Local Ca2+ signaling in the heart enables the triggering and regulation of the time-dependent changes of intracellular Ca2+ concentration ([Ca2+]i) to be flexibly modulated with a high degree of stability and safety. The elementary physiological and pathophysiological components of subcellular Ca2+ signaling are regulated through a process of Ca2+-induced Ca2+ release (CICR), not by store overload–induced Ca2+ release (SOICR). The central element of CICR regulation is the sensitivity of the SR Ca2+ release channel, the RYR type 2 (RYR2), to [Ca2+]i. Here, in terms of past discoveries and future work, we discuss how RYR2 sensitivity to [Ca2+]i is influenced by key factors, including SR luminal Ca2+ ([Ca2+]SR), RYR2 phosphorylation, mutations in RYR2 and its interacting proteins, as well as cellular stretch. Finally, we explore the pathophysiological consequences of dysfunctional tuning of these regulatory factors in the context of dystrophic cardiomyopathy.
The spatial organization of the cardiac ventricular myocyte enables precise regulation of the cardiac [Ca2+]i transient (Bers, 2001; Cheng and Lederer, 2008). The details of this regulation at the subcellular level, however, remain both controversial and exciting. There is consensus that the “global” or cell-wide [Ca2+]i signal in ventricular myocytes is central to cardiac function; it underlies contraction and contributes to the regulation of electrical activity. Despite the certainty and clarity of these contributions, the subcellular details of Ca2+ signaling remain unsettled. How do subcellular organelles contribute to Ca2+ sparks? Although it is clear that sparks originate almost exclusively at the junctional SR (jSR), many aspects of Ca2+ spark signaling are still being investigated. For example, do mitochondria serve as dynamic Ca2+ stores? What “triggers” spontaneous Ca2+ sparks versus synchronized sparks? How does a propagating Ca2+ wave arise? What sustains the propagating wave of elevated [Ca2+]i? Does the cardiac cytoskeleton play both organizational and dynamic roles in the regulation of Ca2+ sparks? How does SR Ca2+ instability arise, and how does it lead to the generation of cardiac arrhythmias? How can mathematical modeling contribute to such investigations? How does Ca2+ signaling dysfunction influence or underlie disease progression? We present here some organizing principles that should help to resolve several issues and lay the foundation for future studies.
Spatial organization of the heart cell and the control of Ca2+ sparks
Subcellular anatomy: key to Ca2+ signaling stability.
The SR includes two primary components, the jSR and the longitudinal SR (also called the free SR or the “network” SR; see Fig. 1) (Brochet et al., 2005). In addition, the SR is connected to the ER and the nuclear Ca2+ store (Wu and Bers, 2006), thus making the entire Ca2+ storage network fully interconnected. The primary Ca2+ release sites are located at the jSR, an extremely small pancake-shaped sub-volume in the system (each jSR contains ∼1 attoliter, ∼1 × 10−18 l, a size ∼4,000 times smaller than a Ca2+ spark). On one face, the jSR contains a paracrystalline cluster of 10–300 SR Ca2+ release channels, the RYR2s that span 15 nm (the “subspace”) to the surface membrane (i.e., the transverse tubule [TT] or surface sarcolemmal [SL] membranes) (Franzini-Armstrong et al., 1999; Baddeley et al., 2009). The other face of the jSR points toward the M line and is tens of nanometers away from the Z-disk end of the mitochondria and is connected through the thin nanoscopic tubules that constitute the longitudinal SR. The L-type Ca2+ channels (or dihydropyridine receptors) are transmembrane proteins that span the SL and TT membranes facing the subspace and thus appose the jSR. The lumen of the jSR also contains the Ca2+-binding protein calsequestrin type 2 (CASQ2) and other regulatory proteins, such as junctin, triadin, junctophilin, and a small amount of other proteins including the ER Ca2+-binding and chaperone protein calreticulin (Bers, 1991; Györke et al., 2007).
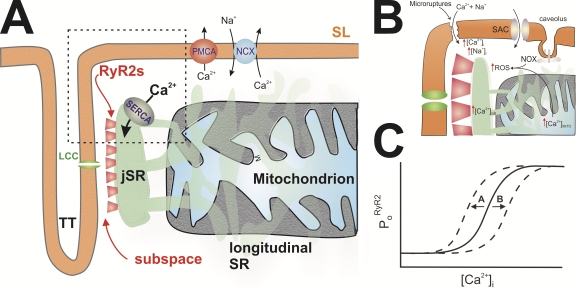
Figure 1.
RYR2s in cardiac ventricular myocytes. (A) Diagram of heart cell illustrating the locations of the TTs and SL with respect to the L-type Ca2+ channels (LCC), the junctional SR (jSR), the longitudinal SR, SERCA, RYR2 cluster, subspace, the Na+/Ca2+ exchanger (NCX), the plasmalemmal Ca2+ ATPase (PMCA), and a mitochondrion. (B) The dashed box (in A) is enlarged to reveal some of the changes that may occur in DMD. Micro-ruptures may lead to an increase in [Ca2+]i and [Na+]i, as may hyperactivity of stretch-activated channels (SAC); overexpression of caveoli, a putative site of NADPH oxidase, may also be involved. (C) Diagrammatic illustration how PoRYR2 sensitivity to [Ca2+]i may be shifted to A (increase in sensitivity) or shifted to B (decrease in sensitivity). See Table I.
The brief opening of either an L-type Ca2+ channel or an RYR2 under diastolic conditions leads to a local elevation of Ca2+ in the subspace ([Ca2+]subspace) from the normal [Ca2+]i of 100 nM to ∼10 µM (Cannell and Soeller, 1997; Soeller and Cannell, 1997; Sobie et al., 2002). This elevation of [Ca2+]subspace, albeit brief (<1 ms), is sufficient to activate the RYR2 cluster to produce a Ca2+ spark, a process termed Ca2+-induced Ca2+ release, or CICR (Fabiato, 1983; Cheng et al., 1993; Cannell et al., 1994a,b; Sobie et al., 2002; Cheng and Lederer, 2008; Györke and Terentyev, 2008; Liu et al., 2010). The spatial organization of the jSR enables reliable activation of Ca2+ sparks by action potentials, but the relative insensitivity of RYR2s to calcium protects the cell from instability and enables Ca2+ sparks during diastole to remain isolated from neighboring jSR spark sites. Thus, Ca2+ sparks do not normally activate other Ca2+ sparks (Cheng et al., 1993). High gain in the signaling pathway is thus created by the spatial organization of RYR2s in a cluster, and stability is maintained by the relative insensitivity of the RYR2s to [Ca2+]i (Cheng and Lederer, 2008).
Ca2+ sparks, Ca2+ blinks, and SR Ca2+ leak.
The “life cycle” of a Ca2+ spark provides clues to its regulation. Should [Ca2+]subspace increase to ∼10 µM, there is good probability that a Ca2+ spark will arise through CICR as noted above. Although Ca2+ spark termination is seen robustly in experiments, our understanding of how this occurs was more challenging. Mathematical modeling of spark behavior suggests that if a significant fraction of the local jSR Ca2+ is depleted during the Ca2+ spark (between 50 and 90%), a robust termination of the spark will be seen (Sobie et al., 2002). Importantly, this occurs without the need for “inactivation” of the RYR2s, unlike in other models (Stern et al., 1999). In support of this model, there is no experimental evidence to date that suggests rapid “fateful” inactivation of RYR2s (Liu et al., 2010). The [Ca2+]SR depletion hypothesis regarding Ca2+ spark termination has been supported by the observation of Ca2+ “blinks” (Brochet et al., 2005). A Ca2+ blink is the [Ca2+]SR depletion signal that occurs when a Ca2+ spark takes place. It is the reciprocal signal of a Ca2+ spark; specifically, [Ca2+]SR depletion is seen whenever sparks occur because the Ca2+ flux that underlies the Ca2+ spark comes from the jSR lumen. SR Ca2+ leak is the term of art now used to describe the non-synchronized loss of Ca2+ from the SR, which may occur as Ca2+ sparks or as very small release events that may be invisible when viewed with a confocal microscope (Sobie et al., 2006). How Ca2+ leak may occur, and how this leak may influence Ca2+ instability and cardiac arrhythmogenesis, is actively under examination (Wehrens et al., 2003; Lehnart et al., 2006, 2008; Sobie et al., 2006).
Triggering Ca2+ sparks and Ca2+ waves.
A Ca2+ spark is the local Ca2+ signal produced by an ensemble of RYR2s at a single jSR (Cheng and Lederer, 2008). Diastolic sparks can occur when a single RYR2 opens probabilistically and increases [Ca2+]subspace to a high level (i.e., ∼10 µM), activating the other RYR2s facing the same subspace and in the same jSR. In a similar manner, the opening of an L-type Ca2+ channel at near-diastolic potentials will also increase [Ca2+]subspace and activate a Ca2+ spark (Cannell et al., 1995; Cheng et al., 1996; Santana et al., 1996). More recently (Jiang et al., 2004), an alternative hypothesis has been put forward suggesting that sparks are triggered by “store overload–induced Ca2+ release,” or SOICR. Although we have not identified experimental evidence that supports the SOICR hypothesis and distinguishes it from CICR, there is strong evidence suggesting that [Ca2+]SR regulates RYR2 [Ca2+]i sensitivity. [Ca2+]SR is certainly an integral feature of CICR because it influences the sensitivity of the RYR2 to be opened by [Ca2+]i (Györke and Györke, 1998). The influence exerted by [Ca2+]SR represents one factor, along with several others such as phosphorylation of the RYR2s, nitrosylation, oxidation, and channel mutations (Wehrens et al., 2003, 2004, 2005; Lehnart et al., 2004; Viatchenko-Karpinski et al., 2004; Kubalova et al., 2005; Terentyev et al., 2005, 2008, 2009; di Barletta et al., 2006; Guo et al., 2006; Györke and Terentyev, 2008), which all can increase the voltage-independent probabilistic openings of RYR2s (Table I and Fig. 1 C). The distinction between the SOICR hypothesis and the CICR hypothesis is important to make. Both support [Ca2+]SR influencing Ca2+ sparks. SOICR, however, suggests that SR Ca2+ overload triggers depolarization-independent Ca2+ sparks (Jiang et al., 2004, 2007, 2008; Jones et al., 2008), underlies Ca2+ waves, and is somehow distinctive from the probabilistic opening of RYR2. Just as the opening of L-type Ca2+ channels apposing the jSR “triggers” Ca2+ sparks by CICR, so does the probabilistic opening of RYR2s in a jSR cluster. Importantly, when a spark initiates a propagating Ca2+ wave or contributes to the conduction of a wave, it is CICR that links the elevation of local [Ca2+]i during the spark produced by one jSR to the triggering of a Ca2+ spark in the next jSR. For Ca2+ waves to arise, it has been hypothesized that an increase in RYR2 sensitivity to [Ca2+]i occurs due to the underlying pathologies, which include SR Ca2+ overload and catecholaminergic polymorphic ventricular tachycardia (CPVT) mutations of RYR2 and CASQ2 (Marks et al., 2002; Eldar et al., 2003; di Barletta et al., 2006; Györke, 2009). By this means, the Ca2+ released by a spark diffuses and elevates the [Ca2+]i in the subspace of a neighboring RYR2 cluster and triggers a spark at that site. This chain reaction can continue to produce the arrhythmogenic Ca2+ wave that activates the Na+/Ca2+ exchanger and produces the arrhythmogenic transient inward current, ITI (Györke et al., 2007). An increase in the sensitivity of the RYR2s to [Ca2+]i is sufficient to enable this sequence. Although elevated [Ca2+]i and [Ca2+]SR are the simplest ways to enable this sequence, changes in RYR2 sensitivity by mutations to its primary sequence or alterations in modulatory proteins (e.g., CASQ2, calstabin2, and others) may also be sufficient. That increases in [Ca2+]SR may increase RYR2 opening rate is a part of CICR that has been well articulated in the literature as noted above. It would thus seem that the CICR hypothesis can fully account for the triggering of Ca2+ sparks and Ca2+ waves in health and disease.
Table I.
Changes in the sensitivity of RYR2 to be opened by [Ca2+]i
Mitochondrial influences on Ca2+ sparks and the [Ca2+]i transient.
To the extent that the mitochondria may buffer Ca2+ or actively take up and release Ca2+, they may significantly affect [Ca2+]i dynamics (Xu et al., 2002; Dedkova and Blatter, 2008; Liu and O’Rourke, 2008; Andrienko et al., 2009; Kettlewell et al., 2009; O’Rourke and Blatter, 2009). The scale of this influence is potentially large because the mitochondria take up approximately one third of the intracellular volume of cardiac myocytes. The largest subset of cardiomyocyte mitochondria are the intermyofibrillar mitochondria that run from the jSR at one Z disk through the M-line structures to the jSR at the next Z disk. Each end of these sarcomere-spanning mitochondria experiences Ca2+ sparks near their source (both ends) and thus may influence the magnitude and kinetics of local [Ca2+]i at their ends and global [Ca2+]i changes in the middle. Although the potential for mitochondria to influence [Ca2+]i is enormous, their actual roles in modulating Ca2+ signals are debated. Experimental evidence for a substantive role (Liu and O’Rourke, 2008, 2009), as well as a minimal role (Andrienko et al., 2009; Kettlewell et al., 2009), has been put forth. Cellular and molecular tools for these investigations are limited because even the primary sequence of virtually every functional structure (channel and transporters) that may influence mitochondrial Ca2+ dynamics remains elusive, despite significant effort. Recently, one of the key transporters was identified, the mitochondrial Na+/Ca2+ exchanger (Palty et al., 2010), which may provide a fundamental tool that is needed to jump-start the investigation and help to resolve the dynamic role that mitochondria may play in cardiac Ca2+ signaling.
Cytoskeleton and Ca2+ sparks.
It has been long hypothesized that the beat-to-beat change in cell length may influence local cardiac [Ca2+]i signals (Allen and Blinks, 1978) and hence Ca2+ sparks . In keeping with this hypothesis, diverse cytoskeletal disruptions have been shown to have profound effects on [Ca2+]i. For example, mutations in the cytoskeletal adaptor protein ankyrin B (AnkB or Ank2) has been shown to affect [Ca2+]i through its actions on Ca2+ transporters and channels (Mohler et al., 2003; Mohler and Bennett, 2005). Consistent with this role, AnkB mutations in human and experimental animals have been shown to underlie long QT syndrome type 4 (Mohler et al., 2003). The investigation, however, suggested that it was not the disruption of a dynamic beat-to-beat regulator that caused the dysfunction. Instead, it was the role played by AnkB in the steady-state organization of the Ca2+ transport and signaling proteins that was the critical cause of the Ca2+ signaling change. Similarly, mutations or knockout of the intermediate filament protein desmin leads to a complex array of cardiac cellular dysfunctions, which may reflect the influence of this cytoskeleton element on diverse steady-state organizational elements such as the mitochondria (Weisleder et al., 2004; Maloyan et al., 2005). In contrast to these findings, recent evidence suggests that there may be a beat-to-beat mechano-transduction pathway for which microtubules and tubulin contribute to stretch activated local Ca2+ signaling (see below) (Iribe et al., 2009).
Cellular stretch, local Ca2+ signaling dysfunction, and muscular dystrophies
Stretch-dependent Ca2+ sparks.
The recent discovery that physiological stretch increases the transient occurrence of Ca2+ sparks (Iribe et al., 2009) reveals an important line of inquiry that can now be investigated for the first time. These findings are important in any reexamination of normal cardiac Ca2+ signaling as well as in the investigation of the pathophysiology of diverse diseases that appear to be linked to cellular contraction and shortening. Iribe et al. (2009) report that a stretch of 8% of the cellular diastolic length leads to a transient increase in the Ca2+ spark rate, which can be blunted specifically by microtubule disruption, but not by inhibitors of L-type Ca2+ channel flux, Na+/Ca2+ exchanger flux, stretch-activated channels, nor NO synthase. Although there was earlier evidence for spatial association of microtubules with the SR and mitochondria (Cartwright and Goldstein, 1985), no functional or contact evidence was provided. In contrast, Iribe and colleagues added to the functional connection noted above with EM tomography, demonstrating co-location of microtubules and the jSR. They thus provide strong evidence for a mechano-transduction pathway that can provide a stretch signal to the jSR. If such a signal were provided to the SR, it could affect all of the RYR2s in the jSR because they are organized in a paracrystalline array. These findings lead to the hypothesis that stretching the ventricular myocyte applies a distortion to the diastolic jSR and thereby increases the sensitivity of RYR2s in the jSR to [Ca2+]i (Iribe et al., 2009). After the stretch is a burst of Ca2+ sparks that relax over seconds. This is the time scale needed for cellular contraction and shortening to influence local Ca2+ signaling and the cardiac [Ca2+]i transient. Note too that such stretch-dependent changes in the open probability of the RYR2 should have analogous effects during all phases of cardiac Ca2+ signaling, including during systole. These exciting and provocative results, still in need of much further investigation, provide us with a lens through which we can view one of the key “stretch-dependent” cardiac diseases, muscular dystrophy. We suspect that there may be important connections between specific kinds of muscular dystrophy and stretch-modulated Ca2+ signaling because four interrelated components are involved in dystrophic cardiomyopathy: (1) Ca2+ entry, (2) Ca2+ release, (3) stretch-dependent modulation, and (4) molecular defects associated with some dystrophies.
Duchenne muscular dystrophy (DMD).
DMD, the most common of the muscle dystrophies (1:3,500 males), is a fatal, X-linked disease characterized by progressive muscle weakness that most commonly leads to respiratory failure (for review see Ervasti and Campbell, 1993). DMD also results in a progressive dilated cardiomyopathy, with nearly all patients exhibiting cardiac manifestations by 20 years of age. As improved treatments for skeletal muscle weakness delay respiratory failure, cardiac complications have become increasingly limiting for the survival of DMD patients (Finsterer and Stöllberger, 2000; Muntoni et al., 2003).
The genetic basis of the DMD pathology is due to a lack of expression or dysfunction of the very large (3,500 amino acids) intracellular muscle protein, dystrophin. Dystrophin associates with the transmembrane β-dystroglycan and other proteins to form the dystrophin–glycoprotein complex and binds to cytosolic actin (Ervasti et al., 1990; Ohlendieck et al., 1993). Similar to human patients, mdx animals lacking dystrophin demonstrate contractile and conductive deficits as well as cardiac dilation and fibrosis that increase with age (Quinlan et al., 2004). Several groups have recently identified local Ca2+ signaling abnormalities in mdx hearts that may underlie the pathology of dystrophic cardiomyopathy (Williams and Allen, 2007a,b; Jung et al., 2008; Ward et al., 2008; Ullrich et al., 2009).
Dysregulated Ca2+ signaling in dystrophic hearts.
Dystrophic myocytes reveal elevated Ca2+ spark activity and the appearance of Ca2+ waves in response to mechanical stress (Yasuda et al., 2005; Jung et al., 2008; Fanchaouy et al., 2009). Of note, among the compensatory adaptations in the mdx heart is an ∼1.4-fold increase in β-tubulin (Wilding et al., 2005). Based on this cytoskeletal adaptation in conjunction with the findings of Iribe et al. (2009), it is attractive to ascribe altered mechano-transduction (via microtubules) to aberrant Ca2+ signals in the DMD heart. In support of this notion, Prins et al. (2009) have discussed dystrophin as a giant “cytolinker” protein, one that stabilizes structures through the ability to link to actin filaments as well as to microtubules.
Work by Yasuda et al. (2005) suggests that the stretch-induced aberrant Ca2+ signals in mdx myocytes depends on Ca2+ (and possibly Na+) entry. Use of the surfactant and putative “membrane sealant” poloxamer 188 (with support from experiments with the extracellular dye FM-143) has led to the hypothesis that “micro-ruptures” in the SL membrane may underlie Ca2+ entry in stressed mdx myocytes (Yasuda et al., 2005; Fanchaouy et al., 2009). Other reports have linked stretch-activated channels to enhanced Ca2+ influx in the dystrophic heart (Williams and Allen, 2007a). These channels have been shown to be overexpressed in the DMD heart, and their inhibition blunts the aberrant local Ca2+ activity associated with stress (Fanchaouy et al., 2009).
Other mechanisms have been reported that could indirectly affect mechano-transduction pathways of altered SR Ca2+ release. The dystrophic heart produces more reactive oxygen species (ROS) (Williams and Allen, 2007b), and mdx myocytes exhibit aggravated ROS production in response to osmotic shock (Jung et al., 2008; Fanchaouy et al., 2009), a commonly used, albeit non-physiological form of mechanical stress. Mechanistically, oxidation sensitizes RYRs to Ca2+ (Pessah et al., 2002), making excessive ROS production and RYR2 sensitization an appealing explanation for a greater degree of Ca2+ instability in the DMD heart. Supporting this hypothesis is the apparent ability of ROS scavengers to protect mdx cells from the Ca2+ signaling consequences of mechanical stress (Jung et al., 2008; Ullrich et al., 2009) and the up-regulation of NADPH oxidase in the DMD heart (Williams and Allen, 2007b). Furthermore, several additional pathways have been linked to dystrophic changes in skeletal muscle, such as RYR nitrosylation and RYR hyperphosphorylation (Bellinger et al., 2009). These pathways could also serve to increase the sensitivity of RYR2 in the DMD heart to [Ca2+]i. If so, this could result in an increase in the mechano-transduction–induced gain of CICR. Clearly, these provocative hypotheses must be tested experimentally.
Conclusions
The regulation of local Ca2+ signaling is critically dependent on the sensitivity of RYR2 to [Ca2+]i and the subcellular anatomy of the heart cell. Physiological and pathophysiologic changes in local cardiac Ca2+ signaling may depend on altered sensitivity of the RYR2s to [Ca2+]i. These critical signaling changes arise from many factors that influence the open probability of RYR2, including [Ca2+]SR, mutations of RYR2 itself, mutations of RYR2-linked proteins, RYR2 protein phosphorylation and nitrosylation, and other molecular and cellular features. Recently, cellular stretch and the activation of mechano-transduction pathways have been shown to contribute to this process. Quantitative molecular and biophysical investigations of the processes that regulate RYR2 behavior are needed to further elucidate cardiac Ca2+ signaling in health and disease.
This Perspectives series includes articles by Gordon, Parker and Smith, Xie et al., Santana and Navedo, and Hill-Eubanks et al.
Acknowledgments
We would like to acknowledge support from National Heart, Lung and Blood Institute (grants P01 HL67849 and R01-HL36974); National Institutes of Health (grant RC2 NR011968); Leducq North American-European Atrial Fibrillation Research Alliance; European Union Seventh Framework Program (FP7) “Identification and therapeutic targeting of common arrhythmia trigger mechanisms”; and support from the Maryland Stem Cell Commission.
Footnotes
Abbreviations used in this paper:
- AnkB
- ankyrin B
- [Ca2+]i
- intracellular Ca2+
- [Ca2+]SR
- SR luminal Ca2+
- CASQ2
- calsequestrin type 2
- CICR
- Ca2+-induced Ca2+ release
- DMD
- Duchenne muscular dystrophy
- jSR
- junctional SR
- ROS
- reactive oxygen species
- RYR2
- RYR type 2
- SL
- surface sarcolemmal
- SOICR
- store overload–induced Ca2+ release
- TT
- transverse tubule
References
- Allen D.G., Blinks J.R. 1978. Calcium transients in aequorin-injected frog cardiac muscle. Nature. 273:509–513 10.1038/273509a0 [DOI] [PubMed] [Google Scholar]
- Andrienko T.N., Picht E., Bers D.M. 2009. Mitochondrial free calcium regulation during sarcoplasmic reticulum calcium release in rat cardiac myocytes. J. Mol. Cell. Cardiol. 46:1027–1036 10.1016/j.yjmcc.2009.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley D., Jayasinghe I.D., Lam L., Rossberger S., Cannell M.B., Soeller C. 2009. Optical single-channel resolution imaging of the ryanodine receptor distribution in rat cardiac myocytes. Proc. Natl. Acad. Sci. USA. 106:22275–22280 10.1073/pnas.0908971106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger A.M., Reiken S., Carlson C., Mongillo M., Liu X., Rothman L., Matecki S., Lacampagne A., Marks A.R. 2009. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat. Med. 15:325–330 10.1038/nm.1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D.M. 1991. Excitation-Contraction Coupling and Cardiac Contractile Force. Kluwer Academic Publishers, Boston: 288 [Google Scholar]
- Bers D.M. 2001. Excitation-Contraction Coupling and Cardiac Contractile Force. Kluwer Academic Publishers, Boston: 452 [Google Scholar]
- Brochet D.X., Yang D., Di Maio A., Lederer W.J., Franzini-Armstrong C., Cheng H. 2005. Ca2+ blinks: rapid nanoscopic store calcium signaling. Proc. Natl. Acad. Sci. USA. 102:3099–3104 10.1073/pnas.0500059102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M.B., Soeller C. 1997. Numerical analysis of ryanodine receptor activation by L-type channel activity in the cardiac muscle diad. Biophys. J. 73:112–122 10.1016/S0006-3495(97)78052-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M.B., Cheng H., Lederer W.J. 1994a. Nifedipine decreases the spatial uniformity of the depolarization-evoked Ca2+ transient in isolated rat cardiac myocytes. J. Physiol. 477:25P [Google Scholar]
- Cannell M.B., Cheng H., Lederer W.J. 1994b. Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys. J. 67:1942–1956 10.1016/S0006-3495(94)80677-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M.B., Cheng H., Lederer W.J. 1995. The control of calcium release in heart muscle. Science. 268:1045–1049 10.1126/science.7754384 [DOI] [PubMed] [Google Scholar]
- Cartwright J., Jr., Goldstein M.A. 1985. Microtubules in the heart muscle of the postnatal and adult rat. J. Mol. Cell. Cardiol. 17:1–7 10.1016/S0022-2828(85)80087-0 [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W.J. 2008. Calcium sparks. Physiol. Rev. 88:1491–1545 10.1152/physrev.00030.2007 [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W.J., Cannell M.B. 1993. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 262:740–744 10.1126/science.8235594 [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer M.R., Lederer W.J., Cannell M.B. 1996. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am. J. Physiol. 270:C148–C159 [DOI] [PubMed] [Google Scholar]
- Chopra N., Yang T., Asghari P., Moore E.D., Huke S., Akin B., Cattolica R.A., Perez C.F., Hlaing T., Knollmann-Ritschel B.E., et al. 2009. Ablation of triadin causes loss of cardiac Ca2+ release units, impaired excitation-contraction coupling, and cardiac arrhythmias. Proc. Natl. Acad. Sci. USA. 106:7636–7641 10.1073/pnas.0902919106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedkova E.N., Blatter L.A. 2008. Mitochondrial Ca(2+) and the heart. Cell Calcium. 44:77–91 [DOI] [PubMed] [Google Scholar]
- di Barletta M.R., Viatchenko-Karpinski S., Nori A., Memmi M., Terentyev D., Turcato F., Valle G., Rizzi N., Napolitano C., Gyorke S., et al. 2006. Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia. Circulation. 114:1012–1019 10.1161/CIRCULATIONAHA.106.623793 [DOI] [PubMed] [Google Scholar]
- Eldar M., Pras E., Lahat H. 2003. A missense mutation in the CASQ2 gene is associated with autosomal-recessive catecholamine-induced polymorphic ventricular tachycardia. Trends Cardiovasc. Med. 13:148–151 10.1016/S1050-1738(03)00025-2 [DOI] [PubMed] [Google Scholar]
- Ervasti J.M., Campbell K.P. 1993. Dystrophin and the membrane skeleton. Curr. Opin. Cell Biol. 5:82–87 10.1016/S0955-0674(05)80012-2 [DOI] [PubMed] [Google Scholar]
- Ervasti J.M., Ohlendieck K., Kahl S.D., Gaver M.G., Campbell K.P. 1990. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 345:315–319 10.1038/345315a0 [DOI] [PubMed] [Google Scholar]
- Fabiato A. 1983. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 245:C1–C14 [DOI] [PubMed] [Google Scholar]
- Fanchaouy M., Polakova E., Jung C., Ogrodnik J., Shirokova N., Niggli E. 2009. Pathways of abnormal stress-induced Ca2+ influx into dystrophic mdx cardiomyocytes. Cell Calcium. 46:114–121 10.1016/j.ceca.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J., Stöllberger C. 2000. Cardiac involvement in primary myopathies. Cardiology. 94:1–11 10.1159/000007039 [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Protasi F., Ramesh V. 1999. Shape, size, and distribution of Ca(2+) release units and couplons in skeletal and cardiac muscles. Biophys. J. 77:1528–1539 10.1016/S0006-3495(99)77000-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T., Zhang T., Mestril R., Bers D.M. 2006. Ca2+/calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ. Res. 99:398–406 10.1161/01.RES.0000236756.06252.13 [DOI] [PubMed] [Google Scholar]
- Györke I., Györke S. 1998. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys. J. 75:2801–2810 10.1016/S0006-3495(98)77723-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S. 2009. Molecular basis of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 6:123–129 10.1016/j.hrthm.2008.09.013 [DOI] [PubMed] [Google Scholar]
- Györke S., Terentyev D. 2008. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc. Res. 77:245–255 10.1093/cvr/cvm038 [DOI] [PubMed] [Google Scholar]
- Györke S., Györke I., Lukyanenko V., Terentyev D., Viatchenko-Karpinski S., Wiesner T.F. 2002. Regulation of sarcoplasmic reticulum calcium release by luminal calcium in cardiac muscle. Front. Biosci. 7:d1454–d1463 10.2741/gyorke [DOI] [PubMed] [Google Scholar]
- Györke S., Hagen B.M., Terentyev D., Lederer W.J. 2007. Chain-reaction Ca2+ signaling in the heart. J. Clin. Invest. 7:1758–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo C., Aracena P., Sanchez G., Donoso P. 2002. Redox regulation of calcium release in skeletal and cardiac muscle. Biol. Res. 35:183–193 10.4067/S0716-97602002000200009 [DOI] [PubMed] [Google Scholar]
- Iribe G., Ward C.W., Camelliti P., Bollensdorff C., Mason F., Burton R.A., Garny A., Morphew M.K., Hoenger A., Lederer W.J., Kohl P. 2009. Axial stretch of rat single ventricular cardiomyocytes causes an acute and transient increase in Ca2+ spark rate. Circ. Res. 104:787–795 10.1161/CIRCRESAHA.108.193334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Xiao B., Yang D., Wang R., Choi P., Zhang L., Cheng H., Chen S.R. 2004. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR). Proc. Natl. Acad. Sci. USA. 101:13062–13067 10.1073/pnas.0402388101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Chen W., Wang R., Zhang L., Chen S.R. 2007. Loss of luminal Ca2+ activation in the cardiac ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc. Natl. Acad. Sci. USA. 104:18309–18314 10.1073/pnas.0706573104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D., Chen W., Xiao J., Wang R., Kong H., Jones P.P., Zhang L., Fruen B., Chen S.R. 2008. Reduced threshold for luminal Ca2+ activation of RyR1 underlies a causal mechanism of porcine malignant hyperthermia. J. Biol. Chem. 283:20813–20820 10.1074/jbc.M801944200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P.P., Jiang D., Bolstad J., Hunt D.J., Zhang L., Demaurex N., Chen S.R. 2008. Endoplasmic reticulum Ca2+ measurements reveal that the cardiac ryanodine receptor mutations linked to cardiac arrhythmia and sudden death alter the threshold for store-overload-induced Ca2+ release. Biochem. J. 412:171–178 10.1042/BJ20071287 [DOI] [PubMed] [Google Scholar]
- Jung C., Martins A.S., Niggli E., Shirokova N. 2008. Dystrophic cardiomyopathy: amplification of cellular damage by Ca2+ signalling and reactive oxygen species-generating pathways. Cardiovasc. Res. 77:766–773 10.1093/cvr/cvm089 [DOI] [PubMed] [Google Scholar]
- Kettlewell S., Cabrero P., Nicklin S.A., Dow J.A., Davies S., Smith G.L. 2009. Changes of intra-mitochondrial Ca2+ in adult ventricular cardiomyocytes examined using a novel fluorescent Ca2+ indicator targeted to mitochondria. J. Mol. Cell. Cardiol. 46:891–901 10.1016/j.yjmcc.2009.02.016 [DOI] [PubMed] [Google Scholar]
- Knollmann B.C., Chopra N., Hlaing T., Akin B., Yang T., Ettensohn K., Knollmann B.E., Horton K.D., Weissman N.J., Holinstat I., et al. 2006. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J. Clin. Invest. 116:2510–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubalova Z., Terentyev D., Viatchenko-Karpinski S., Nishijima Y., Györke I., Terentyeva R., da Cuñha D.N., Sridhar A., Feldman D.S., Hamlin R.L., et al. 2005. Abnormal intrastore calcium signaling in chronic heart failure. Proc. Natl. Acad. Sci. USA. 102:14104–14109 10.1073/pnas.0504298102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnart S.E., Wehrens X.H., Laitinen P.J., Reiken S.R., Deng S.X., Cheng Z., Landry D.W., Kontula K., Swan H., Marks A.R. 2004. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation. 109:3208–3214 10.1161/01.CIR.0000132472.98675.EC [DOI] [PubMed] [Google Scholar]
- Lehnart S.E., Terrenoire C., Reiken S., Wehrens X.H., Song L.S., Tillman E.J., Mancarella S., Coromilas J., Lederer W.J., Kass R.S., Marks A.R. 2006. Stabilization of cardiac ryanodine receptor prevents intracellular calcium leak and arrhythmias. Proc. Natl. Acad. Sci. USA. 103:7906–7910 10.1073/pnas.0602133103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnart S.E., Mongillo M., Bellinger A., Lindegger N., Chen B.X., Hsueh W., Reiken S., Wronska A., Drew L.J., Ward C.W., et al. 2008. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J. Clin. Invest. 118:2230–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Chu G., Kranias E.G., Bers D.M. 1998. Cardiac myocyte calcium transport in phospholamban knockout mouse: relaxation and endogenous CaMKII effects. Am. J. Physiol. 274:H1335–H1347 [DOI] [PubMed] [Google Scholar]
- Liu T., O’Rourke B. 2008. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ. Res. 103:279–288 10.1161/CIRCRESAHA.108.175919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., O’Rourke B. 2009. Regulation of mitochondrial Ca2+ and its effects on energetics and redox balance in normal and failing heart. J. Bioenerg. Biomembr. 41:127–132 10.1007/s10863-009-9216-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Porta M., Qin J., Ramos J., Nani A., Shannon T.R., Fill M. 2010. Flux regulation of cardiac ryanodine receptor channels. J. Gen. Physiol. 135:15–27 10.1085/jgp.200910273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V., Györke I., Györke S. 1996. Regulation of calcium release by calcium inside the sarcoplasmic reticulum in ventricular myocytes. Pflugers Arch. 432:1047–1054 10.1007/s004240050233 [DOI] [PubMed] [Google Scholar]
- Maloyan A., Sanbe A., Osinska H., Westfall M., Robinson D., Imahashi K., Murphy E., Robbins J. 2005. Mitochondrial dysfunction and apoptosis underlie the pathogenic process in alpha-B-crystallin desmin-related cardiomyopathy. Circulation. 112:3451–3461 10.1161/CIRCULATIONAHA.105.572552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks A.R., Priori S., Memmi M., Kontula K., Laitinen P.J. 2002. Involvement of the cardiac ryanodine receptor/calcium release channel in catecholaminergic polymorphic ventricular tachycardia. J. Cell. Physiol. 190:1–6 10.1002/jcp.10031 [DOI] [PubMed] [Google Scholar]
- Marx S.O., Reiken S., Hisamatsu Y., Jayaraman T., Burkhoff D., Rosemblit N., Marks A.R. 2000. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 101:365–376 10.1016/S0092-8674(00)80847-8 [DOI] [PubMed] [Google Scholar]
- Mohler P.J., Bennett V. 2005. Ankyrin-based cardiac arrhythmias: a new class of channelopathies due to loss of cellular targeting. Curr. Opin. Cardiol. 20:189–193 10.1097/01.hco.0000160372.95116.3e [DOI] [PubMed] [Google Scholar]
- Mohler P.J., Schott J.J., Gramolini A.O., Dilly K.W., Guatimosim S., duBell W.H., Song L.S., Haurogné K., Kyndt F., Ali M.E., et al. 2003. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 421:634–639 10.1038/nature01335 [DOI] [PubMed] [Google Scholar]
- Muntoni F., Torelli S., Ferlini A. 2003. Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet Neurol. 2:731–740 10.1016/S1474-4422(03)00585-4 [DOI] [PubMed] [Google Scholar]
- O’Neill S.C., Eisner D.A. 1990. A mechanism for the effects of caffeine on Ca2+ release during diastole and systole in isolated rat ventricular myocytes. J. Physiol. 430:519–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke B., Blatter L.A. 2009. Mitochondrial Ca2+ uptake: tortoise or hare? J. Mol. Cell. Cardiol. 46:767–774 10.1016/j.yjmcc.2008.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendieck K., Matsumura K., Ionasescu V.V., Towbin J.A., Bosch E.P., Weinstein S.L., Sernett S.W., Campbell K.P. 1993. Duchenne muscular dystrophy: deficiency of dystrophin-associated proteins in the sarcolemma. Neurology. 43:795–800 [DOI] [PubMed] [Google Scholar]
- Overend C.L., Eisner D.A., O’Neill S.C. 1997. The effect of tetracaine on spontaneous Ca2+ release and sarcoplasmic reticulum calcium content in rat ventricular myocytes. J. Physiol. 502:471–479 10.1111/j.1469-7793.1997.471bj.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palty R., Silverman W.F., Hershfinkel M., Caporale T., Sensi S.L., Parnis J., Nolte C., Fishman D., Shoshan-Barmatz V., Herrmann S., et al. 2010. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. USA. 107:436–441 10.1073/pnas.0908099107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah I.N., Kim K.H., Feng W. 2002. Redox sensing properties of the ryanodine receptor complex. Front. Biosci. 7:a72–a79 10.2741/pessah [DOI] [PubMed] [Google Scholar]
- Postma A.V., Denjoy I., Hoorntje T.M., Lupoglazoff J.M., Da Costa A., Sebillon P., Mannens M.M., Wilde A.A., Guicheney P. 2002. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ. Res. 91:e21–e26 10.1161/01.RES.0000038886.18992.6B [DOI] [PubMed] [Google Scholar]
- Prins K.W., Humston J.L., Mehta A., Tate V., Ralston E., Ervasti J.M. 2009. Dystrophin is a microtubule-associated protein. J. Cell Biol. 186:363–369 10.1083/jcb.200905048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan J.G., Hahn H.S., Wong B.L., Lorenz J.N., Wenisch A.S., Levin L.S. 2004. Evolution of the mdx mouse cardiomyopathy: physiological and morphological findings. Neuromuscul. Disord. 14:491–496 10.1016/j.nmd.2004.04.007 [DOI] [PubMed] [Google Scholar]
- Rousseau E., Meissner G. 1989. Single cardiac sarcoplasmic reticulum Ca2+-release channel: activation by caffeine. Am. J. Physiol. 256:H328–H333 [DOI] [PubMed] [Google Scholar]
- Santana L.F., Cheng H., Gómez A.M., Cannell M.B., Lederer W.J. 1996. Relation between the sarcolemmal Ca2+ current and Ca2+ sparks and local control theories for cardiac excitation-contraction coupling. Circ. Res. 78:166–171 [DOI] [PubMed] [Google Scholar]
- Sobie E.A., Dilly K.W., dos Santos Cruz J., Lederer W.J., Jafri M.S. 2002. Termination of cardiac Ca(2+) sparks: an investigative mathematical model of calcium-induced calcium release. Biophys. J. 83:59–78 10.1016/S0006-3495(02)75149-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobie E.A., Guatimosim S., Gómez-Viquez L., Song L.S., Hartmann H., Saleet Jafri M., Lederer W.J. 2006. The Ca2+ leak paradox and rogue ryanodine receptors: SR Ca2+ efflux theory and practice. Prog. Biophys. Mol. Biol. 90:172–185 10.1016/j.pbiomolbio.2005.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeller C., Cannell M.B. 1997. Numerical simulation of local calcium movements during L-type calcium channel gating in the cardiac diad. Biophys. J. 73:97–111 10.1016/S0006-3495(97)78051-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M.D., Song L.S., Cheng H., Sham J.S., Yang H.T., Boheler K.R., Ríos E. 1999. Local control models of cardiac excitation–contraction coupling. A possible role for allosteric interactions between ryanodine receptors. J. Gen. Physiol. 113:469–489 10.1085/jgp.113.3.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Yamaguchi N., Xu L., Eu J.P., Stamler J.S., Meissner G. 2008. Regulation of the cardiac muscle ryanodine receptor by O(2) tension and S-nitrosoglutathione. Biochemistry. 47:13985–13990 10.1021/bi8012627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D., Cala S.E., Houle T.D., Viatchenko-Karpinski S., Gyorke I., Terentyeva R., Williams S.C., Gyorke S. 2005. Triadin overexpression stimulates excitation-contraction coupling and increases predisposition to cellular arrhythmia in cardiac myocytes. Circ. Res. 96:651–658 10.1161/01.RES.0000160609.98948.25 [DOI] [PubMed] [Google Scholar]
- Terentyev D., Györke I., Belevych A.E., Terentyeva R., Sridhar A., Nishijima Y., de Blanco E.C., Khanna S., Sen C.K., Cardounel A.J., et al. 2008. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ. Res. 103:1466–1472 10.1161/CIRCRESAHA.108.184457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D., Belevych A.E., Terentyeva R., Martin M.M., Malana G.E., Kuhn D.E., Abdellatif M., Feldman D.S., Elton T.S., Györke S. 2009. miR-1 overexpression enhances Ca(2+) release and promotes cardiac arrhythmogenesis by targeting PP2A regulatory subunit B56alpha and causing CaMKII-dependent hyperphosphorylation of RyR2. Circ. Res. 104:514–521 10.1161/CIRCRESAHA.108.181651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich N.D., Fanchaouy M., Gusev K., Shirokova N., Niggli E. 2009. Hypersensitivity of excitation-contraction coupling in dystrophic cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 297:H1992–H2003 10.1152/ajpheart.00602.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia H.H., Kaplan J.H., Ellis-Davies G.C., Lederer W.J. 1995. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 267:1997–2000 10.1126/science.7701323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venetucci L.A., Trafford A.W., Eisner D.A. 2007. Increasing ryanodine receptor open probability alone does not produce arrhythmogenic calcium waves: threshold sarcoplasmic reticulum calcium content is required. Circ. Res. 100:105–111 10.1161/01.RES.0000252828.17939.00 [DOI] [PubMed] [Google Scholar]
- Viatchenko-Karpinski S., Terentyev D., Györke I., Terentyeva R., Volpe P., Priori S.G., Napolitano C., Nori A., Williams S.C., Györke S. 2004. Abnormal calcium signaling and sudden cardiac death associated with mutation of calsequestrin. Circ. Res. 94:471–477 10.1161/01.RES.0000115944.10681.EB [DOI] [PubMed] [Google Scholar]
- Ward M.L., Williams I.A., Chu Y., Cooper P.J., Ju Y.K., Allen D.G. 2008. Stretch-activated channels in the heart: contributions to length-dependence and to cardiomyopathy. Prog. Biophys. Mol. Biol. 97:232–249 10.1016/j.pbiomolbio.2008.02.009 [DOI] [PubMed] [Google Scholar]
- Wehrens X.H., Lehnart S.E., Huang F., Vest J.A., Reiken S.R., Mohler P.J., Sun J., Guatimosim S., Song L.S., Rosemblit N., et al. 2003. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 113:829–840 10.1016/S0092-8674(03)00434-3 [DOI] [PubMed] [Google Scholar]
- Wehrens X.H., Lehnart S.E., Reiken S.R., Deng S.X., Vest J.A., Cervantes D., Coromilas J., Landry D.W., Marks A.R. 2004. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 304:292–296 10.1126/science.1094301 [DOI] [PubMed] [Google Scholar]
- Wehrens X.H., Lehnart S.E., Marks A.R. 2005. Intracellular calcium release and cardiac disease. Annu. Rev. Physiol. 67:69–98 10.1146/annurev.physiol.67.040403.114521 [DOI] [PubMed] [Google Scholar]
- Weisleder N., Taffet G.E., Capetanaki Y. 2004. Bcl-2 overexpression corrects mitochondrial defects and ameliorates inherited desmin null cardiomyopathy. Proc. Natl. Acad. Sci. USA. 101:769–774 10.1073/pnas.0303202101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding J.R., Schneider J.E., Sang A.E., Davies K.E., Neubauer S., Clarke K. 2005. Dystrophin- and MLP-deficient mouse hearts: marked differences in morphology and function, but similar accumulation of cytoskeletal proteins. FASEB J. 19:79–81 [DOI] [PubMed] [Google Scholar]
- Williams I.A., Allen D.G. 2007a. Intracellular calcium handling in ventricular myocytes from mdx mice. Am. J. Physiol. Heart Circ. Physiol. 292:H846–H855 10.1152/ajpheart.00688.2006 [DOI] [PubMed] [Google Scholar]
- Williams I.A., Allen D.G. 2007b. The role of reactive oxygen species in the hearts of dystrophin-deficient mdx mice. Am. J. Physiol. Heart Circ. Physiol. 293:H1969–H1977 10.1152/ajpheart.00489.2007 [DOI] [PubMed] [Google Scholar]
- Wu X., Bers D.M. 2006. Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ. Res. 99:283–291 [DOI] [PubMed] [Google Scholar]
- Xu L., Jones R., Meissner G. 1993. Effects of local anesthetics on single channel behavior of skeletal muscle calcium release channel. J. Gen. Physiol. 101:207–233 10.1085/jgp.101.2.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Eu J.P., Meissner G., Stamler J.S. 1998. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 279:234–237 10.1126/science.279.5348.234 [DOI] [PubMed] [Google Scholar]
- Xu W., Liu Y., Wang S., McDonald T., Van Eyk J.E., Sidor A., O’Rourke B. 2002. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science. 298:1029–1033 10.1126/science.1074360 [DOI] [PubMed] [Google Scholar]
- Yasuda S., Townsend D., Michele D.E., Favre E.G., Day S.M., Metzger J.M. 2005. Dystrophic heart failure blocked by membrane sealant poloxamer. Nature. 436:1025–1029 10.1038/nature03844 [DOI] [PubMed] [Google Scholar]