2 receptor (B). The blots shown are representative of four experiments. A, coimmunoprecipitation experiments show that α5β1 integrin and EGFR coimmunoprecipitate and that their association can be increased by stimulation of mIMCD-3 cells with 100 nM BK but not with EGF. Inset, representative Western blot with antibody against EGFR showing immunoreactive band at 175 kDa. Blot was stripped and re-probed with antibody against α5 integrin to control for immunoprecipitation and protein loading. Immunoreactive band at 150 kDa is shown. B, BK B2 receptor coimmunoprecipitates with α5β1 integrin. Inset, representative Western blot with antibody against BK B2 receptor showing immunoreactive duplet at 42/40 kDa. Blot was stripped and reprobed with antibody against α5 integrin to control for immunoprecipitation and protein loading. Immunoreactive band at 150 kDa is shown. IP, immunoprecipitation; IB, immunoblot.
Discussion
The current work describes a novel mechanism of EGFR transactivation by the Gq-coupled BK B2 receptor in mIMCD-3 cells that involves integrin α5β1. What is new about this work is that we have (1) characterized the repertoire of integrins in mIMCD-3 cells using reverse transcription-PCR, microarray detection, and Western blotting; (2) implicated integrins as key mediators of BK-induced ERK activation using RGD peptides; (3) provided evidence that integrin α5β1 is involved in BK-induced EGFR transactivation based on results of experiments using neutralizing anti-integrin antibodies and siRNA; and (4) demonstrated for the first time that BK induces formation of a signaling complex among α5β1 integrin, EGFR, the BK B2 receptor, and probably MMP-13 and MMP-8.
In our previous work, we established that the BK B2 receptor stimulates early mitogenic signals associated with activation of ERK1/2 in mIMCD-3 cells and demonstrated that BK-induced cell proliferation was dependent on activation of EGFR (Mukhin et al., 2003). Furthermore, we described a novel mechanism of EGFR transactivation by the Gq-coupled BK B2 receptor that involves activation of MMPs, namely collagenases 2 and 3 (MMP-8 and MMP-13). We demonstrated that collagenases 2 and 3 are activated by the BK B2 receptor in mIMCD-3 cells, and are involved in cross-talk between the B2 receptor and EGFR (Mukhin et al., 2006). In the current study, we looked further into this mechanism by testing the hypothesis that in mIMCD-3 cells, integrins may play a role in BK-induced signaling by targeting collagenases to the membrane, thus forming a functional signaling complex. Several GPCRs have been shown to activate ERK in an integrin-dependent manner: thrombin, BK, and lysophosphatidic acid receptors in PC12 rat pheochromocytoma cells (Della Rocca et al.,1999); gonadotropin-releasing hormone receptors expressed in HEK293 cells (Davidson et al., 2004); P2Y receptors in endothelial cells (Short et al., 2000); histamine receptors in HeLa cells (Litvak et al., 2000); and δ-opioid receptors transfected into HEK293 cells and endogenously expressed in neuroblastoma x glioma hybrid NG108-15 cells (Eisinger and Ammer, 2008).
In our study, we assessed the involvement of integrins in BK-induced signaling in mIMCD-3 cells, using RGD-containing synthetic peptides that inhibit ligand binding to integrins with RGD recognition specificity (e.g., integrins α3β1, α5β1, α8β1, αVβ1, αVβ3, αVβ5, αVβ6, and α2bβ3). RGD peptides decreased BK-induced ERK activation by ∼65% (Fig. 1A) without affecting EGF-induced ERK activation (Fig. 1C), supporting the hypothesis that integrins are involved in BK-induced ERK phosphorylation in mIMCD-3 cells. Next, we aimed to determine specific integrins responsible for mediating BK-induced ERK activation. Using two different methods of mRNA analysis and Western blotting, we established that mIMCD-3 cells express the following integrin subunits: α2, α2b, α3, α5, αV, αX, β1, and β6 (Table and Fig. 2). These subunits may form four integrins with RGD recognition specificity: α3β1, α5β1, αVβ1, and αVβ6. We employed neutralizing antibodies against these integrins to study their possible involvement in BK-induced ERK activation. Neutralizing antibody against α5β1 integrin significantly blocked BK-induced phosphorylation of ERK by ∼60% in mIMCD-3 cells without affecting EGF-induced ERK activation (Fig. 1, B and D), suggesting the involvement of α5β1 integrin in BK-induced ERK phosphorylation. At the same time, neutralizing antibodies against α3, αV, β6, and αVβ6 integrins did not change BK-induced ERK phosphorylation, suggesting a lack of involvement of α3β1, αVβ1, and αVβ6 integrins. We further supported the involvement of α5β1 integrin in BK-induced signaling by using mIMCD-3 cells, in which the expression of α5 and β1 subunits was knocked down by an RNA-mediated interference (Fig. 3A). Because silencing of integrin α5β1 also decreased BK-induced EGFR phosphorylation (Fig. 4A), the present findings indicate that BK-induced ERK activation is mediated by integrin-stimulated EGFR. At the same time, down-regulation of α5β1 integrin, either alone or in combination with MMP-8 and/or MMP-13, did not affect EGF-induced EGFR phosphorylation and ERK activation (Figs. 3B and 4B). Thus, for the first time, we have identified a specific integrin (α5β1) that specifically mediates BK-induced EGFR transactivation and ERK phosphorylation in mIMCD-3 cells.
The ability of integrins to cooperate with receptor tyrosine kinases, including EGFR, to transduce proliferative signals and to regulate cell survival and migration has been discussed previously (Miranti and Brugge, 2002; Schwartz and Ginsberg, 2002). Integrins are able to form physical complexes with EGFR at the cell membrane and to trigger ligand-independent phosphorylation of Tyr845, Tyr1068, Tyr1086, and Tyr1173 residues in the EGFR molecule (Moro et al., 1998). This integrin-dependent EGFR activation seems to be necessary for full EGFR-dependent transcriptional responses (Cabodi et al., 2004). GPCR-dependent transactivation of EGFR is usually mediated by MMP-dependent processing of membrane EGFR pro-ligands (Prenzel et al., 1999). In our previous study, we demonstrated the involvement of MMP-8 and MMP-13 in BK-induced EGFR transactivation in mIMCD-3 cells; at the same time, we found that this transactivation does not require extracellular release of EGF-like growth factors such as HB-EGF and/or TGF-α (Mukhin et al., 2006). Our present data favor the possibility that BK induces association of EGFR with integrin α5β1, thus causing ligand-independent phosphorylation and activation of EGFR. In support of that idea, we showed that α5β1 integrin and EGFR coimmunoprecipitate upon BK treatment (Fig. 5A).
The concept that the association of MMPs with integrins can modify intracellular signaling has been demonstrated in several cell models (for review, see Stefanidakis and Koivunen, 2006). However, most reports describe interactions between integrins and gelatinases (MMP-2 and MMP-9) either in tumor cells (Brooks et al., 1998; Rolli et al., 2003) or in leukocytes (Stefanidakis et al., 2003). The only collagenase described to interact with integrins, collagenase-1 (MMP-1), was reported to be associated with α2β1 integrin in keratinocytes (Dumin et al., 2001) and in human neurons (Conant et al., 2004) and with α1β1 integrin in monocytes (Stricker et al., 2001). In the current work, the involvement of MMP-8 and MMP-13 in BK-induced ERK activation was supported by combined inhibition of MMP-8 and/or MMP-13 and α5β1 integrin by cotransfecting cells with MMP-8 or MMP-13 siRNA in addition to integrin siRNAs. Although differences between cells transfected only with α5β1 integrin siRNA and cells cotransfected with MMP-8 and MMP-13 siRNAs in addition to α5β1 integrin siRNA were not statistically significant, in all experiments, there was a trend that down-regulation of both collagenases in addition to α5β1 integrin resulted in a complete inhibition of BK-induced EGFR phosphorylation and ERK activation. (Figs. 3A and 4A). Thus, the current work suggests that collagenase-2 and -3 (MMP-8 and MMP-13) may act in concert with integrin α5β1 to mediate BK-induced phosphorylation of EGFR and ERK in kidney cells. It is noteworthy that the BK B2 receptor also was coimmunoprecipitated with α5β1 integrin after BK treatment of mIMCD-3 cells (Fig. 5B).
This work is novel in that there are only a few reports regarding interactions between GPCRs and integrins. Colocalization of β1 and β3 integrins with the μ-opioid receptor was detected in trigeminal ganglion neurons by immunocytochemistry and confocal imaging (Berg et al., 2007). These RGD-binding integrins probably regulate the spatial distribution of G proteins in plasma membrane microdomains containing GPCRs; therefore, the relative amounts of activated integrins (β1 or β3) at focal adhesions govern G protein subunit composition (Gαi versus Gαs) coupled to the μ-opioid receptor, thus regulating the signaling of this receptor in sensory neurons. The only GPCR that has been shown to interact directly with integrins, the P2Y2 nucleotide receptor, contains an RGD motif in the first extracellular loop that enables it to interact with αVβ3 and αVβ5 integrins (Erb et al., 2001). These interactions between the P2Y2 nucleotide receptor and αV integrins are necessary for the receptor to activate Go and to initiate Go-mediated signaling events leading to chemotaxis (Bagchi et al., 2005) and also are critical for astrocyte migration (Wang et al., 2005). Our studies provide the first evidence of the interaction of the BK B2 receptor with α5β1 integrin and demonstrate that this interaction leads to both transactivation of EGFR and ERK phosphorylation in cultured mIMCD-3 cells. In conclusion, these studies demonstrate a novel mechanism of EGFR transactivation by the Gq-coupled BK B2 receptor that involves formation of a functional complex between α5β1 integrin, EGFR, and BK B2 receptor.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK52448], the National Institutes of Health National Institute of General Medical Sciences [Grant GM3909], the Department of Veterans Affairs Merit and Research Enhancement Award Program (to J.R.R. and M.N.G.), the American Heart Association [Grant-in-Aid 0655445U], and a laboratory endowment jointly supported by the Medical University of South Carolina Division of Nephrology and Dialysis Clinics, Incorporated.
Parts of this work were presented previously in abstract form: Kramarenko I, Bunni M, Raymond JR, and Garnovskaya MN (2008) Bradykinin B2 receptor interacts with integrin α5/β1 to transactivate epidermal growth factor receptor in kidney cells [abstract]. FASEB J 22:829.5.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.064840.
- BK
- bradykinin
- ERK
- extracellular signal-regulated protein kinase
- EGFR
- epidermal growth factor receptor
- MMP
- matrix metalloproteinase
- GPCR
- G protein-coupled receptor
- FAK
- focal adhesion kinase
- EGF
- epidermal growth factor
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- PCR
- polymerase chain reaction
- RIPA
- radioimmunoprecipitation assay
- ANOVA
- analysis of variance
- siRNA
- small interfering RNA.
References
- Bagchi S, Liao Z, Gonzalez FA, Chorna NE, Seye CI, Weisman GA, Erb L. (2005) The P2Y2 nucleotide receptor interacts with αV integrins to activate Go and induce cell migration. J Biol Chem 280:39050–39057 [DOI] [PubMed] [Google Scholar]
- Berg KA, Zardeneta G, Hargreaves KM, Clarke WP, Milam SB. (2007) Integrins regulate opioid receptor signaling in trigeminal ganglion neurons. Neuroscience 144:889–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PC, Strömblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. (1996) Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell 85:683–693 [DOI] [PubMed] [Google Scholar]
- Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA. (1998) Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell 92:391–400 [DOI] [PubMed] [Google Scholar]
- Cabodi S, Moro L, Bergatto E, Boeri Erba E, Di Stefano P, Turco E, Tarone G, Defilippi P. (2004) Integrin regulation of epidermal growth factor (EGF) receptor and of EGF-dependent responses. Biochem Soc Trans 32:438–442 [DOI] [PubMed] [Google Scholar]
- Conant K, St Hillaire C, Nagase H, Visse R, Gary D, Haughey N, Anderson C, Turchan J, Nath A. (2004) Matrix metalloproteinase 1 interacts with neuronal integrins and stimulates dephosphorylation of Akt. J Biol Chem 279:8056–8062 [DOI] [PubMed] [Google Scholar]
- Davidson L, Pawson AJ, Millar RP, Maudsley S. (2004) Cytoskeletal reorganization dependence of signaling by the gonadotropin-releasing hormone receptor. J Biol Chem 279:1980–1993 [DOI] [PubMed] [Google Scholar]
- Della Rocca GJ, Maudsley S, Daaka Y, Lefkowitz RJ, Luttrell LM. (1999) Pleiotropic coupling of G protein-coupled receptors to the mitogen-activated protein kinase cascade. Role of focal adhesions and receptor tyrosine kinases. J Biol Chem 274:13978–13984 [DOI] [PubMed] [Google Scholar]
- Dumin JA, Dickeson SK, Stricker TP, Bhattacharyya-Pakrasi M, Roby JD, Santoro SA, Parks WC. (2001) Pro-collagenase-1 (matrix metalloproteinase-1) binds the α2β1 integrin upon release from keratinocytes migrating on type I collagen. J Biol Chem 276:29368–29374 [DOI] [PubMed] [Google Scholar]
- Eisinger DA, Ammer H. (2008) δ-Opioid receptors activate ERK/MAP kinase via integrin-stimulated receptor tyrosine kinases. Cell Signal 20:2324–2331 [DOI] [PubMed] [Google Scholar]
- Erb L, Liu J, Ockerhausen J, Kong Q, Garrad RC, Griffin K, Neal C, Krugh B, Santiago-Pérez LI, González FA, et al. (2001) An RGD sequence in the P2Y(2) receptor interacts with alpha(V)beta(3) integrins and is required for G(o)-mediated signal transduction. J Cell Biol 153:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golser R, Gorren AC, Leber A, Andrew P, Habisch HJ, Werner ER, Schmidt K, Venema RC, Mayer B. (2000) Interaction of endothelial and neuronal nitric-oxide synthases with the bradykinin B2 receptor. J Biol Chem 275:5291–5296 [DOI] [PubMed] [Google Scholar]
- Humphries JD, Byron A, Humphries MJ. (2006) Integrin ligands at a glance. J Cell Sci 119:3901–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunger-Glaser I, Salazar EP, Sinnett-Smith J, Rozengurt E. (2003) Bombesin, lysophosphatidic acid, and epidermal growth factor rapidly stimulate focal adhesion kinase phosphorylation at Ser-910: requirement for ERK activation. J Biol Chem 278:22631–22643 [DOI] [PubMed] [Google Scholar]
- Juliano RL. (2002) Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol 42:283–323 [DOI] [PubMed] [Google Scholar]
- Kramarenko II, Bunni MA, Morinelli TA, Raymond JR, Garnovskaya MN. (2009) Identification of a functional bradykinin B2 receptors endogenously expressed in HEK293 cells. Biochem Pharmacol 77:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Tian D, Shaul YD, Lev S. (2000) Targeting of PYK2 to focal adhesions as a cellular mechanism for convergence between integrins and G protein-coupled receptor signaling cascades. J Biol Chem 275:32736–32746 [DOI] [PubMed] [Google Scholar]
- Miranti CK, Brugge JS. (2002) Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol 4:E83–E90 [DOI] [PubMed] [Google Scholar]
- Moro L, Venturino M, Bozzo C, Silengo L, Altruda F, Beguinot L, Tarone G, Defilippi P. (1998) Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J 17:6622–6632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai H, Fitzgibbon WR, Bozeman G, Margolius HS, Ploth DW. (1996) Bradykinin B2 receptor antagonist increases chloride and water absorption in rat medullary collecting duct. Am J Physiol 271:R352–R360 [DOI] [PubMed] [Google Scholar]
- Mukhin YV, Garnovsky EA, Ullian ME, Garnovskaya MN. (2003) Bradykinin B2 receptor activates extra-cellular signal regulated protein kinase in mIMCD-3 cells via epidermal growth factor receptor transactivation. J Pharmacol Exp Ther 304:968–977 [DOI] [PubMed] [Google Scholar]
- Mukhin YV, Gooz M, Raymond JR, Garnovskaya MN. (2006) Collageneses 2 and 3 mediate epidermal growth factor receptor transactivation by Bradykinin B2 receptor in kidney cells. J Pharmacol Exp Ther 318:1033–1043 [DOI] [PubMed] [Google Scholar]
- Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. (2000) Ligand binding to integrins. J Biol Chem 275:21785–21788 [DOI] [PubMed] [Google Scholar]
- Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. (1999) EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402:884–888 [DOI] [PubMed] [Google Scholar]
- Rolli M, Fransvea E, Pilch J, Saven A, Felding-Habermann B. (2003) Activated integrin αvβ3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc Natl Acad Sci USA 100:9482–9487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Ginsberg MH. (2002) Networks and crosstalk: integrin signalling spreads. Nat Cell Biol 4:E65–E68 [DOI] [PubMed] [Google Scholar]
- Short SM, Boyer JL, Juliano RL. (2000) Integrins regulate the linkage between upstream and downstream events in G protein-coupled receptor signaling to mitogen-activated protein kinase. J Biol Chem 275:12970–12977 [DOI] [PubMed] [Google Scholar]
- Slack BE. (1998) Tyrosine phosphorylation of paxillin and focal adhesion kinase by activation of muscarinic m3 receptors is dependent on integrin engagement by the extracellular matrix. Proc Natl Acad Sci USA 95:7281–7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanidakis M, Bjorklund M, Ihanus E, Gahmberg CG, Koivunen E. (2003) Identification of a negatively charged peptide motif within the catalytic domain of progelatinases that mediates binding to leukocyte β2 integrins. J Biol Chem 278:34674–34684 [DOI] [PubMed] [Google Scholar]
- Stefanidakis M, Koivunen E. (2006) Cell-surface association between matrix metalloproteinases and integrins: role of the complexes in leukocyte migration and cancer progression. Blood 108:1441–1450 [DOI] [PubMed] [Google Scholar]
- Stricker TP, Dumin JA, Dickeson SK, Chung L, Nagase H, Parks WC, Santoro SA. (2001) Structural analysis of the α2 integrin I domain/procollagenase-1 (matrix metalloproteinase-1) interaction. J Biol Chem 276:29375–29381 [DOI] [PubMed] [Google Scholar]
- Tomita K, Pisano JJ, Knepper MA. (1985) Control of sodium and potassium transport in the cortical collecting duct of the rat. Effects of bradykinin, vasopressin, and deoxycorticosterone. J Clin Invest 76:132–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Kong Q, Gonzalez FA, Sun G, Erb L, Seye C, Weisman GA. (2005) P2Y2 nucleotide receptor interaction with alphaV integrin mediates astrocyte migration. J Neurochem 95:630–640 [DOI] [PubMed] [Google Scholar]
- Xie P, Browning DD, Hay N, Mackman N, Ye RD. (2000) Activation of NF-κB by Bradykinin through a Gαq- and Gβγ-dependent pathway that involves Phosphoinositide 3-Kinase and Akt. J Biol Chem 275:24907–24914 [DOI] [PubMed] [Google Scholar]
- Zeidel ML, Jabs K, Kikeri D, Silva P. (1990) Kinins inhibit conductive Na+ uptake by rabbit inner medullary collecting duct cells. Am J Physiol 258:F1584–F1591 [DOI] [PubMed] [Google Scholar]