Abstract
Large-conductance voltage- and Ca2+-activated K+ (BKCa) channels play a fundamental role in cellular function by integrating information from their voltage and Ca2+ sensors to control membrane potential and Ca2+ homeostasis. The molecular mechanism of Ca2+-dependent regulation of BKCa channels is unknown, but likely relies on the operation of two cytosolic domains, regulator of K+ conductance (RCK)1 and RCK2. Using solution-based investigations, we demonstrate that the purified BKCa RCK1 domain adopts an α/β fold, binds Ca2+, and assembles into an octameric superstructure similar to prokaryotic RCK domains. Results from steady-state and time-resolved spectroscopy reveal Ca2+-induced conformational changes in physiologically relevant [Ca2+]. The neutralization of residues known to be involved in high-affinity Ca2+ sensing (D362 and D367) prevented Ca2+-induced structural transitions in RCK1 but did not abolish Ca2+ binding. We provide evidence that the RCK1 domain is a high-affinity Ca2+ sensor that transduces Ca2+ binding into structural rearrangements, likely representing elementary steps in the Ca2+-dependent activation of human BKCa channels.
INTRODUCTION
Ca2+ plays a central role as a ubiquitous signaling molecule in fundamental physiological processes and can directly or indirectly modulate the activity of a large number of proteins (Clapham, 1995; Carafoli and Klee, 1999), including several families of ion channels. In some cases, ion channels acquire Ca2+ sensitivity by associating with Ca2+-binding proteins, such as calmodulin, that in turn modulate channel properties (Pitt, 2007). In contrast, the large-conductance voltage- and Ca2+-activated K+ (BKCa or MaxiK) channels (Marty, 1981; Pallotta et al., 1981; Latorre et al., 1982), which regulate a multitude of physiological processes including smooth muscle tone, uresis, immunity, and neurotransmission (Tang et al., 2004b; Lu et al., 2006; Salkoff et al., 2006; Cui et al., 2009), appear to be directly modulated by intracellular Ca2+ that binds to specialized intracellular modules known as regulator of K+ conductance (RCK) domains (Pico, 2003; Niu et al., 2004; Lingle, 2007; Yusifov et al., 2008; Yuan et al., 2010; Wu et al., 2010).
It has been proposed that RCK domains operate as chemo-mechanical transducers that convert the free energy of Ca2+ binding (or other ligands) into mechanical work that ultimately opens the channel pore (Jiang et al., 2001, 2002; Niu et al., 2004; Chakrapani and Perozo, 2007). Significant insights into the structure and function of these domains were provided by crystallographic data of bacterial RCKs (Jiang et al., 2001, 2002). Sequence analysis suggests that two tandem regions of the large cytoplasmic domain of eukaryotic BKCa channels’ protein conformation, RCK1 and RCK2, are structurally homologous to bacterial RCK domains (Jiang et al., 2002; Pico, 2003; Roosild et al., 2004; Fodor and Aldrich, 2006, 2009; Kim et al., 2006, 2008; Latorre and Brauchi, 2006; Yusifov et al., 2008). Indeed, the large BKCa cytosolic C terminus, recently visualized at 17–20-Å resolution by cryo-electron microscopy (Wang and Sigworth, 2009), is thought to comprise a hetero-octameric assembly of RCK1 and RCK2 domains akin to the gating ring of prokaryotic channels. This view is supported by two recent studies that report on the atomic structure of the cytoplasmic domain of the BKCa channel (Wu et al., 2010; Yuan et al., 2010). Tandem RCK1 and RCK2 domains interact with each other within the same subunit; these four RCK1/RCK2 pairs assemble in a gating ring structure through “assembly” interfaces (Wu et al., 2010; Yuan et al., 2010).
RCK domains in BKCa channels are thought to be involved in high-affinity Ca2+ sensing (Niu et al., 2004; Latorre and Brauchi, 2006; Qian et al., 2006; Lingle, 2007; Yusifov et al., 2008). In the RCK2 domain, high-affinity Ca2+ sensing is endowed by a Ca2+-binding region termed the “Ca2+ bowl” consisting of five consecutive aspartates (894–898) (Schreiber and Salkoff, 1997; Schreiber et al., 1999), which are critical for Ca2+ binding (Bian et al., 2001; Braun and Sy, 2001; Bao et al., 2004; Sheng et al., 2005; Yuan et al., 2010) and Ca2+-induced conformational changes (Yusifov et al., 2008). In RCK1, mutations of aspartates 362/367 or methionine 513 reduce the high-affinity Ca2+ sensitivity of the channel (Bao et al., 2002; Xia et al., 2002; Zeng et al., 2005; Sweet and Cox, 2008). Indeed, the concomitant neutralization of D362/367 (RCK1) and the Ca2+ bowl (RCK2) to alanine residues completely abolishes the high-affinity Ca2+ dependence of BKCa channels (Xia et al., 2002; Zeng et al., 2005; Sweet and Cox, 2008). However, no Ca2+-binding sites within the RCK1 domain have yet been observed (Wu et al., 2010; Yuan et al., 2010). Thus, the molecular details of Ca2+ sensing in the RCK1 domain remain unknown.
To shed light on the molecular basis for Ca2+-dependent channel activation, we have expressed and purified the cytosolic part of the BKCa channel corresponding to the RCK1 region and interrogated its function as a Ca2+ sensor, as previously performed for the RCK2 domain (Yusifov et al., 2008). We postulate that a Ca2+ sensor involved in the Ca2+-dependent activation of the channel must satisfy the following criteria: bind Ca2+; undergo Ca2+-dependent conformational transitions (to exert mechanical force to open the pore); operate in a physiologically relevant range of [Ca2+]; and, finally, mutations of critical residues involved in Ca2+-dependent channel activation should perturb either Ca2+ binding, the transduction mechanism, or both. We have used a combination of optical and biochemical methods to test these criteria for the RCK1 domain of the human BKCa (Slo1) channel.
MATERIALS AND METHODS
Expression and purification of the human BKCa RCK1 and RCK2 domains
Expression and site-directed mutagenesis of the human Slo1 RCK1 domain with flanking N- and C-terminal regions (322IIE…HDP667) and the BKCa RCK2 domain (665HDP…ALK1005) were performed as described previously (Yusifov et al., 2008). The protein fractions were solubilized in 50 mM Tris-HCl and 8 M urea, pH 8.0. The supernatant was refolded by dialysis to 50 mM Tris and 2 mM EGTA, pH 7.5, and applied to a Ni-NTA affinity column. The protein fractions were eluted with 250 mM imidazole and dialyzed against 25 mM MOPS, 2 mM EGTA, and 120 mM KCl, pH 7.2. The purity of the expressed proteins was analyzed using a 12.5% SDS-PAGE. Protein concentrations were determined using the Biuret-Lowry assay.
Size-exclusion chromatography
The purified BKCa RCK1 or RCK2 domains were solubilized in 8 M urea. 500 µl of each sample was refolded onto a Superdex 200 10/300 column with a flow rate of 0.5 ml/min, equilibrated with a buffer containing 50 mM Tris and 5 mM EGTA, pH 8.4, or 20 mM MOPS and 2 mM EGTA, pH 7.2. The refolded protein fractions eluted between 10 and 11 ml were collected and reinjected into the sizing column and eluted in equilibrium buffer. The apparent mol wt of RCK1 and RCK2 was determined by constructing a calibration plot from the elution profiles of protein standards of known mol wt (MWGF1000; Sigma-Aldrich).
Multi-angle laser light scattering (MALLS)
MALLS experiments were performed with a DAWN-EOS MALLS detector coupled to an Optilab refractometer (Wyatt Technologies) and a UV-absorption spectrophotometer. The purified RCK1 domain (100 µl; 0.1 mg/ml) in 25 mM MOPS, 2 mM EGTA, and 120 mM KCl, pH 7.2, was loaded onto a QC-PAK GFC 300 column (Tosoh Bioscience). Light-scattering data were acquired from 14 detectors (detectors 1–4 were disabled, as they are unreliable for aqueous solutions) and fit by the following equation based on the Zimm formalism, using the Astra 5.3.4 program (Wyatt Technologies):
where K* is an optical parameter equal to 4π2n2(dn/dc)2/( NA), where n is the solvent refractive index, dn/dc is the refractive index increment (0.185), λ is the wavelength of the scattered light in vacuum (cm), and NA is Avogadro’s number. Parameter c is the sample concentration in g/ml, R(θ) is the excess intensity of scattered light at DAWN angle θ, MW is the weighted-average mol wt, and P(θ) describes the angular dependence of the scattered light. The proportion of eluted protein, determined by UV absorption at 280 nm, was binned according to mol wt, as determined by light scatter analysis.
Circular dichroism (CD) spectroscopy
CD spectra and free [Ca2+] were obtained as described previously (Yusifov et al., 2008). CD data are presented in units of molar ellipticity per residue. Computational analysis of the far-UV CD spectra of the purified proteins was done using the SELCON3 algorithm of the CDPro software package (Sreerama and Woody, 2004). Secondary structure composition was estimated using SMP56 (IBasis 10). Normalized root mean-square deviation is defined as
where θexp and θcal is the experimental and calculated molar ellipticity per amino acid residue, respectively. The number of the α-helix secondary structure segments (nα) was calculated by dividing the number of residues included in the distorted α-helix structure by a factor of four and in the distorted β structure (nβ) by a factor of two.
Time-resolved fluorescence
The time-resolved fluorescence decay of endogenous tryptophan was recorded with a spectrofluorometer (Fluorolog-3; HORIBA) using the time-correlated single-photon counting method at 22°C with a pulsed nanoLED at λex = 296 nm. Ludox was used as scatter solution. The fluorescence intensity decay data were fit with a sum of three exponential functions, using the DAS6 v6.4 software (HORIBA):
where I is the fluorescence intensity and αi and τi are the normalized preexponential factors and decay times, respectively. The average fluorescence lifetimes (τavg) for three exponential iterative fittings are calculated from the decay times and preexponential factors using equations
where fi shows the fractional contribution of each decay time to the steady-state intensity, which is given by
The goodness of the fit was determined from its χ2 value and the variance of the weighted residual distribution.
Steady-state fluorescence
Intrinsic tryptophan fluorescence spectra were recorded with protein concentrations of 0.1 mg/ml in 25 mM MOPS, 2 mM EGTA, and 120 mM KCl, pH 7.2. Fluorescence measurements were obtained using excitation/emission slit widths of 5 nm each, with λex = 295 nm. The tryptophan emission fluorescence spectra were collected in the wavelength range of 300–400 nm. Experiments were fit with a Hill function in the form:
45Ca2+-binding assay
Purified wild-type (WT) and D362/367A-RCK1 (30 µg each) were centrifuged at 13,000 rpm for 5 min and then blotted onto nitrocellulose membranes prestained with 0.1% Ponceau S and 5% acetic acid (Morçöl and Subramanian, 1999). 10 µg troponin and 10 µg glutathione S-transferase (GST) were used as positive and negative controls, respectively. After protein blotting, the membranes were stained again with Ponceau S and washed five times with distilled water to remove nonspecific staining and estimate the relative protein abundance. The membranes were then probed by 45Ca2+ overlay as reported previously (Levitsky et al., 1994). In brief, the membranes were placed in a wash solution containing 60 mM KCl, 10 mM imidazole, and 2 mM MgCl2, pH 7.2, for 30 min, followed by a 60-min wash in the same solution supplemented with 115 µM CaCl2, and different concentrations of EGTA to obtain the desired free [Ca2+]. Then, 6 µC/ml 45Ca2+ (PerkinElmer) was added to this solution, and the membrane was washed for an additional 10 min. The free [Ca2+] was estimated with WebMaxC and measured with a Ca2+-selective electrode (WPI) or by using a calibration curve constructed from the fluorescent Ca2+-sensitive Ca2+ green 5N dye (Takahashi et al., 1999). The membranes were then washed twice in distilled water, dried, and exposed to a phosphor storage screen for 1–3 d. Relative binding was obtained by dividing the pixel values of the 45Ca2+ signal of each protein by the pixel values of its Ponceau S staining using the ImageJ 1.43S program (http://rsb.info.nih.gov/ij/).
Cleavage of N-terminal 6xHis tag of the purified WT-RCK1 domain was performed using the TAGZyme system (QIAGEN). In brief, 50 µg RCK1 was incubated in the presence of 5 U/ml dipeptidyl aminopeptidase I (DAPase) enzyme mixed with 2 mM cysteamine-HCl (QIAGEN) for several time intervals at 37°C. Reaction efficiency of 6xHis tag cleavage was analyzed using InVision His tag in-gel stain (Invitrogen). The WT-RCK1 domain lacking the 6xHis tag was separated from undigested 6xHis-tagged RCK1 and DAPase using NI-NTA immobilized metal affinity chromatography (IMAC; QIAGEN).
RESULTS
Expression and purification of the C terminus region of the human BKCa channel corresponding to the RCK1 domain
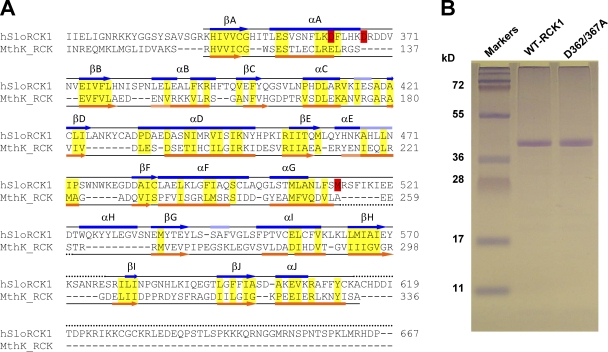
To directly investigate the structure and Ca2+-sensing properties of the human BKCa RCK1 domain in solution, we expressed and purified the region of the human BKCa channel C terminus corresponding to the amino acid sequence 322IIE…HDP667 (Wallner et al., 1995), which includes the S6-RCK1 linker (322IIE…GRK343), the RCK1 domain (344HIV…FYC612), and part of the RCK1-RCK2 interconnecting linker (613KAC…HDP667) (Yuan et al., 2010). In spite of the poor amino acid sequence similarity between the BKCa channel RCK1 domain and the MthK RCK domain (<25%), they share structural similarities (Fig. 1 A).
Figure 1.
Purification of the RCK1 domain of the human BKCa channel. (A) Structure-based sequence alignment of the BKCa channel (GI, 507922) C terminus (encompassing the RCK1 domain) with the MthK (GI, 2622639) RCK domain. The α helices are depicted as bars, and β strands are shown as arrows. The secondary structures (obtained from the DSSP reference set) above the sequences (blue) are obtained from the atomic structure of the BKCa C-terminal domain (Protein Data Bank accession no. 3MT5) (Yuan et al., 2010) and below the sequences (orange) are obtained from the atomic structure of the MthK RCK domain (Protein Data Bank accession no. 2AEF) (Dong et al., 2005). Light blue and light orange bars correspond to 310 helices. Dotted lines are unresolved regions in the respective crystal structures. Semi-conserved residues within ordered structures are highlighted yellow. Residues known to be involved in high-affinity Ca2+ sensitivity in the BKCa RCK1 domain (D362/D367 and M513) are highlighted red. (B) 12.5% SDS-PAGE of purified WT-RCK1 and D362/367A-RCK1 stained with Coomassie blue. Both proteins migrate as an ∼40-kD band, consistent with their expected mol wt (41 kD).
We also expressed and purified a RCK1 mutant carrying the D362A/367A mutations to probe the role of these residues, which are known to be critical for high-affinity Ca2+ sensitivity (Xia et al., 2002; Zeng et al., 2005; Sweet and Cox, 2008). The purity of the two proteins was assessed by SDS-PAGE. The WT and D362/367A-RCK1 domains both migrate as a 40-kD band in denaturating conditions, close to their theoretical mol wt of a monomeric RCK1 domain (41 kD; Fig. 1 B).
The secondary and quaternary structures of the RCK1 domain in solution share similarities with bacterial RCK domains
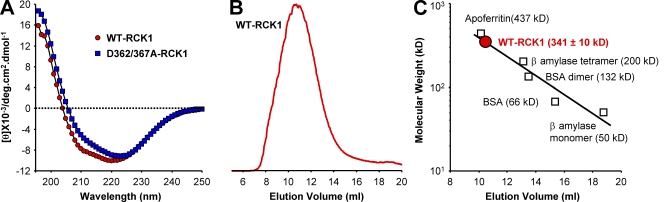
The secondary structure of the purified RCK1 domain was probed by CD spectroscopy, a powerful technique for the determination of secondary structure composition and ligand-induced conformational changes of proteins in solution (Kelly et al., 2005; Greenfield, 2006). The far-UV CD spectrum of WT-RCK1 in nominal Ca2+ (free [Ca2+] = 0.00058 µM) is presented in Fig. 2 A. The CD spectrum of WT-RCK1 shows an inflection at 210 nm and a minimum at 220 nm, characteristic of significant α-helical content (Sreerama and Woody, 2004; Turk et al., 2006). The analysis of the CD spectrum of WT-RCK1 in solution suggests a secondary structure composition of ∼28% α helix and ∼23% β strand, organized in ∼10 α helices and ∼15 β strands. RCK1’s folding pattern, analyzed using the cluster algorithm (Sreerama et al., 2001), revealed an α/β fold (in a consecutive β–α–β pattern; Table I), a common structural motif of the Rossmann fold (Rao and Rossmann, 1973), and an important structural feature of the RCK domains (Jiang et al., 2001, 2002; Wu et al., 2010; Yuan et al., 2010).
Figure 2.
Structural analysis of WT and D362/367A RCK1. (A) Far-UV CD spectra of WT-RCK1 and D362/367A-RCK1 domains obtained in nominal free [Ca2+] (0.00058 µM). The CD spectrum of WT-RCK1 exhibits a strong signal at 220 nm, whereas D362/367A displays different spectral properties with a red-shifted minimum at 223 nm (relative to WT-RCK1), suggesting that the double D362/367A mutation altered its secondary structure. (B) Characteristic size-exclusion column profile of the purified WT-RCK1 domain reveals an elution peak at ∼10.5 ml. (C) A calibration curve is established by plotting the log (mol wt) of proteins with known mol wt versus their elution peak (R2 = 0.91). The purified WT-RCK1 domain is calculated to elute with an apparent mol wt of 341 ± 10.0 kD (n = 7), suggesting a homo-octameric assembly of RCK1 domains (theoretical octameric mol wt = 328 kD).
Table I.
Estimated secondary structure composition of RCK domains
| CD spectroscopy | X-ray diffraction | |||||
| BKCa RCK1, 0 Ca2+ | BKCa RCK1, 35 µM Ca2+ | BKCa RCK1-D362/367A | BKCa RCK1 | MthK RCK | MthK RCK Ca2+ bound | |
| Amino acids | 322IIE…HDP667, 346 aa | 343KHI…IEY570, 577SRI…YCK613, 265 aa | 116RHV…ISA336, 221 aa | |||
| Reference | This study | Yuan et al., 2010 | Ye et al., 2006 | Jiang et al., 2002 | ||
| α helix (%) | 28.1 ± 0.751 | 19.3 ± 0.557 | 24.8 ± 0.0882 | 39.6 (30) | 40.9 | 38.2 |
| nα helix | 10.3 ± 0.415 | 7.81 ± 0.288 | 9.26 ± 0.0302 | 14 | 12 | 13 |
| β strand (%) | 23.2 ± 0.318 | 30.2 ± 0.731 | 25.4 ± 0.208 | 18.5 (14) | 22.3 | 22.3 |
| nβ strand | 15.4 ± 0.181 | 18.3 ± 0.582 | 14.6 ± 0.396 | 12 | 12 | 12 |
| Turn + unordered (%) | 48.9 ± 0.503 | 46.8 ± 0.689 | 48.7 ± 0.306 | 41.9 (56) | 36.8 | 39.5 |
| NRMSD | 0.0630 ± 0.00900 | 0.0827 ± 0.0123 | 0.115 ± 0.00762 | |||
| Fold | α/β | α/β | α/β | α/β | α/β | α/β |
Secondary structure composition of the BKCa RCK1 and MthK RCK domains. Deconvolution of far-UV CD spectra performed on the purified WT-RCK1 in the presence and absence of Ca2+ and D362/367A-RCK1 into percent secondary structural contributions. The WT-RCK1 domain in 0.00058 µM Ca2+ has higher α-helical content than D362/367A-RCK1. The addition of Ca2+ to WT-RCK1 changes the secondary structure fractions of the RCK1 domain, decreasing the α-helix content and increasing the β-strand content (see Fig. 6). Secondary structure fractions of the crystallized BKCa RCK1 domain (Protein Data Bank accession no. 3MT5 estimated from the resolved regions 343KHI…IEY570 and 577SRI…YCK613) and the MthK RCK domain in the presence (Protein Data Bank accession no. 1LNQ) or absence (Protein Data Bank accession no. 2FY8) of Ca2+ are provided for reference, obtained from the DSSP reference set. Note that the purified RCK1 domain in this study is composed of 346 amino acids (322IIE…HDP667, which amounts to an 81–amino acid difference between the RCK1 domain in this study and the BKCa RCK1 x-ray structure, and a 126–amino acid difference with respect to the two MthK RCK domains). This region includes the flanking S6-RCK1 linker and part of the RCK1-RCK2 linker, which were not included or resolved in the BKCa crystal structure. The structure fractions reported in parentheses were estimated by assuming the unresolved RCK1 flaking linker regions possessed no additional α or β structures. Errors represent standard errors of the mean (n = 3). NRMSD, normalized root mean-square deviation.
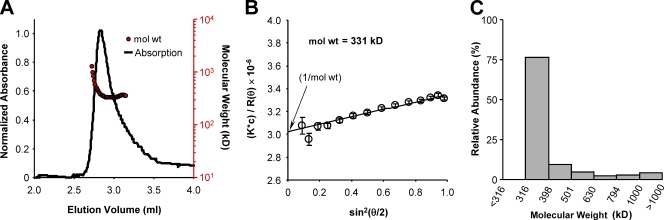
The oligomeric state of the purified RCK1 domain was investigated using size-exclusion chromatography. Fig. 2 B shows the elution profile of purified RCK1 on a Superdex 200 10/300 column. The elution peak (10.4 ± 0.08 ml; n = 7 elutions; errors represent standard errors of the mean) correlates to an apparent mol wt of 341 ± 10.0 kD, very close to the expected mol wt of a RCK1 octamer (theoretical RCK1 × 8 = 328 kD), according to a calibration curve constructed with protein standards of known mol wt (Fig. 2 C). These data suggest that the WT-RCK1 domain in solution preferentially self-assembles into octamers. A MALLS analysis (Wen et al., 1996; Folta-Stogniew and Williams, 1999; Philo, 2006) performed on the RCK1 domain in solution confirms its octameric organization. The elution profile of the WT-RCK1 domain is shown in Fig. 3 A. The corresponding mol wt distribution, estimated from light-scattering analysis, is shown superimposed (red circles). A representative Debye plot of light-scattering data with Zimm formalism, taken from the elution slice 2.893 ml, is shown in Fig. 3 B. The inverse of the y-axis intercept of the fit yields a molecular mass of ∼331 kD (Fig. 3 B). A histogram of the relative abundance of mol wts of the eluted protein between volumes 2.6 and 3.3 ml is shown in Fig. 3 C. The majority of eluted WT-RCK1 (76.3%) had mol wts between 316 and 398 kD (mean = 352 ± 24.2 kD). Thus, the purified RCK1 domain preferentially assembles in solution into homo-octamers, similar to bacterial RCK domains (Jiang et al., 2002; Parfenova et al., 2007).
Figure 3.
Mol wt determination using MALLS. (A) The normalized absorption at 280 nm (continuous line) of eluted WT-RCK1 is superimposed with its mol wt distribution (red circles) calculated from light-scattering data. (B) A Debye plot with Zimm formalism is shown using the light-scattering data from 14 detectors for the sample at elution volume 2.893 ml. Extrapolation of the fit to zero angle reveals a mean mol wt of eluted protein corresponding to 331 kD. (C) Relative protein abundance (calculated from UV absorbance) of samples eluted between volumes 2.6 and 3.3 ml binned according to mol wt, calculated from MALLS analysis. According to this cumulative distribution, 76.3% of eluted protein had mol wt between 316 and 398 kD, with a mean of 352 ± 24 kD.
The neutralization of D362 and D367 alters the structure of the RCK1 domain
Within the RCK1 domain, two aspartates (D362 and D367) are critical for the BKCa channel’s Ca2+ sensitivity in the µM range (Xia et al., 2002; Zeng et al., 2005; Sweet and Cox, 2008). To investigate the structural and functional roles of these residues on RCK1, we introduced mutations D362/367A and probed the secondary structure of the purified D362/367A-RCK1 in solution. Its CD spectrum (shown in Fig. 2 A) is altered as compared with the WT-RCK1 domain. Specifically, a red-shifted minimum from 220 to 223 nm suggests a change in the secondary structure. The analysis of D362/367A-RCK1 CD spectrum reveals that its β content increased from 23 ± 0.3% to 25 ± 0.2%, whereas the α-helix fraction was reduced from 28 ± 0.7% to 25 ± 0.1% compared with WT-RCK1 (Table I). An increase in the β content of proteins has been related to oligomerization (Rotondi and Gierasch, 2006; Zimmer et al., 2006). Interestingly, although WT-RCK1 preferentially self-assembles into an octameric state (Figs. 2, B and C, and 3), we found from size-exclusion chromatography that the D362/367A-RCK1 domain has an apparent mol wt higher than 670 kD (>16-mer). These results suggest that although D362/367A-RCK1 remains soluble and displays an ordered structure, its conformation and oligomeric state are altered.
Time-resolved fluorescence spectroscopy distinguishes between the conformational states of the apo- and Ca2+-bound states of the RCK1 domain
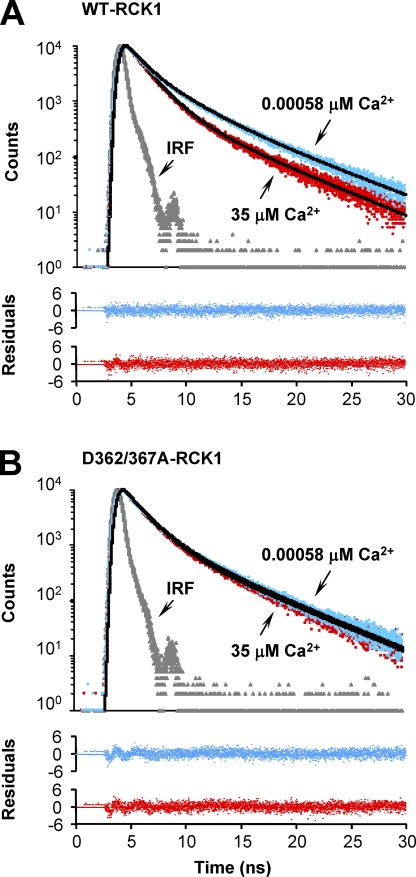
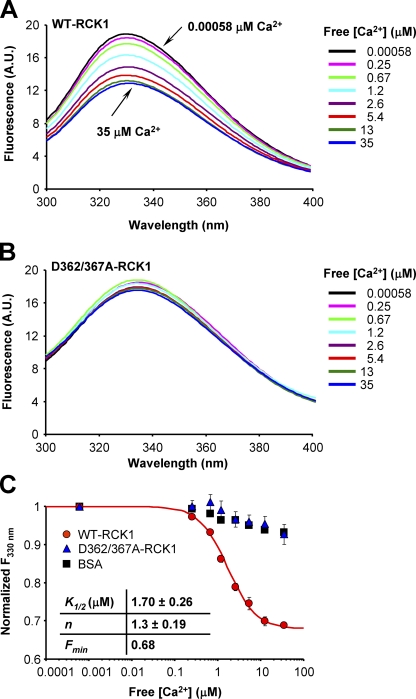
To investigate the Ca2+-dependent properties of the RCK1 domain, we took advantage of the three RCK1 endogenous tryptophan residues (W475/477/524) that can serve as fluorescent probes to resolve possible Ca2+-induced conformational changes. Tryptophan fluorescence lifetime is sensitive to its local environment; therefore, different conformational states of proteins have been reported (Lakowicz, 2006). We used time-correlated single-photon counting spectroscopy to record the excited-state fluorescence lifetime measurements of the endogenous tryptophan residues in the RCK1 domain. The fluorescence intensity decay of WT-RCK1 and D362/367A-RCK1 in the absence and presence of Ca2+ are shown in Fig. 4.
Figure 4.
Ca2+-induced conformational changes revealed by the intrinsic tryptophan fluorescence lifetime. Intrinsic tryptophan fluorescence intensity decay of (A) WT-RCK1 and (B) D362/367A-RCK1 recorded in the absence (free [Ca2+] = 0.00058 µM; blue) and presence (35 µM; red) of Ca2+. Black solid curves are the best fit to a triple-exponential decay (Table II). Residuals are shown below the decay curves. The intensity decay of a scattering solution (IRF) is shown in gray. The goodness of the fit was determined from its χ2 value and the variance of the weighted residual distribution. The triple-exponential analyses gave a χ2 value close to unity (χ2 of ∼0.86–0.95), whereas fitting the fluorescence decays to the sum of four exponential functions did not improve the accuracy of the fit.
The decay of the fluorescence intensity was well fit by the sum of three exponential functions. Mean with standard error fluorescence decay parameters for WT-RCK1 and D362/367A RCK1 in the presence and absence of Ca2+ are shown in Table II. The decay of WT-RCK1 fluorescence in the absence of Ca2+ (free [Ca2+] = 0.00058 µM) is dominated by the intermediate (τ = 2.0 ± 0.068 ns) and the long-lived components (τ3 = 5.4 ± 0.049 ns) that have fractional contributions of f2 = 49% and f3 = 37% of the total fluorescence intensity, respectively (Table II). The fraction of the shortest-lived component (τ1 = 0.62 ± 0.040 ns) is f1 = 14% (n = 3; ±SEM).
Table II.
Time-resolved fluorescence lifetime parameters
| RCK1 | WT | D362/367A | ||
| −Ca2+ | +Ca2+ | −Ca2+ | +Ca2+ | |
| τ1 (ns) | 0.62 ± 0.040 | 0.70 ± 0.018 | 0.68 ± 0.096 | 0.71 ± 0.025 |
| τ2 (ns) | 2.0 ± 0.068 | 1.6 ± 0.011 | 2.0 ± 0.14 | 1.9 ± 0.033 |
| τ3 (ns) | 5.4 ± 0.049 | 5.1 ± 0.030 | 5.4 ± 0.10 | 5.4 ± 0.023 |
| α1 | 0.42 ± 0.032 | 0.29 ± 0.020 | 0.46 ± 0.054 | 0.45 ± 0.017 |
| α2 | 0.45 ± 0.026 | 0.64 ± 0.018 | 0.44 ± 0.052 | 0.45 ± 0.016 |
| α3 | 0.12 ± 0.0052 | 0.068 ± 0.0020 | 0.10 ± 0.0021 | 0.10 ± 0.0012 |
| f1 | 0.14 ± 0.021 | 0.13 ± 0.0088 | 0.18 ± 0.040 | 0.18 ± 0.013 |
| f2 | 0.49 ± 0.011 | 0.65 ± 0.0062 | 0.50 ± 0.033 | 0.50 ± 0.010 |
| f3 | 0.37 ± 0.010 | 0.22 ± 0.0027 | 0.32 ± 0.0073 | 0.31 ± 0.0028 |
| τavg (ns) | 3.1 ± 0.010 | 2.3 ± 0.067 | 2.8 ± 0.049 | 2.8 ± 0.0037 |
| χ2 | 0.95 ± 0.0053 | 0.86 ± 0.0092 | 0.95 ± 0.018 | 0.95 ± 0.0069 |
Triple-exponential fitting parameters of fluorescence lifetime. Lifetime fluorescence was recorded at λem = 330 nm (λex = 296 nm). Fractional preexponential factors αi correspond to lifetimes τi, respectively. fi is fractional contribution of each decay time, and τavg · χ2 values indicate goodness fit. Errors represent standard errors of the mean (n = 3).
In contrast, the WT-RCK1 domain in the Ca2+-bound state (free [Ca2+] = 35 µM) has an intermediate component with a faster time constant of τ2 = 1.6 ± 0.011 ns and an increased fractional component to f2 = 65% when compared with WT-RCK1 in nominal [Ca2+]. Furthermore, the time course of the longest-lived component remained practically unchanged (τ3 = 5.1 ± 0.030 ns), but its fractional contribution decreased to f3 = 22%. The time course and fractional contribution of the shortest-lived component remained practically unchanged (τ1 = 0.7 ± 0.018 ns; f1 = 13%). Also, as shown in Fig. 4 A and Table II, the average excited-state lifetime time constant of the Ca2+-bound protein (free [Ca2+] = 35 µM; τavg = 2.3 ± 0.067 ns; n = 3) is markedly lower than the apo–WT-RCK1 (τavg = 3.1 ± 0.010 ns), suggesting a Ca2+-induced conformational change. On the contrary, the fluorescence lifetime parameters for D362/367A-RCK1 remain similar with the addition of Ca2+, indicating that D362 and D367 are necessary for Ca2+ binding, functional transduction of Ca2+ binding into conformational rearrangements of the RCK1 domain (Fig. 4 B), or both. We have further explored the effects of these mutations on the Ca2+-sensing properties of RCK1.
Steady-state tryptophan fluorescence reports Ca2+-induced conformational changes in the RCK1 domain
The results from the fluorescence lifetime experiments suggest that the conformational state of RCK1 domain in solution is [Ca2+] dependent (Fig. 4). As BKCa channels operate in micromolar [Ca2+], we sought to resolve the Ca2+ dependence of the observed conformational changes in the following experiments. We recorded changes in steady-state tryptophan fluorescence intensity with increasing [Ca2+] to determine RCK1’s apparent affinity for Ca2+. The fluorescence emission spectrum of WT-RCK1 (λex = 295 nm) displays a peak at 330 nm, which undergoes significant Ca2+-dependent quenching as the free [Ca2+] is raised from 0.00058 to 35 µM, suggesting the presence of Ca2+-induced conformational rearrangements (Fig. 5 A). On the other hand, the emission spectrum of D362/367A-RCK1 remains practically unchanged up to 35 µM of free [Ca2+] (Fig. 5 B) (similar to albumin, which was used as a negative control), confirming the results from fluorescence lifetime experiments. The apparent Ca2+ affinity of RCK1 was estimated by fitting the fluorescence intensity at 330 nm in increasing [Ca2+] to a Hill function. The mean of the parameters of the best fit are: K1/2 = 1.7 ± 0.26 µM with n = 1.3 ± 0.19 (n = 3; errors represent standard errors of the mean) (Fig. 5 C).
Figure 5.
Tryptophan fluorescence reports WT- and D362/367A-RCK1 Ca2+ sensitivity. The intrinsic steady-state tryptophan fluorescence emission spectra of (A) WT and (B) D362/367A RCK1 recorded in increasing free [Ca2+]. As free [Ca2+] increases, the fluorescence intensity of WT-RCK1 decreases (A), whereas the fluorescence emission of D362/367A (B) shows no significant sensitivity to [Ca2+]. (C) The normalized fluorescence intensity at λ330nm and free [Ca2+] = 0.00058 µM, plotted against free [Ca2+] for WT-RCK1 (red circles), D362/367A-RCK1 (blue triangles), and albumin (BSA) (black squares). The continuous curve is the least-squares fit to a Hill function, the parameters of which are shown in the panel. Errors represent standard errors of the mean.
The results from both time-resolved and steady-state fluorescence experiments are in agreement and provide evidence for the existence of apo- and Ca2+-bound states of the RCK1 domain, revealing Ca2+-induced structural rearrangements of the RCK1 domain occurring in physiologically relevant [Ca2+]. Moreover, evidence is provided that the residues D362 and D367 are required for Ca2+-induced conformational rearrangements.
Ca2+-induced conformational transitions of the BKCa RCK1 domain
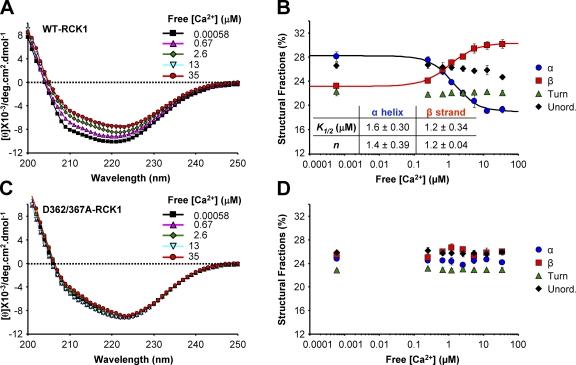
We used CD spectroscopy to characterize the structural changes in terms of the protein’s secondary structure. Far-UV CD spectra of the purified WT-RCK1 domain were recorded in increasing [Ca2+] (Fig. 6). As free [Ca2+] was increased from 0.00058 to 35 µM, the CD spectra of WT-RCK1 displayed a decrease in overall molar ellipticity associated to a red shift of the minimum from 220 to 223 nm (Fig. 6 A). The estimated secondary structure fractions of WT-RCK1 plotted as a function of free [Ca2+] are presented in Fig. 6 B. The elevation of free [Ca2+] resulted in an increase of β-strand content (from ∼23 to 30%), accompanied by a decrease in the α-helical fraction (from ∼28 to 19%), whereas the fraction of turns and unordered secondary structures remain practically unchanged (Fig. 6 B and Table I). The apparent Ca2+ affinity of the structural transitions was estimated by fitting the α-helical and β-strand fractions with a Hill function (K1/2_α-helix = 1.6 ± 0.30 µM; n = 1.4 ± 0.39; and K1/2_β-strand = 1.2 ± 0.34 µM; n = 1.2 ± 0.04). Intriguingly, the apparent affinity of the Ca2+-dependent structural transitions is in the same range of [Ca2+] relevant to physiological BKCa channel activation (Bao et al., 2002; Xia et al., 2002; Zeng et al., 2005; Latorre and Brauchi, 2006).
Figure 6.
CD spectroscopy reveals Ca2+-dependent conformational changes in the WT-RCK1 domain. (A) Far-UV CD spectra of the BKCa WT-RCK1 domain obtained in increasing free [Ca2+]. The spectra exhibit decreased overall amplitude with increasing [Ca2+], and a red shift in the ellipticity minimum from 220 to 223 nm, indicating Ca2+-dependent structural alterations. (B) Estimated secondary structure fractions of WT-RCK1 as a function of free [Ca2+]. The α-helix and β-sheet fraction data obtained at different [Ca2+] are fitted to a Hill function. (C and D) As in A and B, respectively, for purified D362/367A-RCK1. The double mutant exhibits little change in secondary structure up to 35 µM of free [Ca2+].
Unlike WT-RCK1, the CD spectrum of D362/367A-RCK1 was practically unaffected by free Ca2+ (Fig. 6, C and D) up to 35 µM. This is in agreement with fluorescence spectroscopy experiments and further supports the premise that residues D362 and D367 are necessary for Ca2+-induced rearrangements in the µM range.
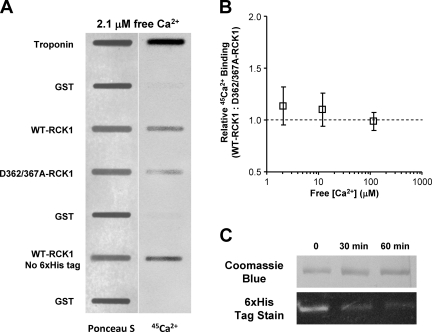
Both WT-RCK1 and D362/367A-RCK1 bind Ca2+
We have presented evidence that RCK1 undergoes Ca2+-induced structural changes and that these conformational rearrangements are no longer detected after the neutralization of residues D362 and D367. An important question is whether these negatively charged aspartates are involved in Ca2+ binding. We directly probed the Ca2+-binding properties of both WT-RCK1 and D362/367A-RCK1 (Fig. 7). Both proteins were blotted onto nitrocellulose membranes along with GST and troponin (for negative and positive controls, respectively) and analyzed for 45Ca2+ binding from 2.1 to 115 µM of free [Ca2+]. Fig. 7 A shows a nitrocellulose membrane stained with Ponceau S (for protein quantification) and its corresponding 45Ca2+ overlay, for 2.1 µM of free Ca2+. Both WT-RCK1 and D362/367A-RCK1 show 45Ca2+ binding at physiologically relevant [Ca2+], whereas GST displays practically no binding. The ratio of WT- to D362/367A-RCK1 45Ca2+ binding is plotted versus free [Ca2+] in Fig. 7 B. Although low-affinity Ca2+-sensing sites E374 and E399 are present in RCK1 (Shi et al., 2002; Zeng et al., 2005; Lingle, 2007; Yang et al., 2007, 2008; Cui et al., 2009), they are unlikely to be responsible for the strong positive 45Ca2+ signal, as they exhibit Ca2+ sensitivity in the mM range (Xia et al., 2002; Zeng et al., 2005).
Figure 7.
45Ca2+ binds to both WT and D362/367A-RCK1 domains. (A) Protein (Ponceau S) staining blotted on nitrocellulose membrane (left) and 45Ca2+ overlay phosphor image of the same blot (right) in 2.1 µM of free [Ca2+]. Signal intensity is proportional to protein amount (left) and Ca2+ binding (right). (B) The ratio of WT to D362/367A-RCK1 Ca2+ binding (normalized by protein amount) is plotted versus free [Ca2+]. (C) Enzymatic removal of the 6xHis from the WT-RCK1 domain. The purified 6xHis-WT-RCK1 is incubated with 5 U/ml DAPase, and then separated on a 12.5% SDS-PAGE and stained with either Coomassie Brilliant Blue (top strip) or InVision His tag in-gel stain (Invitrogen) (bottom strip). Troponin and GST are positive and negative controls, respectively. Note that the removal of the histidine tag did not reduce Ca2+ binding.
To exclude the involvement of the N-terminal 6xHis tag (used for purification of the WT-RCK1 domain) in 45Ca2+ binding, we have enzymatically removed it from the purified protein. Fig. 7 C shows the time-dependent digestion efficiency of the 6xHis tag in WT-RCK1 with DAPase. The 45Ca2+-binding activity of the 6xHis tag–cleaved WT-RCK1 domain is conserved, as presented in Fig. 7 A. We conclude that the 6xHis tag is not responsible for 45Ca2+ binding.
DISCUSSION
The human BKCa RCK1 domain shares structural homology with the bacterial RCK domain
Multiple primary sequence alignments across several species, as well as biochemical and electrophysiological experiments, have suggested that the cytosolic region of the BKCa channel is composed of two modules termed the RCK1 and RCK2 domains (Jiang et al., 2001, 2002; Pico, 2003; Kim et al., 2006; Latorre and Brauchi, 2006; Yusifov et al., 2008). The x-ray structures of the cytoplasmic domain of the BKCa channel have confirmed this view and revealed the structural organization of the RCK1 and RCK2 domains, which form a hetero-octameric superstructure referred to as the gating ring (Wu et al., 2010; Yuan et al., 2010). In addition to Ca2+, this intracellular region has been shown to confer sensitivity to other small signaling molecules, such as carbon monoxide (Hou et al., 2008b), Zn2+ (Hou et al., 2010), Mg2+(Shi et al., 2002; Yang et al., 2007, 2008), H+ (Hou et al., 2008a), heme (Tang et al., 2003; Horrigan et al., 2005), and reactive oxygen species (Tang et al., 2004a), which modulate channel activation.
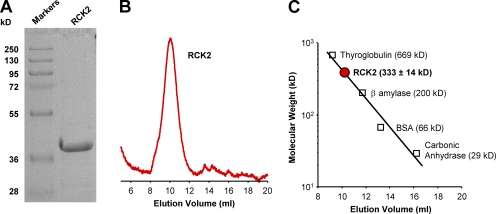
Here, we have probed the structure and investigated the functional properties of the purified BKCa RCK1 domain in solution under conditions physiologically relevant to the operation of BKCa channels. In addition to the α/β fold shared with its bacterial counterparts (Jiang et al., 2001, 2002; Yuan et al., 2010), the human RCK1 domains preferentially self-assemble into octameric structures (Figs. 2, B and C, and 3), as observed for RCK domains of MthK and TvoK channels (Jiang et al., 2002; Parfenova et al., 2007). Although RCK1 homo-octamers likely do not occur in functional BKCa channels, this property of RCK1 domains highlights structural and functional similarities among these ligand-binding domains, emphasizing their significance as modular components of gating ring superstructures. Indeed, the purified human BKCa RCK2 domain was also found to preferentially self-assemble into octamers (Fig. 8). On the other hand, in the intact BKCa channel, structurally homologous RCK1 and RCK2 domains can form a hetero-octameric gating ring complex (Wu et al., 2010; Yuan et al., 2010), which likely represents an evolutionary divergence from prokaryotic channel gating rings.
Figure 8.
The purified BKCa RCK2 domain forms a homo-octamer in solution. (A) 12.5% SDS-PAGE analysis of purified RCK2 reveals a single band with a mol wt of ∼40 kD, consistent with its expected size (39 kD). (B) Size-exclusion profile of the purified RCK2 domain. RCK2 elutes at 10.15 ml on a Superdex 200 10/300 column. (C) The oligomeric state of the purified RCK2 domain was determined using a calibration curve constructed from protein standards. The purified RCK2 domain corresponds to a mol wt of 333 ± 14 kD (n = 8), consistent with a homo-octameric structure of RCK2 (theoretical mol wt = 312 kD). R2 of fit = 0.97.
Secondary structure of RCK1 in solution
The RCK1 domain used in this study spans 346 amino acids, including RCK1 flanking regions (S6-RCK1 linker and a portion of the RCK1-RCK2 linker), which are likely unordered. The CD data of this polypeptide obtained under physiological conditions predicted a secondary structure composition of ∼28% α helix and ∼23% β strand (Table I). This region, with the exception of the flanking linkers, was recently resolved at 3-Å resolution (Yuan et al., 2010). The proportion of α-helical structure (considering the unordered RCK1 flanking sections) is in agreement with the CD data (∼30% α-helical structure) (Table I). However the β-strand content in the crystal structure (14%) is reduced compared with the solution structure. Several factors may account for this discrepancy, including differences in experimental conditions (e.g., buffer, pH, and ionic composition); limited resolving power of CD spectroscopy for β strand (Whitmore and Wallace, 2008); and structural changes resulting from the homo-octameric assembly of RCK1 domains.
Ca2+-induced conformational transitions in the RCK1 domain
Here, we have provided evidence that the purified RCK1 domain undergoes Ca2+-induced structural rearrangement. Our steady-state and time-resolved spectroscopic data (Figs. 4 and 5) reveal Ca2+-induced conformational transitions that correlate with an increase in overall β-sheet content (Fig. 6). Although Ca2+-induced structural preference to β sheet has been observed in other proteins (Hilge et al., 2006; Yousefi et al., 2007; Fan et al., 2008), the physiological meaning of this transition in the RCK domains is unknown. Based on the accepted notion that a gain in β-sheet content (regardless of the cause) may lead to oligomerization or molecular recognition events (Rotondi and Gierasch, 2006; Zimmer et al., 2006), we speculate that in the RCK1 domain, the α-to-β transition may favor or strengthen intra- and/or inter-subunit interactions and possibly the association with other channel partners (Lu et al., 2006). Intriguingly, BKCa RCK2 domains, where Ca2+ binding has been observed in a recent crystal structure (Yuan et al., 2010), also undergo a similar decrease in α/β ratio in solution upon [Ca2+] elevation (Yusifov et al., 2008). Thus, although crystallographic data have not revealed Ca2+ binding to BKCa RCK1, both BKCa RCK1 and RCK2 domains appear to possess similar Ca2+-sensing properties. It is possible that the RCK1 domain is more likely to bind and respond to Ca2+ under conditions resembling those of the cytosolic environment, as those used in this study.
Although Ca2+-induced changes in α/β ratio are exhibited by both BKCa RCK1 and RCK2 domains, such conformational transitions were not observed in the crystal structures of the MthK RCK domain (Jiang et al., 2002; Ye et al., 2006) (Table I), possibly indicating that gating rings in BKCa and MthK channels exhibit different Ca2+-dependent activation mechanisms. The large difference in their Ca2+ sensitivity, which is three orders of magnitude lower in the MthK channel (Li et al., 2007), may also support this view.
Do D362 and D367 participate in a Ca2+-biding site?
Electrophysiological evidence has highlighted the role of aspartates 362 and 367 in the high-affinity Ca2+-dependent activation of BKCa channels. Interestingly, residues D362 and D367 have also been shown to be important for the modulation of BKCa channels by other ligands (Hou et al., 2008a,b); however, the mechanisms by which they exert their function are unknown. The affinity of the putative RCK1 Ca2+-binding site was inferred by allosteric models of BKCa activation to be ∼1–20 µM (depending on the pore state and the membrane potential) (Bao et al., 2002; Xia et al., 2002; Zeng et al., 2005; Sweet and Cox, 2008; Cui et al., 2009), whereas neutralization of D362/367 reduced it into the millimolar range (Xia et al., 2002). Indeed, this view is recapitulated by the apparent Ca2+ sensitivity estimated from steady-state tryptophan fluorescence and CD spectroscopy of the purified WT-RCK1 (K1/2 ≈ 1.5 µM Ca2+) and the evidence that D362/367-RCK1 exhibited no response to Ca2+, as no conformational changes were detected up to 35 µM Ca2+ (Figs. 4–6). Although it is reasonable to consider their involvement in a Ca2+-binding site, the 45Ca2+ assay revealed that the neutralization of D362/367 alone was not sufficient to alter the Ca2+-binding activity of RCK1 in the range of 2.1–115 µM of free Ca2+ (Fig. 7). The minimal [Ca2+] assayed (2.1 µM) is near the experimental K1/2 for the Ca2+-induced conformational changes (Figs. 5 and 6); therefore, conceivably, a change in Ca2+ affinity would have been resolved. The data points in Fig. 7 B exhibit a trend that suggests that WT-RCK1 may have slightly higher Ca2+ affinity than the mutant; however, statistical significance was not achieved. Thus, WT and mutant RCK1 seem to bind Ca2+ with indistinguishable affinity. Collectively, the results favor the hypothesis that D362 and D367 have a major structural role in RCK1, determining their ability to transduce Ca2+ binding into conformational rearrangements. Nevertheless, it cannot be ruled out that D362 or D367 is also an element of a Ca2+-binding site with multiple coordinating residues, so that its neutralization does not substantially impair Ca2+ binding. Furthermore, it cannot be excluded that the RCK1 homo-oligomers in solution possess different Ca2+-dependent properties from RCK1 domains incorporated into the BKCa gating ring, allosterically linked with the pore domain and voltage-sensing apparatus (Horrigan and Aldrich, 2002; Sweet and Cox, 2008).
Structural consequences of D362/367 neutralization
The neutralization of residues D362 and D367 seems to alter the secondary and quaternary structure of the RCK1 domain. The differences in the secondary structure composition of D362/367A-RCK1 relative to the WT are based only on the comparison of the experimental CD data that suggest an increase in β-strand content associated to a decrease in α helix (Fig. 2 A and Table I). CD cannot provide molecular details or information about the region(s) of the protein undergoing this transition, or discriminate between a change occurring in the length of α helices and β strands or in their number, or both. Mutations inducing structural changes that perturb the α/β composition of proteins in solution have been described. For example Ulrih et al. (2008) report a drastic increase in α-helix content after a Tyr>Ala mutation in α synuclein. An Ala>Val mutation greatly increased β-sheet content in N-terminal troponin C (Pinto et al., 2009). In the N-terminal domain of a viral capsid protein, a Pro deletion or Asp>Ala mutation increased the α-helix content by 5% and decreased the β-sheet content by 3–4% (Macek et al., 2009). Increasing the hydrophobicity of residue side chains (as in the D362/367A mutation) can in some cases induce α helix to β-sheet conversion, as reported in a study modeling the energetics of these transitions in simple polypeptides (Imamura and Chen, 2007).
In summary, although a causal relation between the higher β content and loss of Ca2+-induced conformational change is not yet established, the altered structural state of the double mutant retains the ability to bind Ca2+ with high affinity but fails to transduce the free energy of ligand association into structural rearrangements. We speculate that in the intact BKCa channel, these mutations lock RCK1 domains in a conformation state unresponsive to ligand binding, thus hampering the propagation of conformational changes of ligand binding to the gating apparatus.
Both RCK1 and RCK2 are Ca2+ sensors with similar biophysical properties
Investigations of the Ca2+-dependent conformational changes of the purified BKCa RCK1 and RCK2 domains revealed that these two Ca2+ sensors exhibit a different Ca2+ affinity: RCK1 has less apparent affinity for Ca2+ (K1/2 ≈ 1.5 µM) relative to the Ca2+ bowl in RCK2 (K1/2 ≈ 0.4 µM) (Yusifov et al., 2008). In spite of this difference, the RCK1 and RCK2 domains share structural similarity (Wu et al., 2010; Yuan et al., 2010). Here, we report that the nature of Ca2+-induced conformational changes of the RCK1 domain are similar to those described for the purified RCK2 domain (Yusifov et al., 2008). Furthermore, the purified RCK2 domain (Fig. 8 A) also preferentially assembles into homomeric octamers in solution (Fig. 8, B and C), similar to RCK1 (Figs. 2, B and C, and 3). Thus, the RCK1 and RCK2 domains share functional and structural similarities.
In summary, we have presented evidence that Ca2+-induced conformational rearrangements take place within the RCK1 region of the BKCa channel at physiologically relevant [Ca2+]. The Ca2+-dependent properties of this structure seem to correlate well with Ca2+-induced BKCa channel activation. The agreement between the Ca2+ dependence of conformational changes (apparent K1/2 values obtained from CD and tryptophan fluorescence spectroscopy of ∼1.5 µM) with the values obtained from electrophysiological experiments (Bao et al., 2002; Xia et al., 2002; Sweet and Cox, 2008) supports this view. We have demonstrated that the D362/367A mutation, previously shown to impair high-affinity Ca2+ sensing (Xia et al., 2002), causes a structural change in the RCK1 domain, preventing Ca2+-dependent conformational rearrangements.
Acknowledgments
We thank Debora A. Nicoll for her expert advice and assistance; Ligia Toro for the Slo1 clone; Miyeon Kim for MALLS assistance; and Michela Ottolia and the members of the Olcese laboratory for constructive discussions.
This work was supported by National Institutes of Health/National Institute of General Medical Sciences research grant R01GM082289 and the Laubisch Foundation to R. Olcese, and an American Heart Association (Western States Affiliate) Postdoctoral Fellowship to A. Pantazis.
Christopher Miller served as editor.
Footnotes
Abbreviations used in this paper:
- BKCa
- large-conductance voltage- and Ca2+-activated K+
- CD
- circular dichroism
- GST
- glutathione S-transferase
- MALLS
- multi-angle laser light scattering
- RCK
- regulator of K+ conductance
- WT
- wild type
References
- Bao L., Rapin A.M., Holmstrand E.C., Cox D.H. 2002. Elimination of the BKCa channel’s high-affinity Ca2+ sensitivity. J. Gen. Physiol. 120:173–189 10.1085/jgp.20028627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L., Kaldany C., Holmstrand E.C., Cox D.H. 2004. Mapping the BKCa channel’s “Ca2+ bowl”: side-chains essential for Ca2+ sensing. J. Gen. Physiol. 123:475–489 10.1085/jgp.200409052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian S., Favre I., Moczydlowski E. 2001. Ca2+-binding activity of a COOH-terminal fragment of the Drosophila BK channel involved in Ca2+-dependent activation. Proc. Natl. Acad. Sci. USA. 98:4776–4781 10.1073/pnas.081072398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A.P., Sy L. 2001. Contribution of potential EF hand motifs to the calcium-dependent gating of a mouse brain large conductance, calcium-sensitive K(+) channel. J. Physiol. 533:681–695 10.1111/j.1469-7793.2001.00681.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E., Klee C.B. 1999. Calcium As a Cellular Regulator. Oxford University Press, New York: 656 pp [Google Scholar]
- Chakrapani S., Perozo E. 2007. How to gate an ion channel: lessons from MthK. Nat. Struct. Mol. Biol. 14:180–182 10.1038/nsmb0307-180 [DOI] [PubMed] [Google Scholar]
- Clapham D.E. 1995. Calcium signaling. Cell. 80:259–268 10.1016/0092-8674(95)90408-5 [DOI] [PubMed] [Google Scholar]
- Cui J., Yang H., Lee U.S. 2009. Molecular mechanisms of BK channel activation. Cell. Mol. Life Sci. 66:852–875 10.1007/s00018-008-8609-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J., Shi N., Berke I., Chen L., Jiang Y. 2005. Structures of the MthK RCK domain and the effect of Ca2+ on gating ring stability. J. Biol. Chem. 280:41716–41724 10.1074/jbc.M508144200 [DOI] [PubMed] [Google Scholar]
- Fan D., Lakshminarayanan R., Moradian-Oldak J. 2008. The 32kDa enamelin undergoes conformational transitions upon calcium binding. J. Struct. Biol. 163:109–115 10.1016/j.jsb.2008.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor A.A., Aldrich R.W. 2006. Statistical limits to the identification of ion channel domains by sequence similarity. J. Gen. Physiol. 127:755–766 10.1085/jgp.200509419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor A.A., Aldrich R.W. 2009. Convergent evolution of alternative splices at domain boundaries of the BK channel. Annu. Rev. Physiol. 71:19–36 10.1146/annurev.physiol.010908.163124 [DOI] [PubMed] [Google Scholar]
- Folta-Stogniew E., Williams K.R. 1999. Determination of molecular masses of proteins in solution: implementation of an HPLC size exclusion chromatography and laser light scattering service in a core laboratory. J. Biomol. Tech. 10:51–63 [PMC free article] [PubMed] [Google Scholar]
- Greenfield N.J. 2006. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 1:2876–2890 10.1038/nprot.2006.202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilge M., Aelen J., Vuister G.W. 2006. Ca2+ regulation in the Na+/Ca2+ exchanger involves two markedly different Ca2+ sensors. Mol. Cell. 22:15–25 10.1016/j.molcel.2006.03.008 [DOI] [PubMed] [Google Scholar]
- Horrigan F.T., Aldrich R.W. 2002. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J. Gen. Physiol. 120:267–305 10.1085/jgp.20028605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan F.T., Heinemann S.H., Hoshi T. 2005. Heme regulates allosteric activation of the Slo1 BK channel. J. Gen. Physiol. 126:7–21 10.1085/jgp.200509262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S., Xu R., Heinemann S.H., Hoshi T. 2008a. Reciprocal regulation of the Ca2+ and H+ sensitivity in the SLO1 BK channel conferred by the RCK1 domain. Nat. Struct. Mol. Biol. 15:403–410 10.1038/nsmb.1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S., Xu R., Heinemann S.H., Hoshi T. 2008b. The RCK1 high-affinity Ca2+ sensor confers carbon monoxide sensitivity to Slo1 BK channels. Proc. Natl. Acad. Sci. USA. 105:4039–4043 10.1073/pnas.0800304105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S., Vigeland L.E., Zhang G., Xu R., Li M., Heinemann S.H., Hoshi T. 2010. Zn2+ activates large conductance Ca2+-activated K+ channel via an intracellular domain. J. Biol. Chem. 285:6434–6442 10.1074/jbc.M109.069211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura H., Chen J.Z. 2007. Minimum model for the alpha-helix-beta-hairpin transition in proteins. Proteins. 67:459–468 10.1002/prot.21216 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Pico A., Cadene M., Chait B.T., MacKinnon R. 2001. Structure of the RCK domain from the E. coli K+ channel and demonstration of its presence in the human BK channel. Neuron. 29:593–601 10.1016/S0896-6273(01)00236-7 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Lee A., Chen J., Cadene M., Chait B.T., MacKinnon R. 2002. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 417:515–522 10.1038/417515a [DOI] [PubMed] [Google Scholar]
- Kelly S.M., Jess T.J., Price N.C. 2005. How to study proteins by circular dichroism. Biochim. Biophys. Acta. 1751:119–139 [DOI] [PubMed] [Google Scholar]
- Kim H.J., Lim H.H., Rho S.H., Eom S.H., Park C.S. 2006. Hydrophobic interface between two regulators of K+ conductance domains critical for calcium-dependent activation of large conductance Ca2+-activated K+ channels. J. Biol. Chem. 281:38573–38581 10.1074/jbc.M604769200 [DOI] [PubMed] [Google Scholar]
- Kim H.J., Lim H.H., Rho S.H., Bao L., Lee J.H., Cox D.H., Kim H., Park C.S. 2008. Modulation of the conductance-voltage relationship of the BK Ca channel by mutations at the putative flexible interface between two RCK domains. Biophys. J. 94:446–456 10.1529/biophysj.107.108738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowicz J.R. 2006. Principles of Fluorescence Spectroscopy. 3rd ed Springer, New York: 954 pp [Google Scholar]
- Latorre R., Brauchi S. 2006. Large conductance Ca2+-activated K+ (BK) channel: activation by Ca2+ and voltage. Biol. Res. 39:385–401 10.4067/S0716-97602006000300003 [DOI] [PubMed] [Google Scholar]
- Latorre R., Vergara C., Hidalgo C. 1982. Reconstitution in planar lipid bilayers of a Ca2+-dependent K+ channel from transverse tubule membranes isolated from rabbit skeletal muscle. Proc. Natl. Acad. Sci. USA. 79:805–809 10.1073/pnas.79.3.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitsky D.O., Nicoll D.A., Philipson K.D. 1994. Identification of the high affinity Ca(2+)-binding domain of the cardiac Na(+)-Ca2+ exchanger. J. Biol. Chem. 269:22847–22852 [PubMed] [Google Scholar]
- Li Y., Berke I., Chen L., Jiang Y. 2007. Gating and inward rectifying properties of the MthK K+ channel with and without the gating ring. J. Gen. Physiol. 129:109–120 10.1085/jgp.200609655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingle C.J. 2007. Gating rings formed by RCK domains: keys to gate opening. J. Gen. Physiol. 129:101–107 10.1085/jgp.200709739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Alioua A., Kumar Y., Eghbali M., Stefani E., Toro L. 2006. MaxiK channel partners: physiological impact. J. Physiol. 570:65–72 10.1113/jphysiol.2005.098913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macek P., Chmelík J., Krízová I., Kaderávek P., Padrta P., Zídek L., Wildová M., Hadravová R., Chaloupková R., Pichová I., et al. 2009. NMR structure of the N-terminal domain of capsid protein from the mason-pfizer monkey virus. J. Mol. Biol. 392:100–114 10.1016/j.jmb.2009.06.029 [DOI] [PubMed] [Google Scholar]
- Marty A. 1981. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 291:497–500 10.1038/291497a0 [DOI] [PubMed] [Google Scholar]
- Morçöl T., Subramanian A. 1999. A red-dot-blot protein assay technique in the low nanogram range. Anal. Biochem. 270:75–82 10.1006/abio.1999.4057 [DOI] [PubMed] [Google Scholar]
- Niu X., Qian X., Magleby K.L. 2004. Linker-gating ring complex as passive spring and Ca(2+)-dependent machine for a voltage- and Ca(2+)-activated potassium channel. Neuron. 42:745–756 10.1016/j.neuron.2004.05.001 [DOI] [PubMed] [Google Scholar]
- Pallotta B.S., Magleby K.L., Barrett J.N. 1981. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 293:471–474 10.1038/293471a0 [DOI] [PubMed] [Google Scholar]
- Parfenova L.V., Abarca-Heidemann K., Crane B.M., Rothberg B.S. 2007. Molecular architecture and divalent cation activation of TvoK, a prokaryotic potassium channel. J. Biol. Chem. 282:24302–24309 10.1074/jbc.M703650200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philo J.S. 2006. Is any measurement method optimal for all aggregate sizes and types? AAPS J. 8:E564–E571 10.1208/aapsj080365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pico A. 2003. RCK domain model of calcium activation in BK channels. PhD thesis. The Rockefeller University, New York: 106 pp [Google Scholar]
- Pinto J.R., Parvatiyar M.S., Jones M.A., Liang J., Ackerman M.J., Potter J.D. 2009. A functional and structural study of troponin C mutations related to hypertrophic cardiomyopathy. J. Biol. Chem. 284:19090–19100 10.1074/jbc.M109.007021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt G.S. 2007. Calmodulin and CaMKII as molecular switches for cardiac ion channels. Cardiovasc. Res. 73:641–647 10.1016/j.cardiores.2006.10.019 [DOI] [PubMed] [Google Scholar]
- Qian X., Niu X.W., Magleby K.L. 2006. Intra- and intersubunit cooperativity in activation of BK channels by Ca2+. J. Gen. Physiol. 128:389–404 10.1085/jgp.200609486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.T., Rossmann M.G. 1973. Comparison of super-secondary structures in proteins. J. Mol. Biol. 76:241–256 10.1016/0022-2836(73)90388-4 [DOI] [PubMed] [Google Scholar]
- Roosild T.P., Lê K.T., Choe S. 2004. Cytoplasmic gatekeepers of K+-channel flux: a structural perspective. Trends Biochem. Sci. 29:39–45 10.1016/j.tibs.2003.11.008 [DOI] [PubMed] [Google Scholar]
- Rotondi K.S., Gierasch L.M. 2006. Natural polypeptide scaffolds: beta-sheets, beta-turns, and beta-hairpins. Biopolymers. 84:13–22 10.1002/bip.20390 [DOI] [PubMed] [Google Scholar]
- Salkoff L., Butler A., Ferreira G., Santi C., Wei A. 2006. High-conductance potassium channels of the SLO family. Nat. Rev. Neurosci. 7:921–931 10.1038/nrn1992 [DOI] [PubMed] [Google Scholar]
- Schreiber M., Salkoff L. 1997. A novel calcium-sensing domain in the BK channel. Biophys. J. 73:1355–1363 10.1016/S0006-3495(97)78168-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber M., Yuan A., Salkoff L. 1999. Transplantable sites confer calcium sensitivity to BK channels. Nat. Neurosci. 2:416–421 10.1038/8077 [DOI] [PubMed] [Google Scholar]
- Sheng J.Z., Weljie A., Sy L., Ling S.Z., Vogel H.J., Braun A.P. 2005. Homology modeling identifies C-terminal residues that contribute to the Ca2+ sensitivity of a BKCa channel. Biophys. J. 89:3079–3092 10.1529/biophysj.105.063610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Krishnamoorthy G., Yang Y., Hu L., Chaturvedi N., Harilal D., Qin J., Cui J. 2002. Mechanism of magnesium activation of calcium-activated potassium channels. Nature. 418:876–880 10.1038/nature00941 [DOI] [PubMed] [Google Scholar]
- Sreerama N., Woody R.W. 2004. On the analysis of membrane protein circular dichroism spectra. Protein Sci. 13:100–112 10.1110/ps.03258404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreerama N., Venyaminov S.Y., Woody R.W. 2001. Analysis of protein circular dichroism spectra based on the tertiary structure classification. Anal. Biochem. 299:271–274 10.1006/abio.2001.5420 [DOI] [PubMed] [Google Scholar]
- Sweet T.B., Cox D.H. 2008. Measurements of the BKCa channel’s high-affinity Ca2+ binding constants: effects of membrane voltage. J. Gen. Physiol. 132:491–505 10.1085/jgp.200810094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A., Camacho P., Lechleiter J.D., Herman B. 1999. Measurement of intracellular calcium. Physiol. Rev. 79:1089–1125 [DOI] [PubMed] [Google Scholar]
- Tang X.D., Xu R., Reynolds M.F., Garcia M.L., Heinemann S.H., Hoshi T. 2003. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature. 425:531–535 10.1038/nature02003 [DOI] [PubMed] [Google Scholar]
- Tang X.D., Garcia M.L., Heinemann S.H., Hoshi T. 2004a. Reactive oxygen species impair Slo1 BK channel function by altering cysteine-mediated calcium sensing. Nat. Struct. Mol. Biol. 11:171–178 10.1038/nsmb725 [DOI] [PubMed] [Google Scholar]
- Tang X.D., Santarelli L.C., Heinemann S.H., Hoshi T. 2004b. Metabolic regulation of potassium channels. Annu. Rev. Physiol. 66:131–159 10.1146/annurev.physiol.66.041002.142720 [DOI] [PubMed] [Google Scholar]
- Turk E., Gasymov O.K., Lanza S., Horwitz J., Wright E.M. 2006. A reinvestigation of the secondary structure of functionally active vSGLT, the vibrio sodium/galactose cotransporter. Biochemistry. 45:1470–1479 10.1021/bi052160z [DOI] [PubMed] [Google Scholar]
- Ulrih N.P., Barry C.H., Fink A.L. 2008. Impact of Tyr to Ala mutations on alpha-synuclein fibrillation and structural properties. Biochim. Biophys. Acta. 1782:581–585 [DOI] [PubMed] [Google Scholar]
- Wallner M., Meera P., Ottolia M., Kaczorowski G.J., Latorre R., Garcia M.L., Stefani E., Toro L. 1995. Characterization of and modulation by a beta-subunit of a human maxi KCa channel cloned from myometrium. Receptors Channels. 3:185–199 [PubMed] [Google Scholar]
- Wang L., Sigworth F.J. 2009. Structure of the BK potassium channel in a lipid membrane from electron cryomicroscopy. Nature. 461:292–295 10.1038/nature08291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J., Arakawa T., Philo J.S. 1996. Size-exclusion chromatography with on-line light-scattering, absorbance, and refractive index detectors for studying proteins and their interactions. Anal. Biochem. 240:155–166 10.1006/abio.1996.0345 [DOI] [PubMed] [Google Scholar]
- Whitmore L., Wallace B.A. 2008. Protein secondary structure analyses from circular dichroism spectroscopy: methods and reference databases. Biopolymers. 89:392–400 10.1002/bip.20853 [DOI] [PubMed] [Google Scholar]
- Wu Y., Yang Y., Ye S., Jiang Y. 2010. Structure of the gating ring from the human large-conductance Ca(2+)-gated K(+) channel. Nature. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X.M., Zeng X., Lingle C.J. 2002. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 418:880–884 10.1038/nature00956 [DOI] [PubMed] [Google Scholar]
- Yang H., Hu L., Shi J., Delaloye K., Horrigan F.T., Cui J. 2007. Mg2+ mediates interaction between the voltage sensor and cytosolic domain to activate BK channels. Proc. Natl. Acad. Sci. USA. 104:18270–18275 10.1073/pnas.0705873104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Shi J., Zhang G., Yang J., Delaloye K., Cui J. 2008. Activation of Slo1 BK channels by Mg2+ coordinated between the voltage sensor and RCK1 domains. Nat. Struct. Mol. Biol. 15:1152–1159 10.1038/nsmb.1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S., Li Y., Chen L.P., Jiang Y.X. 2006. Crystal structures of a ligand-free MthK gating ring: insights into the ligand gating mechanism of K+ channels. Cell. 126:1161–1173 10.1016/j.cell.2006.08.029 [DOI] [PubMed] [Google Scholar]
- Yousefi R., Imani M., Ardestani S.K., Saboury A.A., Gheibi N., Ranjbar B. 2007. Human calprotectin: effect of calcium and zinc on its secondary and tertiary structures, and role of pH in its thermal stability. Acta Biochim. Biophys. Sin. (Shanghai). 39:795–802 10.1111/j.1745-7270.2007.00343.x [DOI] [PubMed] [Google Scholar]
- Yuan P., Leonetti M.D., Pico A.R., Hsiung Y., MacKinnon R. 2010. Structure of the human BK channel Ca2+-activation apparatus at 3.0 A resolution. Science. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusifov T., Savalli N., Gandhi C.S., Ottolia M., Olcese R. 2008. The RCK2 domain of the human BKCa channel is a calcium sensor. Proc. Natl. Acad. Sci. USA. 105:376–381 10.1073/pnas.0705261105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X.H., Xia X.M., Lingle C.J. 2005. Divalent cation sensitivity of BK channel activation supports the existence of three distinct binding sites. J. Gen. Physiol. 125:273–286 10.1085/jgp.200409239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer J., Li W.K., Rapoport T.A. 2006. A novel dimer interface and conformational changes revealed by an X-ray structure of B. subtilis SecA. J. Mol. Biol. 364:259–265 10.1016/j.jmb.2006.08.044 [DOI] [PubMed] [Google Scholar]