Elemental calcium signals from RYR arrays operating in cardiac myocytes have been extensively characterized with ever-improving optical methods and other innovative techniques. However, the exact nature of elemental calcium signals in terms of RYR gating in situ remains an enigma. Here, we synthesize insights gleaned from recent developments in single-channel resolution of cardiac RYR organization and in visualization of calcium release events that are much smaller than calcium sparks. This synthesis leads to the proposal of a conceptual framework that promises to unify diverse observations in sparkology.
Introduction
Ever since the discovery of calcium sparks some 17 years ago (Cheng et al., 1993), the characterization of elemental calcium signals has unveiled a new paradigm of intracellular calcium signaling. It is now appreciated that nearly all cells use discrete microscopic calcium signals to build up exquisite hierarchical calcium dynamics in space, time, and concentration (Berridge et al., 2000; Cheng and Lederer, 2008). Elemental calcium signals are relevant not only in striated muscle contraction (Cheng et al., 1993; Tsugorka et al., 1995; Klein et al., 1996), smooth muscle relaxation (Nelson et al., 1995), and neuronal secretion (Ouyang et al., 2005), but also in understanding calcium signaling abnormalities in major diseases such as heart failure and calcium-dependent arrhythmias (Litwin et al., 2000; Sipido et al., 2002; Song et al., 2006).
In cardiac myocytes, calcium sparks reflect the gating of RYR calcium release channels in the ER/SR. Because much has been learned about how single RYRs behave in vitro (Fill and Copello, 2002), one might have expected few surprises to originate from the pursuit of sparkology. However, an enduring enigma has been the exact nature of elemental calcium signals in terms of RYR gating in intact cells. Or, stated differently, how can one infer the in situ RYR gating mechanism concealed in the spatiotemporal kinetics of elemental calcium signals?
At the heart of the question lie generic differences between RYRs in cells and those in artificial lipid bilayers or other cell-free preparations. In the first place, RYRs form two-dimensional quasi-crystalline arrays in the membrane of terminal cisterns of the SR. Such array formation imposes physical constraints and enables rich interactions between and among RYRs, creating a new entity that differs from RYRs acting solo. New properties such as the coupled gating of RYRs emerge (Marx et al., 1998, 2001); some behaviors may be abrogated, and still others may be retained, but not without modification. Furthermore, RYR array operation in situ is entangled with extravagant swings of cis (the junctional cleft) and trans (the cisternal lumen) calcium concentration, which may enact positive and negative feedback regulation, including local calcium-induced calcium release (CICR), calcium-dependent channel inactivation/adaptation (Györke and Fill, 1993; Wang et al., 2004), and RYR desensitization by SR calcium depletion (Györke and Györke, 1998; Györke and Terentyev, 2008). Apart from these, generic differences also include decoration of the RYR arrays with molecular partners on the cytosolic side (e.g., calmodulin, calstabin, kinases, and phosphatases), in the lipid membrane (e.g., triadin and junctin), and inside the lumen (e.g., calsequestrin). As such, we should resist the temptation to oversimplify and redefine the laws underlying RYR array operation, including its activation, coordination, termination, and even its pharmacology.
Here, we synthesize insights gleaned from recent developments, particularly in the single-channel resolution of RYR organization in situ (Baddeley et al., 2009) and in visualization of calcium release events that are much smaller than calcium sparks (Brochet et al., 2009). This synthesis leads to a conceptual framework that promises to unify diverse observations in sparkology.
Diversity of elemental calcium signals
RYR array operation in cardiac myocytes has been extensively characterized with ever-improving optical methods and other innovative techniques. Steadfast efforts by many investigators have unraveled rich substructures in a seemingly atomic calcium spark and, more importantly, distinct modes underlying local calcium release.
Quantal calcium release unit (CRU) in a spark.
Although it was initially suggested that calcium sparks are stereotypical, strong evidence indicates that they display remarkable polymorphism. Shen et al. (2004) used the loose-seal patch clamp to acquire calcium sparks at well-defined subsurface locations and demonstrated very broad distributions in spark amplitude and rate of rise. Considerable polymorphism can be found even among events originating from the same release site. Wang et al. (2004) suggested that calcium release flux in a spark consists of quantal units, stochastic recruitment of which gives rise to the polymorphic appearance of the spark. This surprising conclusion was based on the ability to measure calcium release flux in a calcium spark (Ispark) with a novel procedure: (a) measuring calcium “sparklets” produced by unitary L-type calcium currents (iCa) when the SR calcium release is abolished pharmacologically and iCa is enhanced by FPL64176 and high extracellular calcium; (b) determining the iCa under identical experimental conditions with the giga-seal cell-attached patch clamp technique; and (c) estimating Ispark with the sparklet of known iCa as an optical yardstick. The resultant histogram of Ispark exhibits regular or quantal substructures with the quantal unit of 1.24 pA and, on average, 2–3 quanta per calcium spark. The inclusion of tetracaine in the patch pipette reduces the number of quanta per spark while the quantal unit is unchanged.
Calcium quark or “invisible” SR release.
During cardiac excitation–contraction coupling, calcium sparks are readily resolved on the premise that only a small fraction of release sites are active (to ensure adequate signal contrast). However, calcium transients are spatially uniform when the release is activated by low-intensity photolysis of calcium-caged compounds (Lipp and Niggli, 1996) or by the calcium influx of reverse sodium–calcium exchange (Lipp et al., 2002). It has been hypothesized that the sparkless calcium transient results from smaller fundamental events, “calcium quarks,” which represent the independent gating of individual RYRs. Likewise, local calcium release that is smaller than a calcium spark can be triggered photolytically with focal two-photon excitation (Lipp and Niggli, 1998).
An increase of the SR calcium leak can occur with no observable increase in the rate of calcium sparks under certain physiological (Marx et al., 2000; Prestle et al., 2001; Li et al., 2002) and pathological conditions (Gómez et al., 1997; Ono et al., 2000; Shannon et al., 2003). To account for this “invisible” SR calcium efflux, Sobie et al. (2006) postulated the existence of “rogue RYRs,” one or a few RYRs in a cluster that are physically uncoupled from the RYR cluster that underlies spark production. Differing from RYRs in large arrays, rogue RYRs are thought to behave in ways more like the descriptions of single RYRs in terms of gating kinetics and CICR sensitivity. However, ultrastructural evidence for the existence of such rogue RYRs was lacking until recently (see below).
Quarky calcium release
Direct visualization of subspark local release events in resting cells has been made possible by recent technical innovations. Detection of small signals against noise is confounded by the dilemma of rejecting false positive events while preserving high sensitivity to prevent false negatives. Brochet et al. (2009) implemented simultaneous dual imaging of both cytosolic and SR lumenal calcium to cope with this challenge. This approach enhances the detectability by orders of magnitude compared with signal detection in only one imaging channel. In rabbit ventricular myocytes, regular calcium sparks (measured by rhod-2 loaded in the cytosol) are mirrored by “calcium blinks” (measured by fluo-5N loaded in the SR) (Brochet et al., 2005, 2009). Remarkably, there are also tiny events that are only one fifth to one seventh the size of a regular spark–blink pair. This quark-like or “quarky” calcium release can occur at the same sites that support the generation of regular spark–blink pairs. More surprisingly, virtually all regular calcium sparks include quarky calcium release components that last beyond the peak of the spark. Calcium buffering by EGTA exerts little effect on the initial, high-flux release that determines spark amplitude, but it suppresses low-flux, highly variable quarky calcium release, indicating that the latter is sustained by local CICR during an ongoing calcium spark. Together with the polymorphism of calcium sparks noted above, we conclude that distinct modes of elemental calcium release coexist at a single release site.
RYR arrays in cardiac myocytes
Array formation appears to be a property intrinsic to the calcium release channel because purified RYRs can self-assemble into large two-dimensional arrays either in solution (Yin and Lai, 2000) or on the surface of positively charged lipid membrane (Yin et al., 2005). In skeletal muscles of adult mammals, RYRs (type 1) in the junctional cleft or triad are organized in double rows with alternate direct coupling to the sarcolemmal voltage sensor or the dihydropyridine receptor (Franzini-Armstrong et al., 1998). With the advent of optical superresolution microscopy (30-nm resolution), novel and interesting details have been revealed in the subsurface layer of rat ventricular myocytes (Baddeley et al., 2009). First, cardiac RYR clusters are much smaller than previously thought. The mean number of RYRs in a cluster is ∼13.6, or 21.6 in a “supercluster” consisting of clusters within 100 nm edge-to-edge. In contrast, previous estimates, based on thin-section electron microscopy and the intuitive assumption of circular geometry, place 128, 267, and 90 RYRs in a dyad of mouse, rat, and dog ventricular myocytes, respectively (Franzini-Armstrong et al., 1999). Second, there are indeed rogue RYRs as proposed by Sobie et al. (2006), and the cluster size varies over a wide, continuous spectrum. Quantitatively, the curve follows a steep decaying exponential distribution, indicating that there are greater numbers of rogue RYR groups than large RYR arrays. Theoretical analysis suggests that rogue RYRs are simply in transition into the stochastic assembly of large RYR arrays. These newly revealed morphological features of cardiac RYR organization provide a structural framework to unify the perplexing diversity of elemental calcium signals discussed above.
A unifying model
By synthesizing these recent advances in structural and functional studies of RYR arrays in cardiac myocytes, we propose a working model for cardiac elemental calcium release events with the following features (Fig. 1). Structurally, we should treat all RYRs on a terminal cistern or CRU, associated junctional cleft, and cisternal lumen as an integrated nano-assembly. Of these, a CRU contains a main cluster and several surrounding rogue RYRs, and the main cluster may be further divided into subdomains delimited by isthmic or defective connectivity between them. Mechanistically, CICR acts across all RYRs in a CRU, whereas the coupled gating of RYRs is confined to those residing in the same cluster. RYR desensitization by calcium depletion in the shared cisternal lumen or calcium-dependent inhibition in the junctional cleft provides yet another means for inter-RYR and intercluster communication in a CRU, manifesting as use-dependent refractoriness to CICR.
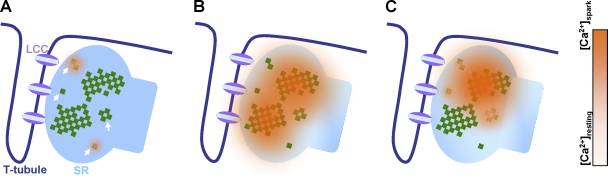
Figure 1.
Distinct modes of elemental calcium release. On a terminal cistern, a main RYR cluster consists of two subdomains with an isthmic connection and is surrounded by a few rogue RYR groups (indicated by arrows in A). (A) Quarky calcium release from rogue RYRs only. (B) Calcium spark with two quantal units. Either or both of the subdomains of the main cluster can be activated in a spark. (C) Calcium spark with quarky substructure. During sparks, the neighbor rogue RYRs are also activated in succession, resulting in prolonging calcium release. Cytosol calcium concentration gradients are roughly displayed by colors (top to bottom of the look-up table corresponds to the calcium concentration from resting to sparked). LCC, L-type calcium channel; SR, sarcoplasmic reticulum.
With this rather complex (but seemingly irreducible) model, we can explain the coexistence of distinct modes of elemental calcium release in a single CRU and predict interactions and interconvertibility of these elemental release modes. The activation of the rogue RYRs gives rise to invisible or quarky calcium release; activation of the main cluster with stochastic recruitment of subdomains produces a calcium spark with quantal units; and the main cluster activation triggers surrounding rogue RYRs in a stochastic manner, resulting in a calcium spark with trailing quarky substructures. That quarky calcium release does not always activate the main cluster is because of the negative cooperativity of rogue RYRs with the main RYR cluster, via lumenal calcium depletion-induced desensitization or a cytosolic calcium-dependent inhibitory mechanism.
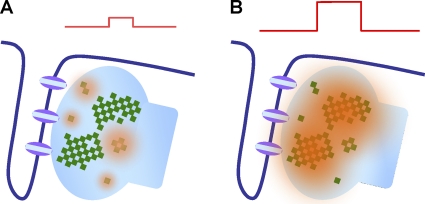
In addition to enabling the coupled gating mechanism, RYR array formation also confers strong use dependence (Wang et al., 2004) and insensitivity to CICR at a low-level calcium stimulus (Sobie et al., 2006). As such, RYR arrays of different size within the same CRU are functionally heterogeneous with respect to CICR and use-dependent refractoriness. This feature can also account for the stimulus intensity dependence of the mode of calcium release: a strong stimulus (iCa injected into the cleft) preferentially evokes a calcium spark (with trailing quarky substructures), whereas a weak stimulus (uncaging calcium, calcium influx via NCX, iCa at near reversal potentials, and calcium at the front of an aborting calcium wave) promotes quarky calcium release (Fig. 2).
Figure 2.
Stimulus intensity dependence of elemental calcium release mode. A weak stimulus preferentially activates rogue RYRs to produce quarky calcium release (A), whereas a strong stimulus activates the main cluster as well as the rogue RYRs (B).
If it can survive future validation, this model bears important ramifications with regard to physiological and pathophysiological modulation of calcium signaling. First, it predicts that there are many more quarky calcium release events than calcium sparks in resting cardiac myocytes (with the weak stimulus afforded by low-level cytosolic calcium); i.e., spontaneous calcium sparks are merely the tip of the iceberg of the SR leak. Second, a multitude of new parameters comes into play in determining the mode of elemental calcium release and hence cellular calcium signaling. These include parameters related to the formation, organization, and turnover of CRUs (size of the main cluster, defects in the main cluster, and proportion of rogue RYRs). For instance, a smaller main cluster and a greater number of rogue RYRs would generate a greater SR leak, even if the total RYRs in a CRU remain constant. A mismatch between L-type calcium currents and CRUs will also alter the strength of a stimulus sensed by RYRs and favor a spark-to-quarky switch of the elemental release mode. Furthermore, the model predicts that depletion of the SR tends to shift the release toward the quarky release mode, whereas SR overload tends to shift it in the opposite direction. Understanding the multiplicity and modulation of the modes of elemental calcium signals not only affords promising new directions to explore the still mysterious “invisible” SR leak in heart failure, but also sheds new light on how the seemingly plain calcium ion acts as a universal and versatile second messenger.
This Perspectives series includes articles by Gordon, Parker and Smith, Prosser et al., Santana and Navedo, and Hill-Eubanks et al.
Acknowledgments
The authors would like to thank Iain C. Bruce for manuscript editing.
This work was supported by the Major State Basic Research Development Program of China (2007CB512100) and the National Natural Science Foundation of China (30628009 and 30900264).
Footnotes
Abbreviations used in this paper:
- CICR
- calcium-induced calcium release
- CRU
- calcium release unit
References
- Baddeley D., Jayasinghe I.D., Lam L., Rossberger S., Cannell M.B., Soeller C. 2009. Optical single-channel resolution imaging of the ryanodine receptor distribution in rat cardiac myocytes. Proc. Natl. Acad. Sci. USA. 106:22275–22280 10.1073/pnas.0908971106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J., Lipp P., Bootman M.D. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1:11–21 10.1038/35036035 [DOI] [PubMed] [Google Scholar]
- Brochet D.X., Yang D., Di Maio A., Lederer W.J., Franzini-Armstrong C., Cheng H. 2005. Ca2+ blinks: rapid nanoscopic store calcium signaling. Proc. Natl. Acad. Sci. USA. 102:3099–3104 10.1073/pnas.0500059102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochet D.X.P., Yang D., Xie W., Cheng H., Lederer W.J. 2009. Quarky calcium sparks in heart. Biophys. J. 96:21a–22a 10.1016/j.bpj.2008.12.101418849415 [DOI] [Google Scholar]
- Cheng H., Lederer W.J. 2008. Calcium sparks. Physiol. Rev. 88:1491–1545 10.1152/physrev.00030.2007 [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W.J., Cannell M.B. 1993. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 262:740–744 10.1126/science.8235594 [DOI] [PubMed] [Google Scholar]
- Fill M., Copello J.A. 2002. Ryanodine receptor calcium release channels. Physiol. Rev. 82:893–922 [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Protasi F., Ramesh V. 1998. Comparative ultrastructure of Ca2+ release units in skeletal and cardiac muscle. Ann. NY Acad. Sci. 853:20–30 10.1111/j.1749-6632.1998.tb08253.x [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Protasi F., Ramesh V. 1999. Shape, size, and distribution of Ca(2+) release units and couplons in skeletal and cardiac muscles. Biophys. J. 77:1528–1539 10.1016/S0006-3495(99)77000-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez A.M., Valdivia H.H., Cheng H., Lederer M.R., Santana L.F., Cannell M.B., McCune S.A., Altschuld R.A., Lederer W.J. 1997. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 276:800–806 10.1126/science.276.5313.800 [DOI] [PubMed] [Google Scholar]
- Györke S., Fill M. 1993. Ryanodine receptor adaptation: control mechanism of Ca(2+)-induced Ca2+ release in heart. Science. 260:807–809 10.1126/science.8387229 [DOI] [PubMed] [Google Scholar]
- Györke I., Györke S. 1998. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys. J. 75:2801–2810 10.1016/S0006-3495(98)77723-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S., Terentyev D. 2008. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc. Res. 77:245–255 10.1093/cvr/cvm038 [DOI] [PubMed] [Google Scholar]
- Klein M.G., Cheng H., Santana L.F., Jiang Y.H., Lederer W.J., Schneider M.F. 1996. Two mechanisms of quantized calcium release in skeletal muscle. Nature. 379:455–458 10.1038/379455a0 [DOI] [PubMed] [Google Scholar]
- Li Y., Kranias E.G., Mignery G.A., Bers D.M. 2002. Protein kinase A phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ. Res. 90:309–316 10.1161/hh0302.105660 [DOI] [PubMed] [Google Scholar]
- Lipp P., Niggli E. 1996. Submicroscopic calcium signals as fundamental events of excitation—contraction coupling in guinea-pig cardiac myocytes. J. Physiol. 492:31–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., Niggli E. 1998. Fundamental calcium release events revealed by two-photon excitation photolysis of caged calcium in guinea-pig cardiac myocytes. J. Physiol. 508:801–809 10.1111/j.1469-7793.1998.801bp.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp P., Egger M., Niggli E. 2002. Spatial characteristics of sarcoplasmic reticulum Ca2+ release events triggered by L-type Ca2+ current and Na+ current in guinea-pig cardiac myocytes. J. Physiol. 542:383–393 10.1113/jphysiol.2001.013382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin S.E., Zhang D., Bridge J.H. 2000. Dyssynchronous Ca(2+) sparks in myocytes from infarcted hearts. Circ. Res. 87:1040–1047 [DOI] [PubMed] [Google Scholar]
- Marx S.O., Ondrias K., Marks A.R. 1998. Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors). Science. 281:818–821 10.1126/science.281.5378.818 [DOI] [PubMed] [Google Scholar]
- Marx S.O., Reiken S., Hisamatsu Y., Jayaraman T., Burkhoff D., Rosemblit N., Marks A.R. 2000. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 101:365–376 10.1016/S0092-8674(00)80847-8 [DOI] [PubMed] [Google Scholar]
- Marx S.O., Gaburjakova J., Gaburjakova M., Henrikson C., Ondrias K., Marks A.R. 2001. Coupled gating between cardiac calcium release channels (ryanodine receptors). Circ. Res. 88:1151–1158 10.1161/hh1101.091268 [DOI] [PubMed] [Google Scholar]
- Nelson M.T., Cheng H., Rubart M., Santana L.F., Bonev A.D., Knot H.J., Lederer W.J. 1995. Relaxation of arterial smooth muscle by calcium sparks. Science. 270:633–637 10.1126/science.270.5236.633 [DOI] [PubMed] [Google Scholar]
- Ono K., Yano M., Ohkusa T., Kohno M., Hisaoka T., Tanigawa T., Kobayashi S., Kohno M., Matsuzaki M. 2000. Altered interaction of FKBP12.6 with ryanodine receptor as a cause of abnormal Ca(2+) release in heart failure. Cardiovasc. Res. 48:323–331 10.1016/S0008-6363(00)00191-7 [DOI] [PubMed] [Google Scholar]
- Ouyang K., Zheng H., Qin X., Zhang C., Yang D., Wang X., Wu C., Zhou Z., Cheng H. 2005. Ca2+ sparks and secretion in dorsal root ganglion neurons. Proc. Natl. Acad. Sci. USA. 102:12259–12264 10.1073/pnas.0408494102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestle J., Janssen P.M., Janssen A.P., Zeitz O., Lehnart S.E., Bruce L., Smith G.L., Hasenfuss G. 2001. Overexpression of FK506-binding protein FKBP12.6 in cardiomyocytes reduces ryanodine receptor-mediated Ca(2+) leak from the sarcoplasmic reticulum and increases contractility. Circ. Res. 88:188–194 [DOI] [PubMed] [Google Scholar]
- Shannon T.R., Pogwizd S.M., Bers D.M. 2003. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ. Res. 93:592–594 10.1161/01.RES.0000093399.11734.B3 [DOI] [PubMed] [Google Scholar]
- Shen J.X., Wang S., Song L.S., Han T., Cheng H. 2004. Polymorphism of Ca2+ sparks evoked from in-focus Ca2+ release units in cardiac myocytes. Biophys. J. 86:182–190 10.1016/S0006-3495(04)74095-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipido K.R., Volders P.G., Vos M.A., Verdonck F. 2002. Altered Na/Ca exchange activity in cardiac hypertrophy and heart failure: a new target for therapy? Cardiovasc. Res. 53:782–805 10.1016/S0008-6363(01)00470-9 [DOI] [PubMed] [Google Scholar]
- Sobie E.A., Guatimosim S., Gómez-Viquez L., Song L.S., Hartmann H., Saleet Jafri M., Lederer W.J. 2006. The Ca2+ leak paradox and rogue ryanodine receptors: SR Ca2+ efflux theory and practice. Prog. Biophys. Mol. Biol. 90:172–185 10.1016/j.pbiomolbio.2005.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L.S., Sobie E.A., McCulle S., Lederer W.J., Balke C.W., Cheng H. 2006. Orphaned ryanodine receptors in the failing heart. Proc. Natl. Acad. Sci. USA. 103:4305–4310 10.1073/pnas.0509324103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugorka A., Ríos E., Blatter L.A. 1995. Imaging elementary events of calcium release in skeletal muscle cells. Science. 269:1723–1726 10.1126/science.7569901 [DOI] [PubMed] [Google Scholar]
- Wang S.Q., Stern M.D., Ríos E., Cheng H. 2004. The quantal nature of Ca2+ sparks and in situ operation of the ryanodine receptor array in cardiac cells. Proc. Natl. Acad. Sci. USA. 101:3979–3984 10.1073/pnas.0306157101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C.C., Lai F.A. 2000. Intrinsic lattice formation by the ryanodine receptor calcium-release channel. Nat. Cell Biol. 2:669–671 10.1038/35023625 [DOI] [PubMed] [Google Scholar]
- Yin C.C., Han H., Wei R., Lai F.A. 2005. Two-dimensional crystallization of the ryanodine receptor Ca2+ release channel on lipid membranes. J. Struct. Biol. 149:219–224 10.1016/j.jsb.2004.10.008 [DOI] [PubMed] [Google Scholar]