Abstract
OBJECTIVE:
To review the epidemiology of selected nonviral enteric illnesses reported in children in Quebec between 1999 and 2006.
METHODS:
Incidence rates were calculated to describe age, sex, temporal and geographical characteristics of the selected nonviral enteric cases reported in children who were between zero and four years of age. Standard descriptive methods were used to analyze the temporal and geographical distributions of the incidence rates.
RESULTS:
A total of 5068 cases were reported. Of these, three pathogens accounted for the majority of the infections: Giardia (32.52%), Salmonella (30.98%) and Campylobacter (30.82%). Salmonella was most frequent in children younger than one year of age, whereas comparable incidence rates for the three pathogens were calculated for children between one and four years of age. For Giardia, the geographical distributions showed that the highest rates were in areas with more than 100,000 inhabitants (except Montreal, Quebec); for Salmonella, the highest rates were in Montreal; and for Campylobacter, the highest rates were in areas with fewer than 10,000 inhabitants. No detectable trends were seen over the study period for the three pathogens. Seasonal summer peaks were noted for Salmonella and Campylobacter, contrasting with late summer to early autumn peaks for Giardia.
CONCLUSION:
Findings suggest that Giardia, Salmonella and Campylobacter were the most common causes of nonviral enteric illnesses reported in children in Quebec. Giardia cases seemed to arise from different sources and transmission routes than the other two pathogens. Characteristics specific to Campylobacter infections in children, namely its predominance in areas with low population densities, and to Salmonella infections, namely predominance in the Greater Montreal area, should be further investigated to better guide prevention and control measures.
Keywords: Campylobacter, Children, Epidemiology, Gastroenteritis, Giardia, Salmonella
Abstract
OBJECTIF:
Analyser l’épidémiologie de maladies non virales sélectionnées déclarées chez des enfants du Québec entre 1999 et 2006.
MÉTHODOLOGIE:
Les chercheurs ont calculé les taux d’incidence pour décrire l’âge, le sexe, les caractéristiques temporelles et géographiques de cas entériques non viraux sélectionnés déclarés chez des enfants de zéro à quatre ans. Ils ont utilisé des méthodes descriptives standards pour analyser la répartition temporelle et géographique de ces taux d’incidence.
RÉSULTATS:
Au total, 5 068 cas ont été déclarés. De ce nombre, trois pathogènes étaient responsables de la majorité des infections : la Giardia (32,52 %), la Salmonella (30,98 %) et le Campylobacter (30,82 %). La Salmonella était plus courante chez les enfants de moins d’un an, tandis que les chercheurs ont calculé des taux d’incidence comparables entre les trois pathogènes chez les enfants de un à quatre ans. Pour ce qui est de la Giardia, la répartition géographique a révélé que les taux les plus élevés se situaient dans des régions où on comptait plus de 100 000 habitants (sauf Montréal, au Québec). Quant à la Salmonella, les taux les plus élevés s’observaient à Montréal, tandis que ceux du Campylobacter étaient constatés dans les régions de moins de 10 000 habitants. Les chercheurs n’ont décelé aucune tendance détectable pour les trois pathogènes pendant la période de l’étude. Ils ont remarqué des pointes saisonnières estivales pour la Salmonella et le Campylobacter, en opposition aux pointes de fin d’été et de début d’automne de la Giardia.
CONCLUSION:
Selon les résultats, la Giardia, la Salmonella et le Campylobacter étaient les principales causes de maladies entériques non virales déclarées chez des enfants du Québec. Les cas de Giardia semblaient découler de sources et de voies de transmission différentes de celles des deux autres pathogènes. Les caractéristiques propres aux infections à Campylobacter chez les enfants, soit leur prédominance dans les régions où la densité de la population est faible, et aux infections à Salmonella, soit leur prédominance dans la grande région de Montréal, devraient faire l’objet de recherches plus approfondies pour mieux orienter les mesures de prévention et de contrôle.
Diarrheal disease remains an important cause of morbidity and mortality in children worldwide (1). The estimated incidence rates in developing countries range from 3.5 to 7.0 cases/child/year during the first two years of life, and from two to five cases/child/year for the first five years (2). In the United States (US), more than 20 million cases of diarrhea are estimated annually in children younger than five years of age, leading to approximately 2.5 million visits to a doctor, 220,000 hospitalizations and approximately 40 deaths (3).
Bacterial enteric pathogens are second to rotaviruses among the known causes of diarrheal disease in infants and young children (4). Campylobacter and Salmonella are commonly identified causes of diarrheal disease in children (4,5). Because of their virulence, infections by Escherichia coli O157 and Yersinia enterocolitica are other important pathogens commonly reported, but occur with relatively less frequency in this age group (6–8).
The most important intestinal parasites infecting North American children include Giardia lamblia (intestinalis) and Cryptosporidium parvum (9,10). Population-based surveillance studies (9,11–13) conducted in the US demonstrated increasing rates of Giardiasis between 1992 and 1997, with the highest national rates found among children between zero and five years of age. The direct and indirect costs of diarrheal disease in children are very high for communities worldwide, and better prevention and control programs could significantly decrease the economic burden on health care systems (3,14,15).
In Quebec, infections attributed to Giardia, Salmonella, Campylobacter, E coli, Yersinia and Cryptosporidium represent up to 90% of all notifiable nonviral gastroenteritis reported in regional public health registries (16).
More insight into the epidemiological characteristics of gastroenteritis caused by these leading pathogens, and specifically those pertinent to young children, may help to enhance current control programs within communities (3). In considering the importance of targeting our interventions for this age class, there are, however, very few published population-based studies to date that give a specific description and analysis of the basic epidemiology pertinent to infectious gastroenteritis in children (17,18), with most of them being case-control studies with small population subsets or data obtained through sentinel surveillance systems.
The objective of the present study was to review the patient characteristics, and the seasonal and geographical patterns of nonviral enteric illnesses in children most commonly reported in Quebec between 1999 and 2006.
METHODS
Data sources
Records of reported cases were extracted from the diseases registry of the Regional Public Health Departments (RPHD) of the Quebec Ministry of Health and Social Services. In Quebec, all notifiable diseases are reported to the RPHD through an electronic database system designed to gather and manage a specific list of diseases that must be reported by physicians or chiefs of microbiological laboratories. Quebec has 18 health regions, each of which is mandated by the provincial Ministry of Health to forward reportable diseases data to a central registry managed by the provincial public health laboratory.
Cases were defined as children who were between zero and four years of age with a laboratory diagnosis of infection by Giardia, Salmonella, Campylobacter, Yersinia, invasive E coli and/or Cryptosporidium (Appendix 1), and a notification by the physician or laboratory director to the RPHD between 1999 and 2006. The total population of children, between zero and four years of age from 1999 to 2000, was estimated and projected by the Quebec Statistics Institute, based on municipal population using the 1996 general census data compiled by Statistics Canada. The estimated population was updated each year using the general census data from 2001 and 2006.
Validation, exclusion and ethics
Cases from northern health regions (northern Quebec, Nunavik and James Bay Cree territories) and those that occurred outside Quebec were excluded from the study. A validation was also performed according to standard disease definitions, geographical location and possible association with known outbreaks (19). Intra- and inter-regional duplication of each case was avoided by verifying the date of onset, postal code, birth date and sex. Physician diagnosis and laboratory results were also cross-checked for all records. A minimum time interval between two episodes for the same child was estimated to avoid any link between reports (one to 10 days for Campylobacter and E coli, three to 10 days for Yersinia, one to four weeks for Giardia, one to 12 days for Cryptosporidium, and three to 90 days for Salmonella). The study has been independently reviewed and approved by the Ethics Review Board of the Centre Hospitalier Universitaire de Québec, Quebec.
Descriptive analyses
Incidence rates of Giardia, Salmonella, Campylobacter, other pathogens (Y enterocolitica, C parvum and invasive E coli) and all selected pathogens (Giardia, Salmonella, Campylobacter, E coli, Yersinia or Cryptosporidium) were calculated according to two age groups (zero to one year, and one to four years), by sex and year, and season and year. The age categorization was mainly designed with two major considerations: statistical efficiency and children behaviour, and vulnerability in relation to the epidemiology of these diseases. Therefore, children between zero and one year of age, and children between one and four years of age were grouped together for descriptive analyses. Rates were expressed as cases per children-years. Seasonal variation and trend analyses were performed using a moving average algorithm available in Microsoft Excel 2003 (Microsoft Corporation, USA). The incidence rates of Giardia, Salmonella, Campylobacter and all selected pathogens were further calculated according to four categories of municipalities based on their population sizes: the census metropolitan area of Montreal; other census metropolitan areas with municipalities of more than 100,000 inhabitants; census agglomerations with municipalities ranging from 10,000 to 100,000 inhabitants; and census areas with municipalities of fewer than 10,000 inhabitants. A descriptive analysis of the geographical distribution for the cumulative incidence rate of cases associated with the three main pathogens was performed using the empirical Bayes smoothing method to account for various population sizes and small denominators. Maps were constructed using the Jenks natural break algorithm to categorize incidence rates per health area (ArcGis version 9.3, ESRI, USA). The Moran’s I statistic was estimated to assess evidence of global spatial autocorrelation (clustering) of incidence rates over the study region. Computation of the Moran’s I statistic was performed using GeoDa software version 0.9.5-I (GeoDa Center for Geospatial Analysis and Computation, USA) (20).
RESULTS
Case occurrence
There were a total of 3,039,085 children-years estimated from 1999 to 2006 in Quebec. Over the study period, a total of 5104 validated cases were extracted from the RPHD registries. From these, 36 were excluded due to their location in the northern regions (northern Quebec, Nunavik and James Bay Cree territories). The remaining 5068 cases were included in the analyses and were divided into two age groups. A group representing cases in children younger than one year of age, which included 277 cases (5.5%); and one representing children between one and four years of age, which included 4791 cases (94.5%). The incidence rates for children younger than one year of age ranged from 0.4 to 5.8 cases/100,000 children-years. Cases attributed to Giardia, Salmonella and Campylobacter represented a total of 94.3% of all studied cases, with similar incidence rates for each of these three pathogens averaging 50 cases/100,000 children-years. In comparison, the total number of cases attributed to Yersinia, Cryptosporidium and E coli represented 5.7% of all cases, with incidence rates of less than 10 cases/100,000 children-years. Higher rates were also observed in the one- to four-year-old group compared with the younger than one-year-old group.
Detailed incidence rates per pathogen and according to age group are presented in Table 1. Incidence rates of all selected pathogens and Campylobacter cases revealed a significant (P<0.0001) difference between sexes, showing higher rates in males than females (Table 2). The incidence rates of Giardia and Salmonella cases were similar for both sexes.
TABLE 1.
Distribution of selected bacterial and parasitic infections in children between zero and four years of age in Quebec (1999 to 2006)
|
(<1 years) |
(1–4 years) |
(0–4 years) |
||||
|---|---|---|---|---|---|---|
| Pathogen | Cases, n | IR* | Cases, n | IR* | Total cases, n | Total IR* (95% CI) |
| Giardia | 11 | 0.4 | 1637 | 53.8 | 1648 | 54.2 (51.6–56.8) |
| Salmonella | 177 | 5.8 | 1393 | 45.8 | 1570 | 51.6 (49.1–54.2) |
| Campylobacter | 74 | 2.5 | 1488 | 48.9 | 1562 | 51.4 (48.8–53.9) |
| Others† | 15 | 0.6 | 273 | 8.9 | 288 | 9.5 (6.9–12.1) |
| All selected pathogens | 277 | 9.3 | 4791 | 157.4 | 5068 | 166.7 (162.1–171.3) |
Per 100,000 children-years;
Including Yersinia enterocolitica, Cryptosporidium parvum and Escherichia coli. IR Incidence rate
TABLE 2.
Variation of selected bacterial and parasitic infection incidence rates (IR) according to year and sex of children between zero and four years of age in Quebec (1999 to 2006)
|
Giardia(n=1648) |
Salmonella(n=1570) |
Campylobacter(n=1562) |
All selected pathogens†(n=5068) |
|||||
|---|---|---|---|---|---|---|---|---|
| Male | Cases, n (n=857) | IR* | Cases, n (n=804) | IR* | Cases, n (n=899) | IR* | Cases, n (n=2705) | IR* |
| 1999 | 114 | 54.1 | 92 | 44.0 | 92 | 43.9 | 316 | 161.6 |
| 2000 | 89 | 43.8 | 100 | 48.7 | 112 | 55.3 | 319 | 151.1 |
| 2001 | 86 | 44.0 | 103 | 52.2 | 108 | 54.8 | 315 | 140.8 |
| 2002 | 131 | 68.2 | 101 | 52.7 | 114 | 59.4 | 365 | 175.3 |
| 2003 | 120 | 63.1 | 99 | 52.2 | 96 | 50.5 | 333 | 158.8 |
| 2004 | 104 | 54.6 | 108 | 56.8 | 137 | 71.9 | 368 | 176.6 |
| 2005 | 114 | 59.0 | 102 | 53.4 | 132 | 69.5 | 365 | 173.3 |
| 2006 | 99 | 51.1 | 99 | 50.7 | 108 | 55.8 | 324 | 147.7 |
| Global rates and 95% CI | 54.6 (51.0–58.2) | 51.2 (47.6–54.8) | 57.6 (53.7–61.5) | 172.4 (169.8–174.9) | ||||
| Female | Cases, n (n=791) | IR* | Cases, n (n=766) | IR* | Cases, n (n=663) | IR* | Cases, n (n=2363) | IR* |
| 1999 | 86 | 42.9 | 92 | 45.9 | 76 | 38.0 | 272 | 131.8 |
| 2000 | 90 | 46.3 | 81 | 42.3 | 82 | 42.7 | 271 | 130.2 |
| 2001 | 77 | 41.3 | 97 | 52.4 | 90 | 48.8 | 282 | 135.2 |
| 2002 | 130 | 71.7 | 112 | 61.6 | 77 | 41.9 | 335 | 158.3 |
| 2003 | 108 | 60.2 | 98 | 54.4 | 64 | 35.5 | 288 | 142.1 |
| 2004 | 107 | 59.3 | 95 | 52.4 | 85 | 47.0 | 305 | 144.3 |
| 2005 | 105 | 58.0 | 93 | 50.9 | 100 | 54.9 | 316 | 149.5 |
| 2006 | 88 | 47.2 | 98 | 52.9 | 89 | 48.2 | 294 | 134.9 |
| Global rates and 95% CI | 53.1 (49.5–56.7) | 51.5 (47.9–55.1) | 44.5 (40.0–48.1) | 158.9 (156.3–161.5) | ||||
Per 100,000 children-years;
Including Giardia, Salmonella, Campylobacter, Escherichia coli, Cryptosporidium and Yersinia
Population size of municipalities and spatial distribution
The incidence rates of all the pathogens, and especially those of Salmonella and Campylobacter, seemed to increase with the population size of the municipalities, except for the incidence rates of areas that included municipalities with fewer than 10,000 inhabitants (Table 3). This ‘v-shape’ pattern was clearer for Campylobacter cases, whose rates in areas including municipalities of fewer than 10,000 inhabitants were significantly higher than those of the census metropolitan area of Montreal. However, the incidence rates of Giardia cases were significantly lower in areas with municipalities of fewer than 10,000 inhabitants, but seemed to be high in other census metropolitan areas with municipalities of more than 10,000 inhabitants.
TABLE 3.
Urban/rural distribution of selected bacterial and parasitic infection incidence rates (IR) of children between zero and four years of age in Quebec (1999 to 2006)
|
Giardia |
Salmonella |
Campylobacter |
All selected pathogens* |
|||||
|---|---|---|---|---|---|---|---|---|
| Areas | Cases, n | IR (95% CI) | Cases, n | IR (95% CI) | Cases, n | IR (95% CI) | Cases, n | IR (95% CI) |
| >1,000,000 inhabitants | 403 | 51.2 (46.2–56.2) | 529 | 67.3 (61.5–73.0) | 392 | 49.8 (44.9–54.8) | 1420 | 180.7 (171.3–190.1) |
| 100,000–1,000,000 inhabitants | 426 | 59.2 (53.6–64.8) | 351 | 48.8 (43.7–53.9) | 318 | 44.2 (39.3–49.1) | 1148 | 159.7 (150.4–168.9) |
| 10,000–100,000 inhabitants | 480 | 58.2 (53.0–63.5) | 316 | 38.3 (34.1–42.6) | 287 | 34.8 (30.8–38.8) | 1149 | 139.5 (131.4–147.5) |
| <10,000 inhabitants | 339 | 47.6 (42.6–52.7) | 374 | 52.6 (47.2–57.9) | 565 | 79.4 (72.9–86.0) | 1351 | 190.0 (179.8–200.1) |
Including Giardia, Salmonella, Campylobacter, Escherichia coli, Cryptosporidium and Yersinia
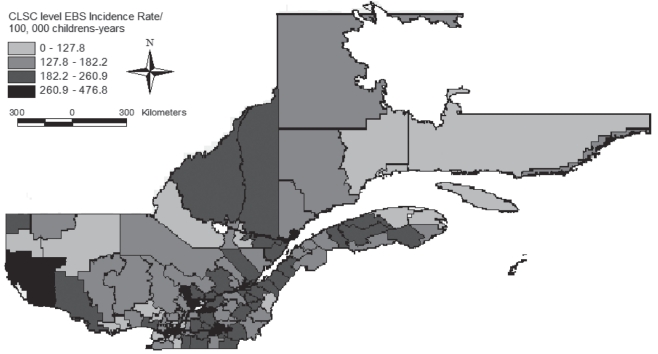
The spatial distribution of incidence rates for all selected pathogens is presented in Figure 1. Visual assessment revealed a heterogeneous distribution of incidence rates in the province, with areas of higher incidence located in the southern health regions. However, the global Moran’s I index of 0.09 indicated a significant (P=0.001) spatial autocorrelation of incidence rates.
Figure 1).
Distribution of empirical Bayes smoothing (EBS) incidence rates of selected enteric bacterial and parasitic pathogens in children between zero and four years of age in Quebec between 1999 and 2006. Data from the notifiable diseases registries of the Regional Public Health Departments, Ministry of Health, Quebec (1999 to 2006). CLSC Centre Local de Santé Communautaire
Trends and seasonal variations
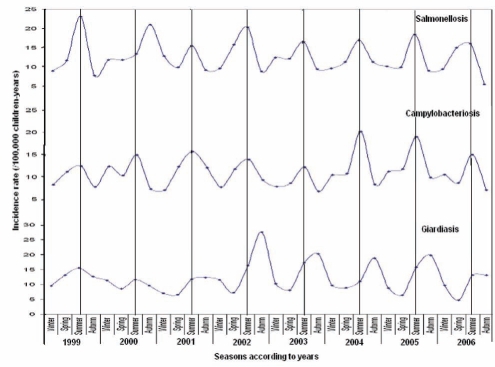
Temporal variations in the incidence rates of Giardia, Salmonella and Campylobacter cases exhibited regular seasonal patterns with consistent peaks during summer for Salmonella and Campylobacter and autumn for Giardia. Seasonal peaks in the incidence rates of Giardia cases were low from 1999 to 2002, and higher from 2003 to 2006. The incidence rates of Campylobacter cases peaked slightly during two summers of the study period – 2005 and 2006. All of these incidence rates were stable throughout 1999 to 2006 (Figure 2).
Figure 2).
Giardia, Salmonella and Campylobacter empirical Bayes smoothing incidence rates (per 100,000 children-years) showing seasonal variation and trends in children between zero and four years of age (1999 to 2006)
DISCUSSION
Excluding viral enteric gastroenteritis, Giardia, Salmonella and Campylobacter were the most common enteric pathogens notified in children younger than five years of age in Quebec, accounting for approximately 50 cases/100,000 children-years for each of these pathogens. The importance of these enteric pathogens in children was also highlighted previously in Quebec (21), and by Koehler et al (22) in the US using FoodNet data collected between 1996 and 1998. The incidence rate of reported cases of Giardia (54.2 cases/100,000 children-years) estimated in the study was approximately five times higher than the estimated rate for the general population (12 cases/100,000 persons-years) in Quebec (23). Similarly, the incidence rate of reported Salmonella cases in the study was approximately 2.5 times greater than the rate estimated for the general population in Quebec (24).
Cases were defined (19) and validated based on the standard definition and time criteria, such as laboratory ascertainment and time frames for each pathogen, to avoid double reporting of the same case (25). Several studies in Canada that reviewed reported cases of Giardia found that children younger than five years of age had the highest incidence rates (26), and that males showed the highest rates among all age groups (26,27). The highest rate of Campylobacter cases was observed in the zero- to four-year age group and the 20 to 39 year age group, with slightly higher rates occurring in males than in females (28). The investigation of age, sex and residence-specific incidence rates of Salmonella enteritidis cases in Michigan (USA) found a higher incidence rate of S enteritidis cases associated with children younger than five years of age compared with the national average incidence rate. Children younger than one year of age and those between one and four years of age were at a higher risk of S enteritidis infection than adults between 15 and 39 years of age (29–31).
Global assessment of the occurrence of reported enteric pathogen cases revealed similar incidence rates in several other studies, with the highest incidence rates of gastroenteritis being in children zero to four years of age (32–35). The reasons behind the higher incidence rates in children for illnesses linked to these pathogens are unclear. However, three broad sets of factors may cause much higher incidence rates of reports in young children for these enteric diseases: greater contact, exposure and infectious doses directly linked to specific behaviours of young children and their interaction with other people and their environment (30,31); greater susceptibility of young children to become sick attributable to gut immaturity and immune system naivety; and greater reporting fraction (less under-reporting) in this age group due to parental concerns and greater severity of symptoms. Indeed, Glass et al (36) estimated that 16.5 million children younger than five years of age experience between 21 and 37 million episodes of diarrhea annually. Of these, 2.1 to 3.7 million lead to a physician visit, a total of 220,000 patients are hospitalized and 325 to 425 children die. The major cost of diarrhea is attributable to the high number and cost of hospitalizations, because approximately 10.6% of hospitalizations in this age group are due to diarrhea (36). Although our study did not investigate these factors and their relative contributions to the final incidence rates of reported cases, these data suggest that preventive and interventional measures aimed specifically at children could bring the greatest benefit with respect to reducing the disease burden caused by these pathogens in the Quebec population.
An interesting relationship seems to emerge in the present study between the incidence rates of Salmonella and Campylobacter infections and the population size of the municipalities where the reported cases resided. For municipalities of more than 10,000 people, our data suggests a proportional increase of reporting incidence rates with the size of the underlying population, which contrasts with municipalities of fewer than 10,000 inhabitants for which incidence rates of these reported cases seemed the highest. This ‘v-shape’ pattern in the relationship between incidence rates and population sizes leads us to believe that there may be two broad influences associated with this factor: one relating to the environmental exposure of populations living in small municipalities where water source, proximity to livestock or wildlife, and lifestyle may contribute to increase infections. This pattern may also be related to various parameters of social and material deprivation and ethno-cultural factors (genetic or behavioural) that seem to be linked to larger municipalities in Quebec. If we simplify the terminology by designating areas as ‘rural’, where municipalities have fewer than 10,000 inhabitants, and remaining areas as ‘urban’, we note large differences in the age structure of incidence rates of Campylobacter cases between the two areas. In rural areas, the incidence rates of Campylobacter cases are higher in almost every age and sex category, with the greatest difference observed in the zero- to four-year age group, in which rates in rural males were 7.3 times higher than in urban males, and 6.95 times higher in rural females compared with urban females (28). Any significant differences in the incidence rates of Salmonella cases were found to occur between urban and rural dwellers or between sexes (29–31). Socioeconomic conditions can explain differences between rural and urban settings, although the association of enteric pathogens (bacteria and protozoa) with low socioeconomic conditions is not clearly established.
Our results were consistent with these findings, but we found that the incidence rates of Salmonella cases were higher in urban areas of Montreal. This may be explained by a larger number of socioeconomically deprived children in urban areas (37). High incidence rates of Giardia cases have also been reported in urban areas, but spatial analysis revealed that the highest incidence rates around Montreal were in areas where agricultural activities were prominent. This is consistent with several studies performed in Ontario (26,38). It may also be explained by the increased awareness, reporting and access to health services in urban areas, which could account for the larger number of cases reported in those areas (26).
We also consistently demonstrated, similar to several other studies, a seasonal pattern peaking in early autumn for Giardia (26,27,39,40). The autumnal pattern could be explained by the long incubation period of Giardia cysts. Most of the Giardia infections also occurred during outdoor activities involving more contact with contaminated water and/or more personal contact between friends and families during the summer holidays.
Different clinical microbiology laboratories may use different methodologies and standards, and reporting differences may also exist. If a given area is served by several laboratories and some of these use more sensitive methodologies than others, this will be reflected in an uneven distribution of cases (41).
Outbreaks caused by the enteric pathogens mentioned in the present study may introduce a separate level of complication in the analyses. Our descriptive geographical analysis was performed with the sole objective of visualizing geographical areas with higher risk of reports. In such an instance, outbreaks need to be taken into account – their presence may bias case distribution because it relates to the local geographical factors (41). In the present study, only cases tagged as ‘sporadic’ were included for analysis and cases known to be related to outbreaks were registered in different databases that were not included in the study.
One common limit inherent to studies such as the present one, which uses public health surveillance data, relates to the under-reporting of cases compiled in administrative systems. Estimating the real magnitude of infections can present challenges when studying acute gastrointestinal infection risk factors, planning health programs and prioritizing health promotion. This bias has been studied in Ontario (42) and British Columbia (43). For each case of acute gastrointestinal infection reported in these provinces, an estimated 313 to 347 cases occur in the community, respectively. In another study, it was estimated that for every reported case of verotoxigenic E coli, Salmonella and Campylobacter, there were approximately 10 to 47, 13 to 37, and 23 to 49 cases, respectively, in the Canadian population (44). In our study, the incidence rates were certainly underestimated and should be considered as conservative measures. Although correcting these values with ratios published for other provinces could be performed, it is likely that there are systematic differences in underestimation fractions across the provinces when reporting cases to public health authorities; this should be further investigated before attempting such corrections. To date, no previous investigation of nonviral enteric gastroenteritis in children between zero and four years of age has been performed in Quebec, and results presented here should be interpreted with caution. Further population-based studies with individual data on risk factors are needed for a better understanding of the epidemiology of these conditions in young children in Canada.
Acknowledgments
The authors thank Professors Yves Brousseau and Marie-Hélène Vandersmissen, and Mr Serge Duchesneau, GIS technician from the Geography Department at Laval University, Laval, Quebec. They also thank Mr Stéfano Biondo, cartographic documents and geospatial data documentation adviser at the Laval University library, and Ms Stéphanie Brazeau and Mr Philippe Martin of the Université de Montreal (Montreal, Quebec). Furthermore, they thank the Directors of the Regional Public Health Departments of the Quebec Ministry of Health and Social Services for providing the data.
APPENDIX 1: DISEASES DEFINITION
Giardiasis
Confirmed case.
Presence of one of the two conditions:
Presence of cysts or trophozoits of Giardia lamblia in the stool, in the duodenal fluid or in the biopsy of small intestine; or
Detection of G lamblia antigens in the stool using the enzyme immunoassay or the direct fluorescent antibody technique.
Infection due to Campylobacter
Confirmed case.
Isolation of one of the Campylobacter species in an appropriate clinical sample.
Salmonellosis
Confirmed case.
Isolation of Salmonella (excluding Salmonella typhi or paratyphi) from an appropriate clinical sample.
Cryptosporidiosis
Confirmed case.
Presence of the following three conditions:
Presence of Cryptosporidium oocysts in stool;
Presence of Cryptosporidium in intestinal fluid or biopsy of the small intestine; or
Detection of Cryptosporidium antigen in stool using reverse passive hemagglutination assay or the enzyme immunoassay or the direct fluorescent antibody technique.
Gastroenteritis due to Yersinia enterocolitica
Confirmed case.
Presence of the following four conditions:
Isolation of Y enterocolitica in an appropriate clinical specimen;
Compatible clinical manifestations and detection of O antigens with a serological titration level of 40 or greater;
Compatible clinical manifestations and detection of OH antigens with a serological titration level of 160 or greater; or
Compatible clinical manifestations and detection of a significant increase of antigens O or OH between serums collected in acute phase and collected in the convalescent.
Invasive infection due to Escherichia coli
Confirmed case.
Presence of the following conditions:
Manifestations compatible with hemolytic uremic syndrome or thrombotic thrombocytopenic purpura; and
Isolation of Escherichia coli O157: H7: O157 serotype or any other verocytotoxin producer in an appropriate clinical specimen.
Footnotes
SUPPORT: The present research was jointly supported by the Fonds québécois de la recherche sur la nature et les technologies (FQRNT) and the Public Health Agency of Canada. The research work was performed at the Institut national de santé publique du Québec.
REFERENCES
- 1.Edgeworth J. Bacterial gastroenteritis. Medicine. 2005;33:73–7. [Google Scholar]
- 2.Black RE. Epidemiology of diarrheal disease: Implications for control by vaccines. Vaccine. 1993;11:100–6. doi: 10.1016/0264-410x(93)90002-f. [DOI] [PubMed] [Google Scholar]
- 3.Graeme L, Eric U, Kerrie B, Ruth F. Aetiology of acute gastroenteritis in hospitalized children in Melbourne, Australia, from April 1980 to March 1993. J Clin Microbiol. 1998;6:133–8. doi: 10.1128/jcm.36.1.133-138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen M. Etiology and mechanisms of acute infectious diarrhea in infants in the United States. J Pediatr. 1991;118:S34–S39. doi: 10.1016/s0022-3476(05)81423-4. [DOI] [PubMed] [Google Scholar]
- 5.Stutman H. Salmonella, Shigella, and Campylobacter: Common bacterial causes of infectious diarrhea. Pediatr Ann. 1994;23:538–43. doi: 10.3928/0090-4481-19941001-07. [DOI] [PubMed] [Google Scholar]
- 6.Besser R, Griffin P, Slutsker L. Escherichia coli O157:H7 gastroenteritis and the hemolytic uremic syndrome: An emerging infectious disease. Annu Rev Med. 1999;50:355–67. doi: 10.1146/annurev.med.50.1.355. [DOI] [PubMed] [Google Scholar]
- 7.Lee L. Yersinia enterocolitica O:3: An emerging cause of pediatric gastroenteritis in the United States. J Infect Dis. 1991;163:660–3. doi: 10.1093/infdis/163.3.660. [DOI] [PubMed] [Google Scholar]
- 8.Abdel-Haq N, Asmar B, Abuhammour W, Brown W. Yersinia enterocolitica infection in children. Pediatr Infect Dis J. 2000;19:954–8. doi: 10.1097/00006454-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Furness B, Beach M, Roberts J. Giardiasis surveillance – United States, 1992–1997. MMWR CDC Surveill Summ. 2000;49:1–13. [PubMed] [Google Scholar]
- 10.Croll N, Gyorkos T. Parasitic disease in humans: The extent in Canada. CMAJ. 1979;120:310–2. [PMC free article] [PubMed] [Google Scholar]
- 11.Dietz V, Roberts J. National surveillance for infection with Cryptosporidium parvum, 1995–1998: What have we learned? Public Health Rep. 2000;115:358–63. doi: 10.1093/phr/115.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Addiss D, Davis J, Roberts J, Mast E. Epidemiology of Giardiasis in Wisconsin: Increasing incidence of reported cases and unexplained seasonal trends. Am J Trop Med Hyg. 1992;47:13–9. doi: 10.4269/ajtmh.1992.47.13. [DOI] [PubMed] [Google Scholar]
- 13.Isaac-Renton J, Philion J. Factors associated with acquiring Giardiasis in British Columbia residents. Can J Public Health. 1992;83:155–8. [PubMed] [Google Scholar]
- 14.Fourquet F, Desenclos J, Maurage C, Baron S. Acute gastro-enteritis in children in France: Estimates of disease burden through national hospital discharge data. Arch Pediatr. 2003;10:861–8. doi: 10.1016/s0929-693x(03)00459-7. [DOI] [PubMed] [Google Scholar]
- 15.Creese AL. Cost effectiveness of potential immunization interventions against diarrhea disease. Soc Sci Med. 1986;23:231–40. doi: 10.1016/0277-9536(86)90343-6. [DOI] [PubMed] [Google Scholar]
- 16.MSSS Surveillance des maladies à déclaration obligatoire au Québec. Rapport annuel. 2002;2005:1–104. [Google Scholar]
- 17.Allos B, Taylor D. Campylobacter infections. Evans A, Brachman P, editors. Bacterial Infections of Humans. Epidemiol Med Hyg. 1998;88:395–9. [Google Scholar]
- 18.Samuel M, Vugia D, Shallow S, et al. Epidemiology of sporadic Campylobacter infection in the United States and declining trend in incidence, FoodNet 1996–1999. Clin Infect Dis. 2004;38:S165–S174. doi: 10.1086/381583. [DOI] [PubMed] [Google Scholar]
- 19.MSSS Surveillance des maladies à déclaration obligatoire au Québec: définitions nosologiques – maladies d’origine infectieuse. 7ème Édition. 2008. pp. 1–115.
- 20.Anselin L.GeoDa 095iUniversity of Illinois<http://geodauiucedu/defaultphp> (consulté en janvier 2009).
- 21.Ministère de la santé et des services sociaux du Québec Surveillance des maladies à déclaration obligatoire au Québec. Rapport annuel. 2002;2005:1–104. [Google Scholar]
- 22.Koehler K, Lasky T, Fein S, et al. Population-based incidence of infection with selected bacterial enteric pathogens in children younger than five years of age, 1996–1998. Pediatr Infect Dis J. 2006;25:129–34. doi: 10.1097/01.inf.0000199289.62733.d5. [DOI] [PubMed] [Google Scholar]
- 23.Louchini R, Douville-Fradet M. Surveillance des maladies infectieuses et des intoxicatioins chimiques à déclaration obligatoire au Québec, de 1990 à 1999. Ministère de la Santé et des Services sociaux, Gouvernement du Québec. 2001. pp. 1–279.
- 24.Agence de la santé publique du canada Éclosion d’infections à salmonella paratyphi B liée à des aquariums dans la province de Québec, 2000. Relevé des maladies transmissibles au canada. 2002;28:89–96. [PubMed] [Google Scholar]
- 25.MSSS Surveillance des maladies à déclaration obligatoire au Québec: guide de saisie des données du registre central des maladies à déclaration obligatoire. DGSP, BSE. 2004. pp. 1–309.
- 26.Greig J, Michel P, Wilson J, Lammerding AM, et al. A descriptive analysis of Giardiasis cases reported in Ontario, 1990–1998. Can J Public Health. 2001;92:361–5. doi: 10.1007/BF03404980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laupland K, Church D. Population-based laboratory surveillance for Giardia sp. and Cryptosporidium sp. infections in a large Canadian health region. BMC Infect Dis. 2005;5:72. doi: 10.1186/1471-2334-5-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green CG, Krause DO, Wylie JL. Spatial analysis of Campylobacter infection in the Canadian province of Manitoba. Int J Health Geogr. 2001;5:1–2. doi: 10.1186/1476-072X-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Younus M, Wilkins MJ, Arshad MM, Rahbar MH, Saeed AM. Demographic risk factors and incidence of Salmonella enteritidis infection in Michigan. Foodborne Path Dis. 2006;3:266–73. doi: 10.1089/fpd.2006.3.266. [DOI] [PubMed] [Google Scholar]
- 30.Arshad MM, Wilkins MJ, Downes FP, et al. A registry-based study on the association between human salmonellosis and routinely collected parameters in Michigan, 1995–2001. Foodborne Path Dis. 2007;4:16–25. doi: 10.1089/fpd.2006.48. [DOI] [PubMed] [Google Scholar]
- 31.Arshad MM, Wilkins MJ, Downes FP, et al. Epidemiologic attributes of invasive non-typhoidal Salmonella infections in Michigan, 1995–2001. Int J Infect Dis. 2008;12:176–82. doi: 10.1016/j.ijid.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Sturkenboom M, Soriano-Gabarró M, Gino P, et al. Incidence and outcomes of acute gastroenteritis in Italian children. Paediatr Infect Dis J. 2008;27:S42–S47. [Google Scholar]
- 33.Majowicz SE, Horrocks J, Bocking K. Demographic determinants of acute gastrointestinal illness in Canada: A population study. BMC Public Health. 2007;7:162. doi: 10.1186/1471-2458-7-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heyworth JS, Baghurst P, McCaul KA. Prevalence of gastroenteritis among 4-year-old children in South Australia. Epidemiol Infect. 2003;130:451. [PMC free article] [PubMed] [Google Scholar]
- 35.de Wit MA, Koopmans MP, Kortbeek LM, et al. Sensor, a population-based cohort study on gastroenteritis in the Netherlands: Incidence and aetiology. Am J Epidemiol. 2001;154:666–74. doi: 10.1093/aje/154.7.666. [DOI] [PubMed] [Google Scholar]
- 36.Glass R, Lew J, Gangarosa R, Le Baron C, Ho M. Estimates of morbidity and mortality rates for diarrheal diseases in American children. J Pediatr. 1991;118:27–33. doi: 10.1016/s0022-3476(05)81422-2. [DOI] [PubMed] [Google Scholar]
- 37.Simonsen J, Frisch M, Ethelberg S. Socioeconomic risk factors for bacterial gastrointestinal infections. Epidemiology. 2008;19:282–90. doi: 10.1097/EDE.0b013e3181633c19. [DOI] [PubMed] [Google Scholar]
- 38.Odoi A, Martin SW, Michel P, Middleton D, Holt J, Wilson J. Investigation of clusters of Giardiasis using GIS and a spatial scan statistic. Int J Health Geogr. 2004;3:11. doi: 10.1186/1476-072X-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naumova E, Jagai J, Matyas B, DeMaria A, Jr, MacNeill I, Griffiths J. Seasonality in six enterically transmitted diseases and ambient temperature. Epidemiol and Infect. 2007;135:281–92. doi: 10.1017/S0950268806006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoque E, Hope V, Scragg R, Baker M, Shrestha R. A descriptive epidemiology of giardiasis in New Zealand and gaps in surveillance data. J NZ Med Assoc. 2004;117:1205. [PubMed] [Google Scholar]
- 41.Jepsen MR, Simonsen J, Ethelberg S. Spatio-temporal cluster analysis of the incidence of Campylobacter cases and patients with general diarrhea in a Danish county, 1995–2004. Int J Health Geogr. 2009;8:1–11. doi: 10.1186/1476-072X-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Majowicz S, Edge V, Fazil A, et al. Estimating the under-reporting rate for infectious gastrointestinal illness in Ontario. Can J Public Health. 2005;96:178–81. doi: 10.1007/BF03403685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacDougall L, Majowicz S, Dore K, et al. Under-reporting of infectious gastrointestinal illness in British Columbia Canada: Who is counted in provincial communicable disease statistics? Epidemiol Infect. 2008;136:248–55. doi: 10.1017/S0950268807008461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thomas MK, Majowicz SE, Sockett PN, et al. Estimated numbers of community cases of illness due to Salmonella, Campylobacter and verotoxicogenic Escherichia coli: Pathogen-specific community rates. Can J Infect Dis Med Microbiol. 2006;17:229–34. doi: 10.1155/2006/806874. [DOI] [PMC free article] [PubMed] [Google Scholar]