Introduction
Cytosolic Ca2+ plays a central role as a universal intracellular messenger, controlling many aspects of physiology such as gene expression, electrical excitability, secretion, and synaptic plasticity in virtually all cell types (Berridge et al., 2000). The rich diversity in information encoded by Ca2+ arises through the mechanisms by which Ca2+ signals are generated and transmitted to act over very different spatial and temporal scales. For example, locally high [Ca2+] can trigger neurotransmitter release within microseconds of Ca2+ entry through voltage-gated channels closely apposed to active release zones (Neher, 1998), whereas globally propagating Ca2+ waves with periods measured in minutes regulate gene expression (Dolmetsch et al., 1998; Bootman et al., 2001). Resting [Ca2+] in the cytosol is maintained at low nanomolar levels, and Ca2+ signals are generated by Ca2+ ions entering the cytosol both across the plasma membrane through voltage- and ligand-gated channels and store-operated calcium channels, and by liberation of Ca2+ from intracellular stores such as the ER through Ca2+ release channels. The inositol trisphosphate (IP3) receptor (IP3R) is one of the two major Ca2+ release channels located on the ER, and it is the focus of our discussion.
The spatiotemporal patterning and transmission of Ca2+ signals involve the inherent properties of the Ca2+ channels themselves, their spatial organization, and how these channels interact via diffusion of Ca2+ both with themselves and with effector proteins. The IP3R channel exhibits particular complexity because its opening requires binding of the second messenger IP3 together with Ca2+ to receptor sites on the cytosolic face. Gating by Ca2+ is biphasic, such that small elevations of cytosolic Ca2+ induce channel opening, whereas larger elevations cause inactivation (Bezprozvanny et al., 1991; Foskett et al., 2007). Thus, there exists the potential for both positive and negative feedback loops, whereby Ca2+ liberated through one channel may modulate its own opening and that of neighboring channels. Channel–channel interactions are delimited by the spatial arrangement of channels, and IP3Rs are organized into tight clusters on the ER membrane. This leads to a hierarchy of calcium signals of differing magnitudes (Lipp and Niggli, 1996a; Parker et al., 1996). “Fundamental” signals (Ca2+ blips) represent Ca2+ flux through individual IP3R channels (Parker and Yao, 1991, 1996; Thomas et al., 2000; Rose et al., 2006); “elementary” Ca2+ transients (Ca2+ puffs) are generated by the openings of multiple IP3R within a cluster, orchestrated by Ca2+-induced Ca2+ release (CICR); and propagating global waves of Ca2+ result from progressive recruitment across multiple clusters by successive cycles of Ca2+ diffusion and CICR (Thorn et al., 1993; Yao et al., 1995; Berridge, 1997; Bootman et al., 1997a,b). The transitions between blips, puffs, and global Ca2+ waves are determined by factors such as stimulus strength ([IP3] and cytosolic [Ca2+]) (Callamaras et al., 1998a), the density of IP3R within a cluster (Shuai et al., 2006), the spacing between clusters (Callamaras et al., 1998b), and the action of mobile and immobile cytosolic Ca2+ buffers to affect the diffusion of Ca2+ ions (Dargan and Parker, 2003; Dargan et al., 2004).
The goal of our laboratory has been to use ever-improving imaging modalities to better resolve the hierarchical components and underlying mechanisms of IP3-mediated Ca2+ signaling in intact cells. This has involved a journey from using wide-field fluorescence microscopy for imaging of global Ca2+ transients, to applying total internal reflection microscopy to resolve the activity and localization of single IP3R channels. Here, we review different techniques that have contributed to an understanding of how IP3Rs are organized and focus on our recent work taking functional imaging studies down to the single-molecule level.
Electrophysiology versus optophysiology
The gold standard for studying single ion channels is the electrophysiological patch clamp, which monitors current flow through single channels with pico ampere and millisecond resolution (Neher and Sakmann, 1976; Hamill et al., 1981). This technique is readily applied to plasmalemmal channels, but intracellular channels such as the IP3R are largely out of reach of the patch pipette within their native environment in intact cells. Electrophysiological studies have, therefore, used reduced systems, such as reconstitution of IP3Rs into lipid bilayers (Ehrlich and Watras, 1988; Bezprozvanny et al., 1991) and patch clamping of IP3Rs on the membrane of excised nuclei (Mak and Foskett, 1994). Both of these approaches allow for high resolution recording of single-channel activity under tightly controlled ionic and electrical conditions. Nevertheless, they suffer from significant drawbacks, including disruption of the spatial organization of IP3Rs, possible loss of the accessory proteins and modulatory molecules, and disruption of channel–channel interactions mediated by CICR and Ca2+ diffusion.
There has thus been a long-standing interest in the development of minimally invasive optical imaging techniques to provide complimentary information about IP3R functioning within intact cells. These approaches have largely been based on the small molecule indicator dyes originally developed by Roger Tsien and, in particular, on the visible wavelength dyes such as fluo-4, which, although they do not allow for true ratiometric measurements, provide bright fluorescence signals with wide dynamic range (Grynkiewicz et al., 1985; Minta et al., 1989; Gee et al., 2000). Because of the very low resting cytosolic concentration of free Ca2+, the opening of even a single Ca2+-permeable channel generates an enormous increase in Ca2+ concentration in the immediate vicinity of the channel pore: from ∼50 nM to tens or even hundreds of µM (Shuai and Parker, 2005). Moreover, optical measurements have, in theory at least, an advantage over electrophysiological recording in that whereas a single Ca2+ ion moving through a channel contributes just two positive charges to the current, a Ca2+-bound fluorophore molecule can be excited to emit many thousands of photons. In practice, however, imaging technology has only recently improved to the point where single-channel activity can reliably be visualized with millisecond resolution. Along the way, advances in technology have been responsible for progressively revealing the hierarchy of IP3-mediated Ca2+ signaling, from waves to elementary puffs to fundamental blips.
Cellular Ca2+ imaging: from global waves to fundamental blips
The Xenopus oocyte has long been a favored model cell to study IP3-mediated Ca2+ signaling, owing to its large size that facilitates microinjection and its lack of ER Ca2+ release channels other than IP3Rs (i.e., RYRs and cADP-ribose receptors) (Parys et al., 1992). Early experiments using confocal microscopy at a slow frame rate of 1 s−1 revealed propagating Ca2+ waves evoked by IP3-mobilizing agonists, which often self-organized into beautiful and complex spiral patterns (Lechleiter et al., 1991). Contemporaneously, wide-field fluorescence imaging using a camera at faster frame rate (30 s−1), in conjunction with flash photolysis of caged IP3 to permit precise regulation of cytosolic [IP3], led to the discovery of transient, localized puffs (Parker and Yao, 1991; Yao and Parker, 1994; Yao et al., 1995), the first member of an ever-growing bestiary of elementary Ca2+ signals, including the analogous sparks generated by RYR in cardiac muscle (Cheng et al., 1993; Cheng and Lederer, 2008). More detailed analyses of puffs were then accomplished using video-rate (30 s−1) frame-scanning confocal microscopy (Yao et al., 1995) and by line scan confocal imaging, whereby temporal resolution is improved to as much as 1,000 scans s−1 at the expense of capturing information in only one spatial dimension. Puffs in both oocytes (Yao et al., 1995) and cultured mammalian cells (Thorn et al., 1993; Bootman et al., 1997a; Thomas et al., 2000) displayed apparently monotonic rising phases lasting tens of milliseconds while Ca2+ was liberated, followed by a slower decay as the Ca2+ microdomain at the puff site dissipated after channel closure (Thorn et al., 1993; Yao et al., 1995; Bootman et al., 1997a). Estimates of the amount of Ca2+ released during puffs already suggested that they must involve the opening of more than a single IP3R channel, and this view was reinforced by the resolution of yet smaller events, Ca2+ blips, that were first detected using a point confocal microscope (Parker and Yao, 1996) and were subsequently spatially resolved in HeLa cells by line scan imaging (Bootman et al., 1997b). Blips were interpreted as fundamental events, arising from the openings of individual IP3R, and in parallel, the discovery of analogous Ca2+ quarks (Lipp and Niggli, 1996b) suggested that these are the fundamental RYR channel events from which sparks are constituted. However, although these and other reports describe specific instances of single IP3R channel fluorescence signals (Parker and Yao, 1996; Bootman et al., 1997b; Rose et al., 2006), none of these imaging modalities provided the spatiotemporal resolution necessary for detailed quantitative studies nor to dissect the contributions of individual channels during puffs.
Issues in optical resolution of single-channel activity
What then is required to image single-channel Ca2+ signals with good fidelity? The opening of a single Ca2+-permeable channel or tightly grouped cluster of channels establishes an extremely steep gradient of cytosolic [Ca2+]. Modeling studies show that the concentration may reach hundreds of µM at nanometer distances from the channel pore, yet fall to only tens of nM above the resting level at distances of a few µm (Shuai and Parker, 2005). Moreover, there are steep temporal gradients. The Ca2+ concentration near the pore rises and falls within microseconds of channel opening and closing, whereas concentrations a few µm away change over tens or hundreds of milliseconds. Ideally, we would like to be able to monitor the local [Ca2+] in the immediate vicinity of the channel pore with sub-millisecond temporal resolution. The genetic targeting of fast indicators to channel proteins holds promise (Giepmans et al., 2006), but at present, the best results have been achieved using soluble small molecule dyes such as fluo-4. Given that both Ca2+ ions and, to a greater extent, Ca2+-bound dye molecules are motile in the cytosol, the fluorescence signal arising from a single-channel opening becomes spatially and temporally blurred by the combined effects of diffusion and the kinetics of Ca2+ binding and unbinding to the indicator. Imaging the fluorescence then introduces yet a further blurring by the diffraction-limited point-spread function of the microscope. In terms of instrumentation, the temporal resolution and signal amplitude can be improved by reducing the cytosolic volume from which fluorescence signals are monitored. But this involves a compromise because smaller volumes encompass progressively fewer Ca2+-bound dye molecules, so that statistical fluctuations (“molecular shot noise”) increasingly degrade the signal/noise ratio. Simulations suggest that sampling volumes of the order of a few tens of atto liters (10–50 × 10−18 l) would be optimal (Shuai and Parker, 2005). In parallel, cells can be loaded with a nonfluorescent Ca2+ chelator with slower binding kinetics than the indicator dye, such as EGTA, into the cytosol. This competes with the indicator for Ca2+ and both speeds the decay of fluorescence after channel closure and “sharpens” the spatial profile of the fluorescence signal (Shuai and Parker, 2005; Zeller et al., 2009). Again, a compromise is required because too much chelator will unacceptably reduce the amplitude of the fluorescence signal.
Technologies to resolve elementary and fundamental Ca2+ signals
As noted above, the key to obtaining good optical single-channel Ca2+ records is to monitor fluorescence from a very small cytosolic volume and to accomplish this rapidly enough to resolve channel gating on a millisecond timescale. A recurring theme has been that both engineering and fundamental limitations have necessitated a trade-off between either spatial or temporal resolution. Wide-field fluorescence microscopy can be used with cameras having fast frame rates (hundreds of frames s−1) (Zou et al., 2004), but it typically provides little or no depth (z-axis) discrimination; although schemes using fast z-axis stepping (Kirber et al., 2001) or multiple cameras with offset focal planes (Demuro and Parker, 2008) can mitigate that limitation. Confocal and multiphoton microscopy achieve a tightly delimited 3-D point-spread function (typical dimensions of ∼300 nm laterally and 800 nm axially with objectives of high numerical aperture), with sub-femtoliter volume. However, these are inherently point-scanning systems, wherein mechanical constraints may limit the speed at which the laser spot can be scanned. Although technologies such as resonant scanning mirrors (Nguyen et al., 2001) and accousto-optic deflectors provide faster scans (Lechleiter et al., 2002), they have their own specific drawbacks, and all approaches are ultimately limited by the need to detect sufficient fluorescence photons. At the shot-noise limit, the signal/noise ratio varies as the square root of light intensity, so the image quality degrades with increasing scan rates and pixel resolution as the dwell-time at each pixel becomes shorter.
A popular compromise for imaging elementary Ca2+ events has been the use of confocal line scan imaging, in which the confocal spot is repeatedly scanned along a single line to derive an image representing the temporal evolution of Ca2+ signals in one spatial dimension. Advantages include high temporal resolution and relatively long pixel dwell times because only a small number of pixels are scanned along the single line, as well as the ability to present results as an individual image with Ca2+ levels encoded by color and/or height representation. Drawbacks include the restriction of sampling to a single line through a cell and, more seriously, the difficulty of ascertaining whether a signal is in focus or arose from a site distant from the scan line. Nonfocal fluorescence signals will be of smaller amplitude and display slowed kinetics, complicating interpretation of experimental results (Izu et al., 1998), with the exception of instances such as a channel trapped at the tip of a patch pipette where the location is precisely known and can be aligned with the scan line (Wang et al., 2001).
Total internal reflection fluorescence (TIRF) microscopy: a good way to image Ca2+ signals near the plasma membrane
TIRF microscopy has more recently been applied to image local Ca2+ signals, and it combines the advantages of fast camera-based imaging together with an optical sectioning effect (z-axis resolution) even finer than confocal microscopy (Demuro and Parker, 2004, 2005; Navedo et al., 2006). TIRF works by directing excitation light through a glass substrate toward an aqueous specimen at a sufficiently shallow angle so that total internal reflection occurs due to the refractive index decrease at the glass–water interface. However, a very thin electromagnetic field (evanescent wave) is created in the liquid with the same wavelength as the incident light and decays exponentially with distance from the interface (typically over one or a few hundred nm). Because this field is able to excite fluorophores near the interface while avoiding excitation further into the aqueous phase, it provides an “optical sectioning” effect similar to, but even narrower, than that achieved by a confocal microscope (Axelrod, 2003). Although the idea of TIRF microscopy is old, its biological utility has expanded greatly in the last few years after the commercial introduction of specialized oil-immersion objective lenses with very high numerical aperture (1.45 or greater). These allow the excitation light to be directed to the specimen at the necessarily shallow angle through the very edge of the lens while collecting emitted fluorescence back through the same objective. In conjunction with developments in low noise electron-multiplied CCD cameras, this enables two-dimensional records to be acquired at high frame rates (1,000 s−1 or better with pixel binning) and good signal/noise ratio. Measurements from small (sub-µm) regions of interest can then be used to monitor fluorescence from attoliter cytosolic volumes centered on active Ca2+ release sites. The main disadvantage of TIRF microscopy is that it can image only in the immediate vicinity of the cover glass and is thus applicable exclusively to signals arising close to the cell membrane. TIRF has thus been used primarily to study Ca2+ flux through plasmalemmal channels (Demuro and Parker, 2004, 2005; Navedo et al., 2006), although it is also applicable to intracellular Ca2+ release channels that lie close to the membrane (Smith and Parker, 2009; Smith et al., 2009a,b; Wiltgen et al., 2010).
How should we interpret local Ca2+ fluorescence signals?
Fluorescence signals originating from single or tightly clustered Ca2+ channels provide only a distorted impression of the underlying Ca2+ gradient and are heavily smoothed in both space and time. Thus, it is worth emphasizing the complications that arise when attempting to interpret and quantify local fluorescence signals, especially because the interpretation depends strongly upon the method used to acquire the images. We can consider two extreme cases.
In the first (approximated by wide-field imaging), fluorescence is collected from a large volume around a localized Ca2+ source, such as a single channel or tight cluster of channels (Zou et al., 2004). The fluorescence signal then represents an integral of Ca2+ release and, neglecting possible indicator saturation near the channel, is not affected by diffusion of Ca2+ ions or Ca2+-bound indicator because little escapes outside the large imaging volume. Given that Ca2+ sequestration mechanisms (pumps and transporters) are slow on the timescale of elementary and fundamental events, the slope of the rising phase of the fluorescence signal provides a measure of Ca2+ flux (current), and the peak signal provides a measure of the total amount (moles) of Ca2+ entering the cytosol. The latter measure of “signal mass” was first applied by considering line scan records as a diametric slice through a 3-D volume (Sun et al., 1998; Hollingworth et al., 2001), and it was subsequently applied to wide-field images (Zou et al., 2004). Although in principle the rising phase reflects Ca2+ flux, differentiation of the fluorescence record usually results in a signal that is too noisy to provide useful information about channel gating kinetics.
The second case is when fluorescence is monitored from an exceedingly small volume around the channel pore. Ideally, this would then provide a signal directly analogous to a patch clamp record; i.e., the fluorescence amplitude would be directly and linearly proportional to instantaneous Ca2+ flux (current). TIRF microscopy approaches this condition, but only approximately, as a result of factors including indicator binding kinetics and diffusion together with the finite imaging volume. Nevertheless, single-channel records obtained using TIRF as an “optical patch clamp” closely resemble electrophysiological recordings and can track channel gating with a resolution of ∼2 ms (Demuro and Parker, 2005). Calibration of the fluorescence amplitude in terms of underlying Ca2+ current is more problematic, but an in situ estimate may be obtained by measuring fluorescence signals under conditions that correspond to a known Ca2+ current as measured by parallel patch clamp recording.
Local measurements of elementary and fundamental Ca2+ signals obtained by confocal microscopy lie intermediate between the above two extreme situations. That is to say, the fluorescence can be considered as a “leaky integral,” reflecting a balance between Ca2+ efflux into the cytosol and its subsequent diffusion out of the region of interest from which fluorescence is measured. The rising phase thus approximates the duration of Ca2+ flux (i.e., when channels are open), whereas the falling phase largely reflects diffusive spread of Ca2+ ions in the cytosol. The peak amplitude may be affected both by the magnitude and the duration of Ca2+ flux. We argue in particular that it is not valid to apply steady-state calibrations of indicator fluorescence to estimate the peak local cytosolic free Ca2+ concentrations during elementary events, given that the fluorescence is a highly blurred reflection of the underlying steep Ca2+ concentration gradient. For the same reason, the spatial extent of local fluorescence signals does not directly report the spatial spread of free Ca2+ or the distribution of Ca2+ channels within a small cluster, although this information can be applied in simulation and computational studies to estimate these parameters (Shuai and Parker, 2005; Ventura et al., 2005).
Applications of TIRF microscopy for imaging elementary and fundamental Ca2+ signals in mammalian cells.
By imaging the local fluorescence changes with a high speed (500 frames s−1), high sensitivity (back-illuminated electron-multiplied CCD) camera, we have achieved recordings of voltage- and ligand-gated Ca2+-permeable channels expressed in the plasma membrane of Xenopus oocytes with a 2-ms temporal resolution and throughput of >500 simultaneous channels (Demuro and Parker, 2004, 2006). We initially assumed that the intracellular location of IP3Rs would preclude their study using this approach because the puff sites in the oocyte are located a few µm inward from the membrane (Callamaras and Parker, 1999). However, we subsequently found that cultured human neuroblastoma SH-SY5Y cells and other mammalian cell lines possess puff sites that are located sufficiently close to the plasma membrane to be visualized by TIRF microscopy (Smith and Parker, 2009; Smith et al., 2009a).
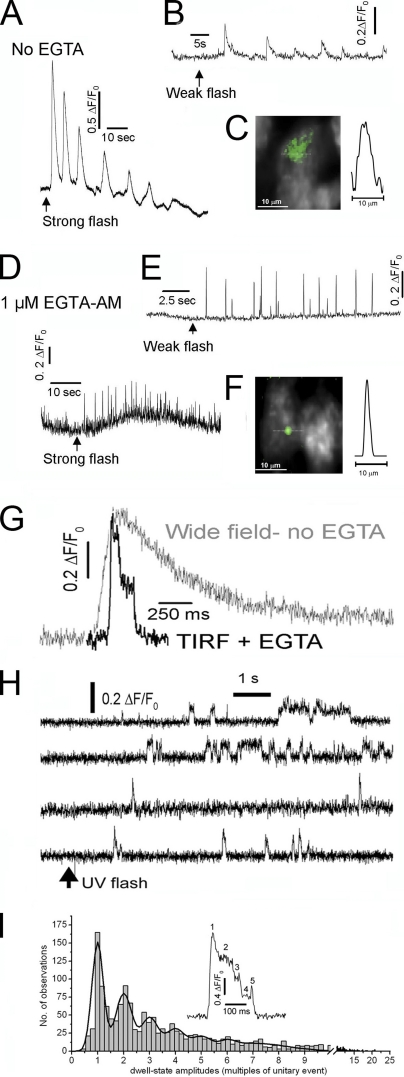
A remaining problem was then how to achieve good control of cytosolic [IP3]. Several studies in HeLa and other cultured cell lines had used extracellular agonists (Bootman et al., 1997a,b; Tovey et al., 2001) or membrane-permeant IP3 (Thomas et al., 2000) to evoke Ca2+ events, but it was problematic to adjust the stimulus intensity to evoke puffs without triggering global Ca2+ waves. The injection of caged IP3 into oocytes allows precise regulation of evoked Ca2+ signals through adjustment of the intensity and duration of UV photolysis flashes (Yao et al., 1995). But although caged IP3 can be introduced into small cells via whole cell patch clamping (Stutzmann et al., 2003, 2004, 2006), this method is technically demanding and laborious. The recent commercial availability of a membrane-permeant ester of caged IP3 (ciIP3AM; SiChem) thus represented an important advance, facilitating precise control over [IP3]i, as illustrated in Fig. 1, where a strong UV flash evoked a global Ca2+ signal (Fig. 1 A), whereas a weaker flash (Fig. 1 B) evoked transient localized puffs. Nevertheless, the window of flash strengths over which discrete puffs were evoked was narrow, and puffs showed a wide spatial spread (Fig. 1 C). To mitigate these effects, we further loaded cells by incubation with a membrane-permeant form of the slow Ca2+ buffer EGTA (Smith et al., 2009a) to “balkanize” global waves into local signals by disrupting CICR between puff sites (Callamaras and Parker, 2000; Dargan and Parker, 2003) and to sharpen the spatial and temporal profiles of puffs (Smith et al., 2009a; Zeller et al., 2009). This is illustrated in Fig. 1 D, where a global Ca2+ signal previously evoked by a strong UV flash devolved into discrete transients at individual puff sites.
Figure 1.
Dissecting the hierarchy of IP3-mediated Ca2+ signaling from global cellular oscillations to single-channel events. (A) Fluorescence traces illustrate global Ca2+ oscillations evoked by a strong (800-ms) UV photolysis flash in an SH-SY5Y cell loaded with fluo-4 and ciIP3. (B) Local Ca2+ puffs evoked by weaker (100-ms flash) photorelease of i-IP3 in another cell. (C) Image shows a Ca2+ puff (pseudocolored in green) captured at the time of peak amplitude from a single video frame and superimposed on a monochrome image of resting fluo-4 fluorescence in an SH-SY5Y cell. The trace shows a profile of Ca2+-dependent fluorescence measured along the dotted line passing through the center of the puff. (D and E) Ca2+ puffs evoked, respectively, by strong and weak photolysis flashes equivalent to those in A and B after intracellular loading with EGTA. Global responses were abolished, and the traces show local signals measured from small regions of interest centered over puff sites. (F) Image shows a Ca2+ puff and corresponding fluorescence profile as in C, but recorded in the presence of EGTA. (G) Comparison of representative puffs recorded in SH-SY5Y cells after photorelease of iIP3 using wide-field fluorescence microscopy (gray trace), and using TIRF microscopy together with EGTA loading (black trace). Both traces show fluorescence ratio changes (ΔF/F0) averaged within 1 × 1–µm regions of interest centered on puff sites. (H) Examples of sites that displayed exclusively single-channel blip activity. (I) Inset shows a Ca2+ signal from a 1-µm2 region of interest, showing five discrete amplitude levels during a single puff. The histogram shows the distributions of event and step-level amplitudes derived from measurements at 87 sites in 20 cells, revealing “quantal” components at integer multiples of the single-channel level. A–F are adapted from Smith et al. (2009a), and G–I are from Smith and Parker (2009).
The combination of TIRF imaging and cytosolic Ca2+ buffering by EGTA accomplished a dramatic improvement in the resolution of elementary and fundamental Ca2+ signals. Puffs imaged by wide-field microscopy show smoothly rising and falling phases on which it is not possible to resolve the step-wise changes that would be expected to arise from the openings and closings of individual IP3R channels (Fig. 1 G, gray trace). However, in cells loaded with EGTA and visualized by TIRF microscopy, abrupt transitions in fluorescence levels are apparent on the falling (and sometimes rising) phase of the Ca2+ transient (Fig. 1 G, black trace). Several further observations support the interpretation that these steps arise from the openings and closings of individual IP3R during a puff (Smith and Parker, 2009). (a) In addition to the puffs, sites often show smaller blips, with unitary fluorescence amplitudes. (b) Other sites, likely representing “lone” IP3R channels, show only blips, giving fluorescence records that closely resemble single-channel patch clamp recordings (Fig. 1 H). (c) The durations of blips are exponentially distributed, with a mean closely matching the mean open time of IP3R channels as measured by patch clamp (Smith and Parker, 2009). (d) A quantal analysis (Del Castillo and Katz, 1954) of step-wise fluorescence amplitude levels during puffs reveals a multimodal distribution, with recurring peaks at integer levels of the unitary blip amplitude (Fig. 1 I).
Quantal dissection of single-channel events underlying puffs.
The ability to image puffs with single-channel resolution now allows us to dissect the quantal contributions of Ca2+ flux through individual IP3Rs. By counting unitary steps, or by simply dividing the peak fluorescence of a Ca2+ puff by that of the unitary blip, one can determine the minimum number of functional IP3Rs at a site. Our results indicate that puffs typically involve simultaneous opening of five to six IP3Rs, although there is considerable heterogeneity between sites, with some containing >20 active IP3Rs (Smith and Parker, 2009). Further, kinetic resolution of channel openings and closings during a puff allows the investigation of puff initiation and termination mechanisms. The rapid upstroke of puffs reflects a fast recruitment of IP3Rs, with an opening rate of 1 channel ms−1 during the first 10 ms, decreasing abruptly to a rate 40 times lower within 20 ms. This rapid coordination likely arises because the high local Ca2+ resulting from the stochastic opening of an initial “trigger” channel evokes CICR from neighboring channels at the puff site. The mechanisms of puff termination still remain unclear, but we can rule out stochastic attrition (failure of CICR because all channels happen to randomly close at the same time) as the sole explanation as, contrary to observations, this predicts that puff durations would increase as an extremely steep function of the number of open channels. Further, depletion of luminal Ca2+ in the ER is unlikely to account for puff termination, as the step-wise decay of puffs continues at integer quantal levels. Instead, the progressive closure of IP3R channels after the peak of a puff, with relatively few subsequent openings, is consistent with the rapid onset of a strong inhibitory process, likely resulting from the Ca2+-dependent inhibition exhibited by the type 1 IP3R predominant in SH-SY5Y cells (Smith and Parker, 2009).
Localization and motility–or immotility–of IP3R channels.
Optical single-channel imaging not only enables channel gating to be monitored, but also permits channel locations to be mapped with nano-scale precision, something that is not possible by patch clamp recording (Demuro and Parker, 2005; Smith et al., 2009b). We have applied this capability to study the clustered organization of IP3Rs that underlies the generation of puffs and, in particular, to investigate whether these clusters are stable entities or may dynamically organize in response to stimulation.
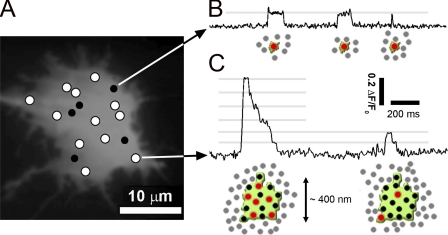
Observations in many cell lines and in Xenopus oocytes have demonstrated numerous puffs arising over many minutes at fixed locations within a cell, suggesting that IP3R clusters are relatively stable entities (Thomas et al., 1998; Tovey et al., 2001; Dargan and Parker, 2003; Smith et al., 2009a). By imaging blip sites we have found that lone IP3R channels (blip sites) are similarly immotile within the ER membrane (Smith et al., 2009b; Wiltgen et al., 2010). These results present a paradox, as imaging studies using GFP-tagged or immunostained IP3Rs have shown that a substantial proportion of IP3Rs can diffuse freely within the ER membrane (Wilson et al., 1998; Fukatsu et al., 2004; Cruttwell et al., 2005; Ferreri-Jacobia et al., 2005; Iwai et al., 2005; Tateishi et al., 2005; Chalmers et al., 2006; Gibson and Ehrlich, 2008; Tojyo et al., 2008), and that they aggregate into clusters after sustained (minutes) activation of IP3 signaling and/or cytosolic Ca2+ elevation (Wilson et al., 1998; Iwai et al., 2005; Tateishi et al., 2005; Chalmers et al., 2006; Tojyo et al., 2008; Taufiq-Ur-Rahman et al., 2009), or even undergo clustering in response to IP3 within just a few seconds (Taufiq-Ur-Rahman et al., 2009). Moreover, there is a marked disparity between immunostaining patterns that show IP3Rs distributed densely throughout the entirety of the cytoplasm (Wilson et al., 1998; Tateishi et al., 2005; Chalmers et al., 2006) versus Ca2+ release at just a few discrete puff and blip sites in mammalian cells (Fig. 2 A) (Bootman et al., 1997a,b; Thomas et al., 1998; Tovey et al., 2001; Smith and Parker, 2009; Smith et al., 2009a,b). As illustrated schematically in Fig. 2, we speculate that the difference may lie in the fact that different experimental approaches monitor different populations of IP3Rs. Specifically, we propose that a majority of IP3Rs (represented by gray dots in Fig. 2) may be motile but are either functionally unresponsive or mediate Ca2+ liberation only during sustained global elevations of cytosolic [Ca2+] (Smith et al., 2009b). Local Ca2+ signals arise, instead, from a smaller subset of IP3Rs that are anchored, individually or in clusters, by association with static cytoskeletal structures (represented in green) and which, possibly as a consequence of this anchoring, display high sensitivity to IP3 to generate Ca2+ blips (Fig. 2 B) and puffs (Fig. 2 C).
Figure 2.
Schematic portrayal of the proposed arrangement of IP3Rs underlying the generation of blips and puffs. (A) Monochrome image of resting fluo-4 fluorescence in an SH-SY5Y cell observed using TIRF microscopy, mapping sites that displayed single-channel blip activity in response to photoreleased IP3 (black filled circles) and sites that displayed puffs (white circles). (B) The trace shows experimental data of activity recorded from a blip site. Cartoons illustrate our suggested arrangement and activity of IP3R channels corresponding to each of the three blips during the trace. Channels open during each blip are shown in red, and closed channels are shown in black. Both are sensitized to IP3 by binding to an as yet unidentified anchoring site (depicted in green). Motile, functionally unresponsive IP3Rs are shown in gray. (C) Corresponding trace and cartoons illustrate the localization and activity of IP3R channels during puffs. Gray lines indicate fluorescence levels corresponding to integer multiples of the single-channel (blip) level. The first puff (left) involves simultaneous opening of six channels at its peak, and the second puff (right) only two channels. The channels contributing to puffs are believed to be distributed across clusters with a diameter of roughly 400 nm.
Summary
Optical single-channel recording is a novel tool for the study of individual Ca2+-permeable channels within intact cells under minimally perturbed physiological conditions. As applied to the functioning and spatial organization of IP3Rs, this approach complements our existing knowledge, which derives largely from reduced systems—such as reconstitution into lipid bilayers and patch clamping of IP3Rs on the membrane of excised nuclei—where the spatial arrangement and interactions among IP3Rs via CICR are disrupted. The ability to image the activity of single IP3R channels with millisecond resolution together with localization of their positions with a precision of a few tens of nanometers both raises several intriguing questions and holds promise of answers. In particular, what mechanism underlies the anchoring of puffs and blips to static locations; why do these Ca2+ release events appear to involve only a very small fraction of the IP3Rs within a cell; and how can we reconcile the relative immotility of functional IP3Rs with numerous studies reporting free diffusion of IP3R protein in the ER membrane?
This Perspectives series includes articles by Gordon, Xie et al., Prosser et al., Santana and Navedo, and Hill-Eubanks et al.
Footnotes
Abbreviations used in this paper:
- CICR
- Ca2+-induced Ca2+ release
- IP3
- inositol trisphosphate
- IP3R
- IP3 receptor
- TIRF
- total internal reflection fluorescence
References
- Axelrod D. 2003. Total internal reflection fluorescence microscopy in cell biology. Methods Enzymol. 361:1–33 10.1016/S0076-6879(03)61003-7 [DOI] [PubMed] [Google Scholar]
- Berridge M.J. 1997. Elementary and global aspects of calcium signalling. J. Physiol. 499:291–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J., Lipp P., Bootman M.D. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1:11–21 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B.E. 1991. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 351:751–754 10.1038/351751a0 [DOI] [PubMed] [Google Scholar]
- Bootman M.D., Berridge M.J., Lipp P. 1997a. Cooking with calcium: the recipes for composing global signals from elementary events. Cell. 91:367–373 10.1016/S0092-8674(00)80420-1 [DOI] [PubMed] [Google Scholar]
- Bootman M.D., Niggli E., Berridge M., Lipp P. 1997b. Imaging the hierarchical Ca2+ signalling system in HeLa cells. J. Physiol. 499:307–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman M.D., Lipp P., Berridge M.J. 2001. The organisation and functions of local Ca2+ signals. J. Cell Sci. 114:2213–2222 [DOI] [PubMed] [Google Scholar]
- Callamaras N., Parker I. 1999. Radial localization of inositol 1,4,5-trisphosphate–sensitive Ca2+ release sites in Xenopus oocytes resolved by axial confocal linescan imaging. J. Gen. Physiol. 113:199–213 10.1085/jgp.113.2.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callamaras N., Parker I. 2000. Phasic characteristic of elementary Ca2+ release sites underlies quantal responses to IP3. EMBO J. 19:3608–3617 10.1093/emboj/19.14.3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callamaras N., Marchant J.S., Sun X.P., Parker I. 1998a. Activation and co-ordination of InsP3-mediated elementary Ca2+ events during global Ca2+ signals in Xenopus oocytes. J. Physiol. 509:81–91 10.1111/j.1469-7793.1998.081bo.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callamaras N., Sun X.P., Ivorra I., Parker I. 1998b. Hemispheric asymmetry of macroscopic and elementary calcium signals mediated by InsP3 in Xenopus oocytes. J. Physiol. 511:395–405 10.1111/j.1469-7793.1998.395bh.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers M., Schell M.J., Thorn P. 2006. Agonist-evoked inositol trisphosphate receptor (IP3R) clustering is not dependent on changes in the structure of the endoplasmic reticulum. Biochem. J. 394:57–66 10.1042/BJ20051130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Lederer W.J. 2008. Calcium sparks. Physiol. Rev. 88:1491–1545 10.1152/physrev.00030.2007 [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W.J., Cannell M.B. 1993. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 262:740–744 10.1126/science.8235594 [DOI] [PubMed] [Google Scholar]
- Cruttwell C., Bernard J., Hilly M., Nicolas V., Tunwell R.E., Mauger J.P. 2005. Dynamics of the Ins(1,4,5)P3 receptor during polarization of MDCK cells. Biol. Cell. 97:699–707 10.1042/BC20040503 [DOI] [PubMed] [Google Scholar]
- Dargan S.L., Parker I. 2003. Buffer kinetics shape the spatiotemporal patterns of IP3-evoked Ca2+ signals. J. Physiol. 553:775–788 10.1113/jphysiol.2003.054247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargan S.L., Schwaller B., Parker I. 2004. Spatiotemporal patterning of IP3-mediated Ca2+ signals in Xenopus oocytes by Ca2+-binding proteins. J. Physiol. 556:447–461 10.1113/jphysiol.2003.059204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Castillo J., Katz B. 1954. Quantal components of the end-plate potential. J. Physiol. 124:560–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuro A., Parker I. 2004. Imaging the activity and localization of single voltage-gated Ca2+ channels by total internal reflection fluorescence microscopy. Biophys. J. 86:3250–3259 10.1016/S0006-3495(04)74373-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuro A., Parker I. 2005. “Optical patch-clamping”: single-channel recording by imaging Ca2+ flux through individual muscle acetylcholine receptor channels. J. Gen. Physiol. 126:179–192 10.1085/jgp.200509331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuro A., Parker I. 2006. Imaging single-channel calcium microdomains. Cell Calcium. 40:413–422 10.1016/j.ceca.2006.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuro A., Parker I. 2008. Multi-dimensional resolution of elementary Ca2+ signals by simultaneous multi-focal imaging. Cell Calcium. 43:367–374 10.1016/j.ceca.2007.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch R.E., Xu K., Lewis R.S. 1998. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 392:933–936 10.1038/31960 [DOI] [PubMed] [Google Scholar]
- Ehrlich B.E., Watras J. 1988. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature. 336:583–586 10.1038/336583a0 [DOI] [PubMed] [Google Scholar]
- Ferreri-Jacobia M., Mak D.O., Foskett J.K. 2005. Translational mobility of the type 3 inositol 1,4,5-trisphosphate receptor Ca2+ release channel in endoplasmic reticulum membrane. J. Biol. Chem. 280:3824–3831 10.1074/jbc.M409462200 [DOI] [PubMed] [Google Scholar]
- Foskett J.K., White C., Cheung K.H., Mak D.O. 2007. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 87:593–658 10.1152/physrev.00035.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukatsu K., Bannai H., Zhang S., Nakamura H., Inoue T., Mikoshiba K. 2004. Lateral diffusion of inositol 1,4,5-trisphosphate receptor type 1 is regulated by actin filaments and 4.1N in neuronal dendrites. J. Biol. Chem. 279:48976–48982 10.1074/jbc.M408364200 [DOI] [PubMed] [Google Scholar]
- Gee K.R., Brown K.A., Chen W.N., Bishop-Stewart J., Gray D., Johnson I. 2000. Chemical and physiological characterization of fluo-4 Ca2+-indicator dyes. Cell Calcium. 27:97–106 10.1054/ceca.1999.0095 [DOI] [PubMed] [Google Scholar]
- Gibson C.J., Ehrlich B.E. 2008. Inositol 1,4,5-trisphosphate receptor movement is restricted by addition of elevated levels of O-linked sugar. Cell Calcium. 43:228–235 10.1016/j.ceca.2007.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giepmans B.N., Adams S.R., Ellisman M.H., Tsien R.Y. 2006. The fluorescent toolbox for assessing protein location and function. Science. 312:217–224 10.1126/science.1124618 [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R.Y. 1985. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 260:3440–3450 [PubMed] [Google Scholar]
- Hamill O.P., Marty A., Neher E., Sakmann B., Sigworth F.J. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391:85–100 10.1007/BF00656997 [DOI] [PubMed] [Google Scholar]
- Hollingworth S., Peet J., Chandler W.K., Baylor S.M. 2001. Calcium sparks in intact skeletal muscle fibers of the frog. J. Gen. Physiol. 118:653–678 10.1085/jgp.118.6.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M., Tateishi Y., Hattori M., Mizutani A., Nakamura T., Futatsugi A., Inoue T., Furuichi T., Michikawa T., Mikoshiba K. 2005. Molecular cloning of mouse type 2 and type 3 inositol 1,4,5-trisphosphate receptors and identification of a novel type 2 receptor splice variant. J. Biol. Chem. 280:10305–10317 10.1074/jbc.M413824200 [DOI] [PubMed] [Google Scholar]
- Izu L.T., Wier W.G., Balke C.W. 1998. Theoretical analysis of the Ca2+ spark amplitude distribution. Biophys. J. 75:1144–1162 10.1016/S0006-3495(98)74034-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirber M.T., Etter E.F., Bellve K.A., Lifshitz L.M., Tuft R.A., Fay F.S., Walsh J.V., Fogarty K.E. 2001. Relationship of Ca2+ sparks to STOCs studied with 2D and 3D imaging in feline oesophageal smooth muscle cells. J. Physiol. 531:315–327 10.1111/j.1469-7793.2001.0315i.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleiter J.D., Girard S., Peralta E., Clapham D. 1991. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 252:123–126 10.1126/science.2011747 [DOI] [PubMed] [Google Scholar]
- Lechleiter J.D., Lin D.T., Sieneart I. 2002. Multi-photon laser scanning microscopy using an acoustic optical deflector. Biophys. J. 83:2292–2299 10.1016/S0006-3495(02)73989-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., Niggli E. 1996a. A hierarchical concept of cellular and subcellular Ca2+-signalling. Prog. Biophys. Mol. Biol. 65:265–296 10.1016/S0079-6107(96)00014-4 [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. 1996b. Submicroscopic calcium signals as fundamental events of excitation—contraction coupling in guinea-pig cardiac myocytes. J. Physiol. 492:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.O., Foskett J.K. 1994. Single-channel inositol 1,4,5-trisphosphate receptor currents revealed by patch clamp of isolated Xenopus oocyte nuclei. J. Biol. Chem. 269:29375–29378 [PubMed] [Google Scholar]
- Minta A., Kao J.P., Tsien R.Y. 1989. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J. Biol. Chem. 264:8171–8178 [PubMed] [Google Scholar]
- Navedo M.F., Amberg G.C., Nieves M., Molkentin J.D., Santana L.F. 2006. Mechanisms underlying heterogeneous Ca2+ sparklet activity in arterial smooth muscle. J. Gen. Physiol. 127:611–622 10.1085/jgp.200609519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. 1998. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 20:389–399 10.1016/S0896-6273(00)80983-6 [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. 1976. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 260:799–802 10.1038/260799a0 [DOI] [PubMed] [Google Scholar]
- Nguyen Q.T., Callamaras N., Hsieh C., Parker I. 2001. Construction of a two-photon microscope for video-rate Ca2+ imaging. Cell Calcium. 30:383–393 10.1054/ceca.2001.0246 [DOI] [PubMed] [Google Scholar]
- Parker I., Yao Y. 1991. Regenerative release of calcium from functionally discrete subcellular stores by inositol trisphosphate. Proc Biol Sci. 246:269–274 10.1098/rspb.1991.0154 [DOI] [PubMed] [Google Scholar]
- Parker I., Yao Y. 1996. Ca2+ transients associated with openings of inositol trisphosphate-gated channels in Xenopus oocytes. J. Physiol. 491:663–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Choi J., Yao Y. 1996. Elementary events of InsP3-induced Ca2+ liberation in Xenopus oocytes: hot spots, puffs and blips. Cell Calcium. 20:105–121 10.1016/S0143-4160(96)90100-1 [DOI] [PubMed] [Google Scholar]
- Parys J.B., Sernett S.W., DeLisle S., Snyder P.M., Welsh M.J., Campbell K.P. 1992. Isolation, characterization, and localization of the inositol 1,4,5-trisphosphate receptor protein in Xenopus laevis oocytes. J. Biol. Chem. 267:18776–18782 [PubMed] [Google Scholar]
- Rose H.J., Dargan S., Shuai J., Parker I. 2006. ‘Trigger’ events precede calcium puffs in Xenopus oocytes. Biophys. J. 91:4024–4032 10.1529/biophysj.106.088872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai J., Parker I. 2005. Optical single-channel recording by imaging Ca2+ flux through individual ion channels: theoretical considerations and limits to resolution. Cell Calcium. 37:283–299 10.1016/j.ceca.2004.10.008 [DOI] [PubMed] [Google Scholar]
- Shuai J., Rose H.J., Parker I. 2006. The number and spatial distribution of IP3 receptors underlying calcium puffs in Xenopus oocytes. Biophys. J. 91:4033–4044 10.1529/biophysj.106.088880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I.F., Parker I. 2009. Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells. Proc. Natl. Acad. Sci. USA. 106:6404–6409 10.1073/pnas.0810799106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I.F., Wiltgen S.M., Parker I. 2009a. Localization of puff sites adjacent to the plasma membrane: functional and spatial characterization of Ca2+ signaling in SH-SY5Y cells utilizing membrane-permeant caged IP3. Cell Calcium. 45:65–76 10.1016/j.ceca.2008.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith I.F., Wiltgen S.M., Shuai J., Parker I. 2009b. Ca2+ puffs originate from preestablished stable clusters of inositol trisphosphate receptors. Sci. Signal. 2:ra77 10.1126/scisignal.2000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann G.E., LaFerla F.M., Parker I. 2003. Ca2+ signaling in mouse cortical neurons studied by two-photon imaging and photoreleased inositol triphosphate. J. Neurosci. 23:758–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann G.E., Caccamo A., LaFerla F.M., Parker I. 2004. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer’s-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J. Neurosci. 24:508–513 10.1523/JNEUROSCI.4386-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutzmann G.E., Smith I., Caccamo A., Oddo S., Laferla F.M., Parker I. 2006. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. J. Neurosci. 26:5180–5189 10.1523/JNEUROSCI.0739-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X.P., Callamaras N., Marchant J.S., Parker I. 1998. A continuum of InsP3-mediated elementary Ca2+ signalling events in Xenopus oocytes. J. Physiol. 509:67–80 10.1111/j.1469-7793.1998.067bo.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi Y., Hattori M., Nakayama T., Iwai M., Bannai H., Nakamura T., Michikawa T., Inoue T., Mikoshiba K. 2005. Cluster formation of inositol 1,4,5-trisphosphate receptor requires its transition to open state. J. Biol. Chem. 280:6816–6822 10.1074/jbc.M405469200 [DOI] [PubMed] [Google Scholar]
- Taufiq-Ur-Rahman, Skupin A., Falcke M., Taylor C.W. 2009. Clustering of InsP3 receptors by InsP3 retunes their regulation by InsP3 and Ca2+. Nature. 458:655–659 10.1038/nature07763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D., Lipp P., Berridge M.J., Bootman M.D. 1998. Hormone-evoked elementary Ca2+ signals are not stereotypic, but reflect activation of different size channel clusters and variable recruitment of channels within a cluster. J. Biol. Chem. 273:27130–27136 10.1074/jbc.273.42.27130 [DOI] [PubMed] [Google Scholar]
- Thomas D., Lipp P., Tovey S.C., Berridge M.J., Li W., Tsien R.Y., Bootman M.D. 2000. Microscopic properties of elementary Ca2+ release sites in non-excitable cells. Curr. Biol. 10:8–15 10.1016/S0960-9822(99)00258-4 [DOI] [PubMed] [Google Scholar]
- Thorn P., Lawrie A.M., Smith P.M., Gallacher D.V., Petersen O.H. 1993. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell. 74:661–668 10.1016/0092-8674(93)90513-P [DOI] [PubMed] [Google Scholar]
- Tojyo Y., Morita T., Nezu A., Tanimura A. 2008. The clustering of inositol 1,4,5-trisphosphate (IP3) receptors is triggered by IP3 binding and facilitated by depletion of the Ca2+ store. J. Pharmacol. Sci. 107:138–150 10.1254/jphs.08021FP [DOI] [PubMed] [Google Scholar]
- Tovey S.C., de Smet P., Lipp P., Thomas D., Young K.W., Missiaen L., De Smedt H., Parys J.B., Berridge M.J., Thuring J., et al. 2001. Calcium puffs are generic InsP3-activated elementary calcium signals and are downregulated by prolonged hormonal stimulation to inhibit cellular calcium responses. J. Cell Sci. 114:3979–3989 [DOI] [PubMed] [Google Scholar]
- Ventura A.C., Bruno L., Demuro A., Parker I., Dawson S.P. 2005. A model-independent algorithm to derive Ca2+ fluxes underlying local cytosolic Ca2+ transients. Biophys. J. 88:2403–2421 10.1529/biophysj.104.045260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.Q., Song L.S., Lakatta E.G., Cheng H. 2001. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 410:592–596 10.1038/35069083 [DOI] [PubMed] [Google Scholar]
- Wilson B.S., Pfeiffer J.R., Smith A.J., Oliver J.M., Oberdorf J.A., Wojcikiewicz R.J. 1998. Calcium-dependent clustering of inositol 1,4,5-trisphosphate receptors. Mol. Biol. Cell. 9:1465–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen S.M., Smith I.F., Parker I. 2010. Super-resolution localization of single functional inositol trisphosphate receptors channels utilizing calcium flux as a readout. Biophys. J. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Parker I. 1994. Ca2+ influx modulation of temporal and spatial patterns of inositol trisphosphate-mediated Ca2+ liberation in Xenopus oocytes. J. Physiol. 476:17–28 [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Choi J., Parker I. 1995. Quantal puffs of intracellular Ca2+ evoked by inositol trisphosphate in Xenopus oocytes. J. Physiol. 482:533–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller S., Rüdiger S., Engel H., Sneyd J., Warnecke G., Parker I., Falcke M. 2009. Modeling of the modulation by buffers of Ca2+ release through clusters of IP3 receptors. Biophys. J. 97:992–1002 10.1016/j.bpj.2009.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H., Lifshitz L.M., Tuft R.A., Fogarty K.E., Singer J.J. 2004. Using total fluorescence increase (signal mass) to determine the Ca2+ current underlying localized Ca2+ events. J. Gen. Physiol. 124:259–272 10.1085/jgp.200409066 [DOI] [PMC free article] [PubMed] [Google Scholar]