Abstract
The leading cause of nosocomial enteric infections in the US is a potentially lethal condition that influences the daily care of medical and surgical patients across all specialties. The incidence is increasing because of the emergence of a new virulent strain, the development of antibiotic resistance, and an increase in infection rates within populations once believed to be at low risk. Current strategies for the prevention, diagnosis, and treatment are cited. Transmission can be minimized with the use of gloves and gowns; proper hand washing with soap and water (alcohol-based washes do not prevent transmission); careful use and proper cleaning of shared patient equipment, such as blood-pressure cuffs, thermometers, and stethoscopes; and the use of bactericidal cleaning solutions. Restricted or judicious antibiotic use will also reduce the incidence of Clostridium difficile infections.
Introduction
As the leading cause of nosocomial enteric infections (diarrhea) in the US, Clostridium difficile infection is a potentially lethal condition that influences the daily care of medical and surgical patients across all specialties and across settings involved in the continuum of health care. Several recent outbreaks since the late 1990s have refocused attention on C difficile, highlighting that the incidence of infection is increasing because of the emergence of a new virulent strain, the development of antibiotic resistance, and an increase in infection rates within populations once believed to be at low risk.1 In parallel, morbidity and mortality rates associated with C difficile infection have risen,1,2 with an increasing number of complications, colectomies, and deaths being reported.3–5 This growing epidemic has heightened our awareness of this infectious organism and its impact on patient care and routine medical practices. In light of these changes, this review article emphasizes the shifting nature of C difficile infections and outlines current strategies for the prevention, diagnosis, and treatment of C difficile infection.
Background and Pathophysiology
C difficile was first described in 1935 by researchers who were studying how bacterial flora are established in the gastrointestinal (GI) tract.6 Hall and O'Toole found an anaerobic Gram-positive rod that was present but, interestingly, harmless in 30% to 50% of newborns.6,7 Although clindamycin-associated colitis was described in 1974, it was not until 1978 that the toxin of C difficile was found in patients with pseudomembranous colitis.8 Now, approximately 300,000 cases of C difficile colitis occur annually in the US, and the associated health care costs are high.9
C difficile colonizes the GI tract after the normal gut flora are altered, typically after antibiotic use. C difficile can release two exotoxins (toxins A and B), both of which can cause tissue damage. Binding to intestinal epithelial cells can lead to cell necrosis and shedding (ulceration), fluid secretion (diarrhea), and inflammation (colitis). The overall risk of developing C difficile–associated diarrhea (CDAD) after antibiotic use is unknown. It is known that the use of all antibiotics can lead to CDAD, even vancomycin and metronidazole, the two primary antibiotics used for the treatment of CDAD. Interestingly, only one-third of colonized patients develop symptoms. It is unclear why two-thirds of colonized patients remain asymptomatic, though this may be due to the development of an appropriate immune response with the production of immunoglobulin G (IgG) antibodies directed against the toxins.
Transmission can be minimized with the use of gloves and gowns; proper hand washing with soap and water (alcohol-based washes do not prevent transmission) …
Clinical Presentation
The clinical presentation of CDAD is classically a watery diarrhea, but can also include voluminous and, less commonly (~5%), bloody diarrhea. Although most patients have symptoms of lower abdominal pain, fever, and leukocytosis (sometimes with white blood cell counts of >30,000/μL), the spectrum of complaints includes mild diarrhea to abdominal distension, dehydration, metabolic acidosis, toxic megacolon with a notable absence of diarrhea, and sepsis with multisystem organ failure. Extra-intestinal involvement, including cellulitis, necrotizing fasciitis, and reactive polyarticular arthritis, has been reported as well. Because of the notable difference in the severity of symptoms, physicians must have a high index of suspicion, especially for at-risk patients with diarrhea.
It is important to distinguish CDAD from antibiotic-associated diarrhea, which is an osmotic diarrhea unrelated to C difficile. Antibiotic-associated diarrhea results from a reduced ability of intestinal flora to break down unabsorbed carbohydrate, which causes an osmotic load, leading to diarrhea. Stopping oral feeding will stop osmotic, antibiotic-associated diarrhea but not CDAD.
Characteristics of a Hypervirulent Strain
Outbreaks of high-mortality CDAD have been reported in the US and Canada since the early 2000s.3,4 The causative agent is a new, more virulent strain of C difficile, designated alternatively as B1, NAP1, or ribotype 027 toxinotype III. This specific strain is highly resistant to fluoroquinolones, is associated with fluoroquinolone and cephalosporin use,10 and expresses a binary toxin that is of unclear significance.11 Most notably, this strain is characterized by the partial deletion of the tcdC gene, which normally functions to down-regulate the expression of toxins A and B. Deletion of the tcdC gene leads to the production of 16 to 23 times more toxin A and B.11,12 Although BI/NAP1/027 isolates existed previously, historic strains were less resistant to fluoroquinolones and were not associated with outbreaks of disease. The emergence of this strain now is likely related to its selective advantage in the presence of widespread increasing use of fluoroquinolones. A similar phenomenon was observed with the clindamycin-resistant “J strain,” which caused outbreaks in the late 1980s and early 1990s.13
Populations at Risk
The most important risk factors for the development of nosocomial CDAD include prolonged hospitalization, age >65 years, antibiotic use (especially clindamycin, penicillins, cephalosporins, and fluoroquinolones), underlying medical conditions, neoplastic disease, GI surgery, use of nasogastric tubes, and GI disorders including inflammatory bowel disease.14,15 Proton-pump inhibitors have also been implicated because the loss of gastric acid that they cause, allowing for the survival of ingested bacteria.16
Given the aforementioned risk factors, it is not surprising that historically C difficile has affected older, hospitalized patients and residents of facilities for long-term care. However, recent reports indicate there has been an increasing incidence of CDAD in populations previously believed to be at low risk.4 Because reports of infection in healthy adults with minimal or no exposure to a health care facility have been emerging, C difficile should be considered a possible culprit in cases of community-acquired diarrhea as well. Infections have also been reported in pregnant women and in the peripartum period17 and in an increasing number of children who have disrupted normal microflora, are of a young age, or who have underlying medical conditions, infections, or cancer.18
Prevention
Transmission occurs via the fecal-oral route. Therefore, effective infection-control practices could largely control the transmission and development of C difficile infection. Transmission can be minimized with the use of gloves and gowns; proper hand washing with soap and water (alcohol-based washes do not prevent transmission); careful use and proper cleaning of shared patient equipment, such as blood-pressure cuffs, thermometers, and stethoscopes; and the use of bactericidal cleaning solutions. Restricted or judicious antibiotic use will also reduce the incidence of C difficile infections.
As already mentioned, up to two-thirds of patients who are colonized with C difficile remain asymptomatic. However, these carriers remain an important reservoir for C difficile, highlighting the point that proper infection-control practices should be implemented for all patients. C difficile spores can persist on contaminated surfaces for several months. McFarland et al demonstrated hospital acquisition of C difficile in 21% of patients screened, 63% of whom were asymptomatic.19 In one study that tested for the presence of spores in the hospital rooms of patients whose test results were negative for C difficile, analysis revealed a contamination rate of 8%.20 Contamination of hospital environments and personnel, along with the ability of spores to persist for up to 20 weeks after seeding,21 explains why C difficile infections remain a dangerous and persistent health issue.
Diagnosis
The definitive tool for diagnosis of C difficile infection has long been the cell cytotoxicity assay, with a sensitivity of 94% to 100% and a specificity of 99%. Although the specificity is high, this assay is expensive and time-consuming, with a test time of two to three days, making it impractical for routine use. Recently, the toxigenic culture and polymerase chain reaction have replaced the cell cytotoxicity culture assay as the preferred assessment tools. The toxigenic culture has increased sensitivity,22 and polymerase chain reaction produces markedly faster results.23
It is important to point out that false-positive and false-negative test results can occur. False-negative results have been reported to occur in 29% to 56% of cases,24,25 so if the level of clinical suspicion is high, treatment should be initiated despite negative results. A false-negative test result can be generated by failure to test a specimen promptly, failure to keep the stool sample on ice to prevent toxin degradation at room temperature, or failure to generate an adequate sample size. A toxin assay finding may remain positive for several months, so a repeat toxin assay is of limited value in assessing recurrent or persistent diarrhea after treatment.
Diagnosis can also be made by flexible sigmoidoscopy. This may be indicated if there is a high degree of suspicion regarding a patient whose test results for C difficile toxin were negative or in patients requiring a rapid diagnosis that would preclude a delay in laboratory testing. Endoscopy is generally contraindicated in patients with confirmed disease or in patients with fulminant colitis, as there is a risk of perforation with the procedure. Endoscopic features include the presence of pseudomembranes, which are yellow-white raised plaques with localized edema and hyperemia, surrounded by intervening areas of normal mucosa. Pseudomembranes are seen in roughly 50% of patients, but because they may be right-sided, evaluation by flexible sigmoidoscopy might miss the diagnosis. It is important to note that C difficile can infect the small bowel as well, so the absence of a colon does not exclude the diagnosis.
Treatment
After the diagnosis has been confirmed, basic treatment strategies involve stopping the inciting antibiotic, correcting any fluid and electrolyte imbalances, avoiding antiperistaltic agents, initiating contact precautions to limit spread, and treatment of patients with antibiotics if there is evidence of colitis (fever, elevated white blood cell (WBC) count, computed tomography scan findings [Figure 1]), if there is persistent diarrhea despite stopping antibiotics, or if the inciting antibiotic must be continued because of a coexisting infection.
Figure 1.
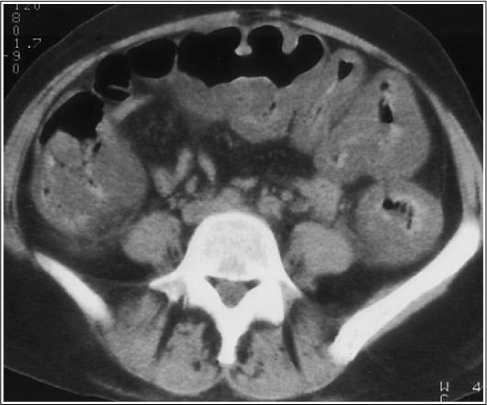
The most common finding on computed tomography scans is thickening of the colon wall, from 3 mm to 3 cm. The thickening is pan-colonic here, although it can also be focal. Contrast trapped within very thick haustra, producing alternating bands of high and low density, is called the accordion sign. Minimal pericolonic stranding and ascites are present.
Standard Treatment Regimens
Standard treatment regimens include the administration of metronidazole, 500 mg orally three times a day for 10 to 14 days; metronidazole, 250 mg orally four times a day for 10 to 14 days; and oral vancomycin, 125 mg four times a day for 10 to 14 days. Metronidazole (but not vancomycin) can also be given intravenously for patients with an ileus. Rectal vancomycin can be administered as a retention enema. Important considerations are that vancomycin use can lead to vancomycin-resistant Enterococcus and that vancomycin tablets are more expensive than metronidazole tablets: For inpatient hospital setting in the US, in 2010, a 500 mg metronidazole tablet costs 7 cents and a 250 mg tablet of vancomycin cost $29. However, C difficile has become more refractory to treatment with metronidazole, and there is a new, less-expensive liquid form of vancomycin available.
… the changing character of C difficile infections and highlight the need for more rapid and reliable diagnostic tools, better treatments, and the implementation of better prevention strategies and infection-control practices.
Treatment of Colonized, Asymptomatic Patients
Treatment of colonized, asymptomatic patients is not effective.26 Asymptomatic fecal excretion is not affected by metronidazole, and though the toxin can be eliminated by vancomycin, most carriers have positive culture findings after therapy is discontinued.
Relapse
Relapse occurs in 10% to 25% of cases. Although it is suspected that noncompliance by patients and misdiagnosis contribute to recurrence, the true etiology of recurrence remains unknown. True relapse represents a persistence of spores, a failure to mount an immune response (IgG), or both. Once the diagnosis has been confirmed, treatment is reinitiated. A tapered or pulse vancomycin regimen should be considered and should last several weeks (125 mg four times a day for one week, then twice a day for one week, once a day for one week, every other day for one week, and finally, every three days for one week), on the basis of the concept that persistent spores convert to toxin producers and are killed when antibiotics are given repeatedly over time.27
Antibiotic Resistance
In 1983, a prospective randomized trial demonstrated no difference in treatment success between vancomycin and metronidazole. Current research data suggest that metronidazole has become less effective in treating CDAD, with higher rates of resistance (9%–25%) and recurrence (20%–40%) in recent years compared with historical controls.28–30 Other studies have suggested that treatment success with metronidazole depends on patient age, with patients older than 65 years having the highest rates of recurrence and treatment failure compared with younger patients.30 Current evidence supports the use of either vancomycin or metronidazole for treatment of mild C difficile colitis, whereas vancomycin has been found to be superior for the treatment of severe C difficile colitis.31 In the study by Zar et al,31 mild CDAD was successfully treated with metronidazole or vancomycin in 90% and 98% of cases, respectively. However, in patients with severe CDAD, treatment was successful with metronidazole or vancomycin in 76% and 97%, respectively. For high-risk patients (immunosuppressed, elderly, etc), vancomycin should be used an initial therapy, possibly in combination with metronidazole.
Alternative Therapies
In addition to the treatment strategies already discussed, there are alternative therapies for recurrent or severe CDAD. Anion-binding resins, such as colestipol (5 g every 12 hours) and cholestyramine (4 mg 3 or 4 times a day), work by binding to C difficile toxins, promoting their fecal excretion. These are generally given for 1 to 2 weeks with vancomycin. However, these resins must be given 2 to 3 hours apart from vancomycin because they bind not only the C difficile toxins but vancomycin as well.
Probiotics, including Saccharomyces boulardii, Lactobacillus rhamnosus, and L plantarum, have been suggested because of the theory that restoration of the normal bacterial flora will reduce C difficile infection. Unfortunately, study results analyzing the use of probiotics for CDAD have been generally inconclusive, with insufficient evidence to support their use.32
With increasing antibiotic resistance, alternative antibiotics (eg, rifaximin, nitazoxanide, tinidazole) have been considered in addition to metronidazole and vancomycin. Various studies have reported high treatment success and low rates of remission.33
One of the most frequently discussed but seldom used treatments for recurrent or refractory CDAD is fecal bacteriotherapy, also playfully termed a “fecal transplant.” Like probiotics, this therapy is based on the concept that restoration of the normal bacterial flora will effectively treat CDAD. At least 17 studies have been reported of roughly 150 participants, with success rates reported in the range of 70% to 90%. Fecal bacteriotherapy can be administered in an enema form or via a nasogastric tube. One proposed regimen involves a mechanical bowel preparation (eg, use of GoLYTELY), followed by a fecal enema consisting of the donor feces (200–300 g) diluted in 250 mL of normal saline. The enema, which should be retained for 6 to 8 hours, should be given within 2 hours of preparation. The fecal enema is administered daily or twice daily for 5 to 14 days.
Lastly, use of intravenous immunoglobulin has also been reported in the treatment of severe, recurrent, or refractory CDAD and has been shown to be effective in various case series.34,35
Surgery
The need for colectomy in patients with C difficile colitis has increased in parallel with the increasing incidence of fulminant colitis and toxic megacolon.36 Fulminant colitis typically manifests as severe lower quadrant pain or diffuse abdominal pain, diarrhea, abdominal distension, fever, hypovolemia, lactic acidosis, and marked leukocytosis (WBC count of ≥40,000/μL).37,38 Because many patients have prolonged ileus due to pooling of secretions in the dilated, atonic colon, they may have minimal diarrhea. Fulminant colitis can progress to toxic megacolon, characterized by colonic dilation >7 cm in association with signs of sepsis, and even bowel perforation necessitating emergency surgery.39 Surgery for C difficile colitis has been associated with a reported mortality of 35% to 80%.36 Poor surgical outcomes have many causes but are related in part to an older, sicker patient population. The key decision regards the timing of surgery. When the need for surgery becomes obvious to all caregivers (eg, in the setting of perforation or multisystem organ failure), the decision has probably come too late, and surgical mortality in this setting will be high. However, the timing of an earlier intervention must weigh the potential advantages of reduced surgical mortality against the possibility that surgery might not have been necessary. This decision requires careful judgment and experience and is made easier by vigilant monitoring of the patient's clinical course, by frequent serial examinations, and by a high level of suspicion, as the patient's condition may rapidly deteriorate. At the time of surgery, the external appearance of the colon may be deceptively normal. Despite this, surgical treatment should be aggressive and include subtotal colectomy rather than hemicolectomy.40
Emerging Treatment Strategies
Despite generally effective treatment strategies, new, promising treatments are necessary in an era of new virulent C difficile strains, worsening antibiotic resistance, and an ever-aging US population that is at higher risk for infection. One such treatment is the drug tolevamer, a non-antimicrobial styrene derivative toxin-binding agent that does not have any antibiotic properties and does not affect the normal gut flora. The drug is currently in testing but has not yet been approved by the US Food and Drug Administration. Another alternative in trials is active immunization with a parenteral toxoid vaccine.41 Use of this vaccine is based on the concept that the humoral immune response (ie, the production of antibody directed against toxins A and B) influences the clinical response to C difficile infection. Preliminary data demonstrate that the vaccine does in fact lead to the production of antitoxin A and B IgG. Its effectiveness for preventing CDAD is currently unknown. A recently studied drug that appears to be promising is a monoclonal antibody, which, when studied with vancomycin or metronidazole, was found to produce a decreased rate of C difficile recurrence.42
Conclusion
Despite historically effective diagnostic and therapeutic tools, and our potential ability to limit transmission with appropriate infection-control practices, C difficile infection remains a prevalent health concern. Recent outbreaks of C difficile due to the development of new virulent strains and antibiotic resistance, and the emergence of infections in previously low-risk populations, demonstrate the changing character of C difficile infections and highlight the need for more rapid and reliable diagnostic tools, better treatments, and the implementation of better prevention strategies and infection-control practices.
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Acknowledgments
Katharine O'Moore-Klopf, ELS, of KOK Edit provided editorial assistance.
References
- Morris AM, Jobe BA, Stoney M, Sheppard BC, Deveney CW, Deveney KE. Clostridium difficile colitis: an increasingly aggressive iatrogenic disease? Arch Surg. 2002 Oct;137(10):1096–100. doi: 10.1001/archsurg.137.10.1096. [DOI] [PubMed] [Google Scholar]
- Pépin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005 Oct 25;173(9):1037–42. doi: 10.1503/cmaj.050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005 Dec 8;353(23):2442–9. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005 Dec 8;353(23):2433–41. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- Muto CA, Pokrywka M, Shutt K, et al. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005 Mar;26(3):273–80. doi: 10.1086/502539. [DOI] [PubMed] [Google Scholar]
- Hall I, O'Toole E. Intestinal flora in newborn infants with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child. 1935;49:390. [Google Scholar]
- Poxton IR. Molecular techniques in the diagnosis and management of infectious diseases: do they have a role in bacteriology? Med Princ Pract. 2005;14(Suppl 1):20–6. doi: 10.1159/000086181. [DOI] [PubMed] [Google Scholar]
- Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978 Mar 9;298(10):531–4. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002 Feb 1;34(3):346–53. doi: 10.1086/338260. [DOI] [PubMed] [Google Scholar]
- O'Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology. 2009 May;136(6):1913–24. doi: 10.1053/j.gastro.2009.02.073. [DOI] [PubMed] [Google Scholar]
- Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005 Sep 24–30;366(9491):1079–84. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- Antikainen J, Pasanen T, Mero S, et al. Detection of virulence genes of Clostridium difficile by multiplex PCR. APMIS. 2009 Aug;117(8):607–13. doi: 10.1111/j.1600-0463.2009.02509.x. [DOI] [PubMed] [Google Scholar]
- Johnson S, Samore MH, Farrow KA, et al. Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficile in four hospitals. N Engl J Med. 1999 Nov 25;341(22):1645–51. doi: 10.1056/NEJM199911253412203. [DOI] [PubMed] [Google Scholar]
- McFarland LV, Clarridge JE, Beneda HW, Raugi GJ. Fluoroquinolone use and risk factors for Clostridium difficile-associated disease within a Veterans Administration health care system. Clin Infect Dis. 2007 Nov 1;45(9):1141–51. doi: 10.1086/522187. [DOI] [PubMed] [Google Scholar]
- Issa M, Vijayapal A, Graham MB, et al. Impact of Clostridium difficile on inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007 Mar;5(3):345–51. doi: 10.1016/j.cgh.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005 Dec 21;294(23):2989–95. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]
- Rouphael NG, O'Donnell JA, Bhatnagar J, et al. Clostridium difficile-associated diarrhea: an emerging threat to pregnant women. Am J Obstet Gynecol. 2008 Jun;198(6):635.e1–6. doi: 10.1016/j.ajog.2008.01.062. [DOI] [PubMed] [Google Scholar]
- McFarland LV, Brandmarker SA, Guandalini S. Pediatric Clostridium difficile: a phantom menace or clinical reality? J Pediatr Gastroenterol Nutr. 2000 Sep;31(3):220–31. doi: 10.1097/00005176-200009000-00004. [DOI] [PubMed] [Google Scholar]
- McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989 Jan 26;320(4):204–10. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- Mutters R, Nonnenmacher C, Susin C, Albrecht U, Kropatsch R, Schumacher S. Quantitative detection of Clostridium difficile in hospital environmental samples by real-time polymerase chain reaction. J Hosp Infect. 2009 Jan;71(1):43–8. doi: 10.1016/j.jhin.2008.10.021. [DOI] [PubMed] [Google Scholar]
- Kim KH, Fekety R, Batts DH, et al. Isolation of Clostridium difficile from the environment and contacts of patients with antibiotic-associated colitis. J Infect Dis. 1981 Jan;143(1):42–50. doi: 10.1093/infdis/143.1.42. [DOI] [PubMed] [Google Scholar]
- Barbut F, Kajzer C, Planas N, Petit JC. Comparison of three enzyme immunoassays, a cytotoxicity assay, and toxigenic culture for diagnosis of Clostridium difficile-associated diarrhea. J Clin Microbiol. 1993 Apr;31(4):963–7. doi: 10.1128/jcm.31.4.963-967.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg RJ, Bruijnesteijn van Coppenraet LS, Gerritsen HJ, Endtz HP, van der Vorm ER, Kuijper EJ. Prospective multicenter evaluation of a new immunoassay and real-time PCR for rapid diagnosis of Clostridium difficile-associated diarrhea in hospitalized patients. J Clin Microbiol. 2005 Oct;43(10):5338–40. doi: 10.1128/JCM.43.10.5338-5340.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hookman P, Barkin JS. Clostridium difficile associated infection, diarrhea and colitis. World J Gastroenterol. 2009 Apr 7;15(13):1554–80. doi: 10.3748/wjg.15.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan C. Detection of Clostridium difficile toxins by enzyme immunoassay. J Hyg (Lond) 1986 Feb;96(1):5–12. doi: 10.1017/s0022172400062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S, Homann SR, Bettin KM, et al. Treatment of asymptomatic Clostridium difficile carriers (fecal excretors) with vancomycin or metronidazole. A randomized, placebo-controlled trial. Ann Intern Med. 1992 Aug 15;117(4):297–302. doi: 10.7326/0003-4819-117-4-297. [DOI] [PubMed] [Google Scholar]
- McFarland LV, Elmer GW, Surawicz CM. Breaking the cycle: treatment strategies for 163 cases of recurrent Clostridium difficile disease. Am J Gastroenterol. 2002 Jul;97(7):1769–75. doi: 10.1111/j.1572-0241.2002.05839.x. [DOI] [PubMed] [Google Scholar]
- Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005 Jun 1;40(11):1586–90. doi: 10.1086/430311. [DOI] [PubMed] [Google Scholar]
- Gordon RS. Metronidazole or vancomycin for Clostridium difficile associated diarrhoea. Lancet. 1983 Dec 17;2(8364):1417. doi: 10.1016/s0140-6736(83)90941-8. [DOI] [PubMed] [Google Scholar]
- Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis. 2005 Jun 1;40(11):1591–7. doi: 10.1086/430315. [DOI] [PubMed] [Google Scholar]
- Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis. 2007 Aug 1;45(3):302–7. doi: 10.1086/519265. [DOI] [PubMed] [Google Scholar]
- McFarland LV. Evidence-based review of probiotics for antibiotic-associated diarrhea and Clostridium difficile infections. Anaerobe. 2009 Dec;15(6):274–80. doi: 10.1016/j.anaerobe.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Bartlett JG. New antimicrobial agents for patients with Clostridium difficile infections. Curr Infect Dis Rep. 2009 Jan;11(1):21–8. doi: 10.1007/s11908-009-0004-8. [DOI] [PubMed] [Google Scholar]
- O'Horo J, Safdar N. The role of immunoglobulin for the treatment of Clostridium difficile infection: a systematic review. Int J Infect Dis. 2009 Nov;13(6):663–7. doi: 10.1016/j.ijid.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Koulaouzidis A, Tatham R, Moschos J, Tan CW. Successful treatment of Clostridium difficile colitis with intravenous immunoglobulin. J Gastrointestin Liver Dis. 2008 Sep;17(3):353–5. [PubMed] [Google Scholar]
- Longo WE, Mazuski JE, Virgo KS, Lee P, Bahadursingh AN, Johnson FE. Outcome after colectomy for Clostridium difficile colitis. Dis Colon Rectum. 2004 Oct;47(10):1620–6. doi: 10.1007/s10350-004-0672-2. [DOI] [PubMed] [Google Scholar]
- Wanahita A, Goldsmith EA, Marino BJ, Musher DM. Clostridium difficile infection in patients with unexplained leukocytosis. Am J Med. 2003 Nov;115(7):543–6. doi: 10.1016/s0002-9343(03)00420-0. [DOI] [PubMed] [Google Scholar]
- Bulusu M, Narayan S, Shetler K, Triadafilopoulos G. Leukocytosis as a harbinger and surrogate marker of Clostridium difficile infection in hospitalized patients with diarrhea. Am J Gastroenterol. 2000 Nov;95(11):3137–41. doi: 10.1111/j.1572-0241.2000.03284.x. [DOI] [PubMed] [Google Scholar]
- Lamontagne F, Labbé AC, Haeck O. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg. 2007 Feb;245(2):267–72. doi: 10.1097/01.sla.0000236628.79550.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss K, Clark MA, Sanders DS, Morton D, Keighley MR, Goh J. The outcome of surgery in fulminant Clostridium difficile colitis. Colorectal Dis. 2006 Feb;8(2):149–54. doi: 10.1111/j.1463-1318.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- Sougioultzis S, Kyne L, Drudy D, et al. Clostridium difficile toxoid vaccine in recurrent C difficile-associated diarrhea. Gastroenterology. 2005 Mar;128(3):764–70. doi: 10.1053/j.gastro.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010 Jan 21;362(3):197–205. doi: 10.1056/NEJMoa0907635. [DOI] [PubMed] [Google Scholar]


