Abstract
BACKGROUND:
The frequency of Chlamydia trachomatis and Neisseria gonorrhoeae coinfection can vary depending on their individual incidence and prevalence rates.
OBJECTIVE:
To determine the frequency of C trachomatis and N gonorrhoeae coinfections by evaluating the results of testing in 2007 and 2008 to better inform testing and treatment decisions.
METHODS:
Specimens from the same patient submitted on the same day served as the basis for the present study. The age, sex and the source of the specimen were also linked to the accession number. Infection and coinfection rates were analyzed in both males and females.
RESULTS:
Concurrent testing was performed on 41,567 female specimens and 1827 male specimens, of which, 1495 female samples (3.6%) tested positive for C trachomatis infection and 88 (0.2%) tested positive for N gonorrhoeae infections. Only 31 females were coinfected; however, for those between 11 and 25 years of age, 25 of 61 females (40.1%) with N gonorrhoeae infection also tested positive for C trachomatis infection; conversely, 25 of 1248 females (2.0%) with C trachomatis infection also tested positive for N gonorrhoeae infection. For males, 213 (11.7%) tested positive for C trachomatis infection, and 59 (3.2%) tested positive for N gonorrhoeae infection. In 30 males with N gonorrhoeae between 11 and 25 years of age, and 149 males with C trachomatis, eight coinfections were observed (26.7% and 5.3%, respectively). Of those older than 25 years of age, only five of 905 men and six of 19,465 women were coinfected. None of the 10,935 women who were 30 years of age or older had coinfections.
CONCLUSION:
The N gonorrhoeae coinfection rate in males with C trachomatis may justify empirical antimicrobials; however, in females, the proportion of coinfected may not justify empirical treatment for N gonorrhoeae infection when the C trachomatis test is positive and N gonorrhoeae testing has not been performed.
Keywords: Chlamydia trachomatis, Coinfection, Neisseria gonorrhoeae
Abstract
HISTORIQUE:
La fréquence de co-infections par le Chlamydia trachomatis et la Neisseria gonorrhoeae peut varier selon l’incidence et le taux de prévalence de chaque infection.
OBJECTIF:
Déterminer la fréquence de co-infections en évaluant les résultats des tests effectués en 2007 et 2008 afin de mieux éclairer les décisions à l’égard des tests et des traitements.
MÉTHODOLOGIE:
L’auteur s’est servi des échantillons d’un même patient soumis la même journée pour mener la présente étude. Il a également lié l’âge, le sexe et la source de l’échantillon au numéro d’entrée. Il a analysé les taux d’infections et de co-infections chez les hommes et les femmes.
RÉSULTATS:
Des tests concomitants ont été exécutés sur 41 567 échantillons de femmes et 1 827 échantillons d’hommes. De ce nombre, 1 495 échantillons de femmes (3,6 %) étaient positifs à l’infection par le C trachomatis et 88 (0,2 %), à l’infection par la N gonorrhoeae. Seulement 31 femmes étaient co-infectées. Toutefois, 25 des 61 femmes de 11 à 25 ans (40,1 %) infectées par la N gonorrhoeae étaient également positives à l’infection par le C trachomatis, mais seulement 25 des 1 248 femmes (2,0 %) infectées par le C trachomatis étaient également positives à l’infection par la N gonorrhoeae. Chez les hommes, 213 (11,7 %) étaient positifs à l’infection par le C trachomatis, et 59 (3,2%), à l’infection par la N gonorrhoeae. Chez les 30 hommes de 11 à 25 ans infectés par la N gonorrhoeae et les 149 hommes infectés par le C trachomatis, on a observé huit co-infections (26,7 % et 5,3 %, respectivement). Cependant, seulement cinq des 905 hommes et six des 19 465 femmes de 25 ans et plus étaient co-infectés. Aucune des 10 935 femmes de 30 ans ou plus n’était co-infectée.
CONCLUSION:
Le taux de co-infections par la N gonorrhoeae chez les hommes infectés par le C trachomatis peut justifier la prescription empirique d’antimicrobiens, mais chez les femmes, la proportion de co-infections ne justifie peut-être pas un traitement empirique contre l’infection par la N gonorrhoea lorsque le test du C trachomatis est positif, mais que le test de la N gonorrhoeae n’a pas été effectué.
Both Chlamydia trachomatis and Neisseria gonorrhoeae are commonly occurring sexually transmitted bacteria that produce broadly overlapping clinical syndromes. In men, infection is most often manifested as urethritis; women most often develop cervicitis. The large majority of infections in women are asymptomatic. Asymptomatic infections are less commonly seen in men, but still represent an important reservoir for transmission. Our laboratory tests approximately 25,000 Nova Scotia clients yearly for C trachomatis, and about 4% of these tests are positive; of the 15,000 cultures for N gonorrhoeae, about 0.2% are positive. The prevalence of both C trachomatis and N gonorrhoeae infections in males and females of different age groups varies considerably. Many physicians tend to only test for C trachomatis; guidelines suggest empirical treatment of N gonorrhoeae in those patients testing positive for the former. Recommendations to treat patients for concurrent infections would be better informed if there was recent Canadian research relating to the frequency of coinfections in males versus females, and in younger versus older adults.
We evaluated the results of testing in our laboratory over a two-year period to precisely determine the frequency of coinfection in males and females, and at different ages, to better inform testing and treating decisions.
METHODS
Specimens for C trachomatis and N gonorrhoeae testing submitted together and registered in the laboratory information system served as the basis for the present study. The specimens were primarily obtained from the Capital District Health Authority (Halifax, Nova Scotia), and the vast majority of tests were performed by physicians in their private offices. There is one sexually transmitted infection clinic, which runs for approximately 6 h/week and is physician based. Testing in Nova Scotia is seldom performed by nurse practitioners; no testing was performed for jurisdictions outside of Nova Scotia. These specimens were assigned the same laboratory accession number. The results of both C trachomatis and N gonorrhoeae testing were extracted from the database, stripped of unique identifiers, and joined using the common accession number, as well as the age and sex of the patients. Results were abstracted from the laboratory information system database.
Specimens for N gonorrhoeae testing were submitted using the Copan M40 Transystem specimen transport system (Copan Diagnostics Inc, USA) and were processed on the day of receipt. Cultures were performed using modified Thayer-Martin media incubated in 10% CO2 at 37°C for 48 h and identified using conventional means (1). Genital swabs and urine samples (the most common sample in men) were tested using the Cobas Amplicor C trachomatis analyzer (Roche Diagnostics, Canada) as described in the manufacturer’s package insert. Female urine sample testing was very rarely performed. Internal controls were used for female urethral but not cervical specimens. The Cobas Amplicor analyzer automatically performed all of the amplification, hybridization and detection steps. All results were interpreted according to the manufacturer’s guidelines, based on signal cut-off readings.
A copy of the protocol was reviewed and approved by the Capital Health Research Review Board. The present study did not receive any funding.
RESULTS
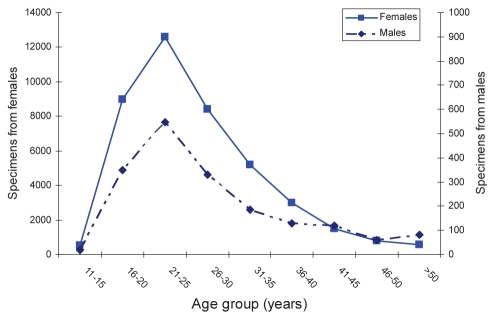
In total, 41,567 specimen pairs were obtained from females and 1827 specimen pairs from males (Figure 1). Of which, 1495 female samples tested positive for C trachomatis, and 88 (0.2%) tested positive for N gonorrhoeae. Females between 16 and 20 years of age had C trachomatis detected in 7.1% of samples and N gonorrhoeae in only 0.3%. Two hundred forty-seven (1.3%) specimens received from women older than 25 years of age were positive for C trachomatis, and 0.14% of cases tested positive for N gonorrhoeae (Table 1). Only six women older than 25 years of age were coinfected with both N gonorrhoeae and C trachomatis.
Figure 1).
The number of specimens received for both Chlamydia trachomatis and Neisseria gonorrhoeae testing by age group
TABLE 1.
The number and proportion of females and males tested and positive for Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) infections
|
Females |
Males |
|||
|---|---|---|---|---|
| Age | ≤25 years | >25 years | ≤25 years | >25 years |
| Both negative, n | 20,818 | 19,197 | 747 | 821 |
| CT positive/NG negative, n (%) | 1,223 (5.53) | 241 (1.24) | 141 (15.36) | 59 (6.49) |
| CT negative/NG positive, n (%) | 36 (0.16) | 21 (0.11) | 22 (2.4) | 24 (2.64) |
| Coinfected, n (%) | 25 (0.11) | 6 (0.03) | 8 (0.87) | 5 (0.55) |
| Total tests performed, n | 22,102 | 19,465 | 918 | 909 |
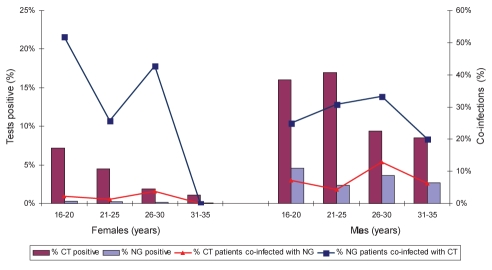
Almost one-half of the testing was performed on men 26 years of age or older (Table 1). C trachomatis infection was more common in males younger than 26 years of age; however, the proportion testing positive for N gonorrhoeae infection was not significantly different. Patients with N gonorrhoeae infection commonly tested positive for C trachomatis infection. Twenty-five of the 61 (41%) women younger than 26 years of age who had N gonnorhoeae infection also had C trachomatis infection (Figure 2). Twenty-five of the 1248 (2.0%) females 25 years of age or younger with C trachomatis also had N gonnorhoeae infection. In women older than 25 years of age, six of 27 (22.2%) with N gonorrhoeae infection also had C trachomatis infection; six of the 247 women with C trachomatis infection also had N gonnorhoeae infection. There were no coinfections in women older than 30 years of age, and only one coinfection in a man older than 30 years of age.
Figure 2).
The proportion of specimens from males and females of different ages that were positive for Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG), and the proportion in each age group that was coinfected
DISCUSSION
The testing practices of Nova Scotia physicians vary widely. The tests that we received represented a composite of patients with symptoms suggestive of a sexually transmitted infection who may have been in contact with an infected partner, who may have been tested because they fell within established guidelines or who may have been tested as part of a regular sexual health checkup (for instance, with an annual contraceptive renewal visit). As is the case in many other centres, women were tested much more frequently than men, and the lower positivity rate in women was more a reflection of prevalence; whereas, in men, more testing was performed for symptoms and for contacts. Irrespective of how patients were identified, this should not fundamentally affect our observations regarding the proportion that was coinfected.
Other studies (2–6) have observed that the frequency of coinfection can vary dramatically, depending on the setting and the background prevalence of each of these sexually transmitted infections. Creighton et al (2) found that 24% of heterosexual men and 38% of women with gonorrhea also had chlamydia. The opposite was less often true, ie, heterosexual men with chlamydia had gonorrhea in 18% of cases; whereas, 13% of women also had gonorrhea. Most of the coinfected were between 15 and 19 years of age. The high prevalence in this setting suggested a policy of epidemiological treatment for chlamydia in patients known to have or be in contact with gonorrhea. In a very high-prevalence setting, a sexually trasmitted disease clinic in Edinburgh, Scotland, identified chlamydia in 24% of men who had sex with men and who had rectal gonorrhea (3). Nsuami et al (4) studied approximately 6000 high school students in Louisiana (USA). In this relatively high-prevalence setting, gonorrhea was detected in 11% of students with chlamydia; conversely, 42% of students with gonorrhea also had chlamydia.
Furthermore, two studies within a low-prevalence setting were reported. The first from Sydney, Australia, by Tapsall and Kinchington (5), identified only four of 124 men with chlamydia infections with concurrent N gonorrhoeae (5). van Bergen et al (6) recently described their experience in the Netherlands (6), and found a very low rate of coinfection in a community where gonorrhea had a very low prevalence. Based on their evidence, they suggested that population screening for asymptomatic gonorrhea may no longer be indicated in Holland. Rather than suggest empirical treatment, they suggested that patients be tested for N gonorrhoeae infection if C trachomatis tests were positive. Canadian treatment guidelines suggest ‘empirical cotreatment when a diagnosis of N gonorrhoeae is made without waiting for test results of C trachomatis due to the significant probability of coinfection (20% to 42%)’ (7). The coinfection rates that are cited in the Canadian guidelines are not, however, based on Canadian data (2,8).
Testing for N gonorrhoeae infection is infrequently performed alone, and it is unlikely that clinicians will encounter a patient with N gonorrhoeae infection without results of C trachomatis testing. On the rare occasions when this situation arises, the very high coinfection rate in both males and females should easily justify cotreatment. The opposite situation is often encountered; ie, C trachomatis testing is positive, but N gonorrhoeae testing is not performed. The 5.4% coinfection rate in males younger than 26 years of age might justify empirical treatment, especially when follow-up is not reasonably assured. Only 2.0% of females younger than 26 years of age diagnosed with C trachomatis infection were coinfected with N gonorrhoeae. Whether these patients should empirically receive an antibiotic or be tested for gonorrhea should be the subject of further analysis.
REFERENCES
- 1.Murray PR. In: Manual of Clinical Microbiology. 9th edn. Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Washington, DC: ASM Press; 2007. [Google Scholar]
- 2.Creighton S, Tenant-Flowers M, Taylor CB, Miller R, Low N. Co-infection with gonorrhoea and chlamydia: How much is there and what does it mean? Int J STD AIDS. 2003;142:109–13. doi: 10.1258/095646203321156872. [DOI] [PubMed] [Google Scholar]
- 3.McMillan A, Manavi K, Young H. Concurrent gonococcal and chlamydial infections among men attending a sexually transmitted diseases clinic. Int J STD AIDS. 2005;16:357–61. doi: 10.1258/0956462053888925. [DOI] [PubMed] [Google Scholar]
- 4.Nsuami M, Cammarata CL, Brooks BN, Taylor SN, Martin DH. Chlamydia and gonorrhea co-occurrence in a high school population. Sex Transm Dis. 2004;31:424–7. doi: 10.1097/01.olq.0000130535.96576.d3. [DOI] [PubMed] [Google Scholar]
- 5.Tapsall JW, Kinchington M. The frequency of co-infection with Neisseria gonorrhoeae and Chlamydia trachomatis in men and women in eastern Sydney. Pathology. 1996;28:84–7. doi: 10.1080/00313029600169603. [DOI] [PubMed] [Google Scholar]
- 6.van Bergen JE, Spaargaren J, Götz HM, et al. Population prevalence of Chlamydia. trachomatis and Neisseria gonorrhoeae in the Netherlands. Should asymptomatic persons be tested during population-based chlamydia screening also for gonorrhoea or only if chlamydial infection is found? BMC Infect Dis. 2006;6:42. doi: 10.1186/1471-2334-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Public Health Agency of Canada Section V: Management and treatment of specific infections: Chlamydial infections. Canadian Guidelines on Sexually Transmitted Infections<http://www.phac-aspc.gc.ca/std-mts/sti-its/guide-lignesdir-eng.php> (Accessed on March 24, 2010).
- 8.Lyss SB, Kamb ML, Peterman TA, et al. Project RESPECT Study Group Chlamydia trachomatis among patients infected with and treated for Neisseria gonorrhoeae in sexually transmitted disease clinics in the United States. Ann Intern Med. 2003;139:178–85. doi: 10.7326/0003-4819-139-3-200308050-00007. [DOI] [PubMed] [Google Scholar]