Abstract
Objectives
Esophagectomy is the standard treatment for T1 esophageal cancer (EC). With an increasing interest in endoscopic therapies particularly for T1 EC, our objectives were to evaluate the long term outcomes following esophagectomy and to examine the pathological features of T1 cancer in detail to determine the suitability for potential endoscopic therapy.
Methods
We reviewed the outcomes of esophagectomy in 100 consecutive patients with T1 EC. The primary endpoints studied were overall survival (OS) and disease-free survival (DFS). In addition to detailed pathology review, we evaluated prognostic variables associated with survival.
Results
Esophagectomy was performed in 100 patients (79 men, 21 women; median age 68 years) for T1 EC (adenocarcinoma 91, squamous 9; intramucosal (T1a):29, submucosal (T1b):71). The 30 day mortality was 0%. Resection margins were microscopically negative in 99% (99/100) of patients. N1 disease was present in 21 patients (T1a:2/29(7%); T1b:19/71(27%)), associated high-grade dysplasia in 64/100 (64%) and angiolymphatic invasion in 19/100 (19%) of patients. At a median follow-up of 66 months, estimated 5-year OS and 3-year DFS were 62% and 80%, respectively, for all patients (including N1). Nodal status and tumor size were significantly associated with overall survival and disease-free survival, respectively.
Conclusions
Esophagectomy can be performed safely in patients with T1 cancer with good long term results. Many patients with T1 EC have several risk factors which may preclude adequate treatment with endoscopic therapy. Further prospective studies are required to evaluate endoscopic therapies. Esophagectomy should continue to remain the standard treatment in patients with T1 EC.
Introduction
The incidence of esophageal carcinoma has increased dramatically over the past three decades. In the United States, adenocarcinoma now surpasses squamous cell carcinoma as the most common histological sub-type. This epidemiological shift is thought to be secondary to changes in dietary habits, lifestyle, and an increase in the incidence of obesity, gastroesophageal reflux disease and Barrett’s esophagus (BE)(1). The outcome for patients with esophageal carcinoma continues to be poor with a 5-year survival rate of 14% (1). With increasing use of endoscopic surveillance and early detection, more early stage T1 cancers are being diagnosed (2). These early stage cancers present the best opportunity for cure.
Esophagectomy is the standard treatment for T1 esophageal cancer (EC) and, although debated, should be strongly considered in patients with high-grade dysplasia (HGD) due to the presence of occult cancer in about 40% of patients (3). Recently, however, there has been increasing interest in endoscopic therapies particularly for T1 EC. These modalities include ablative techniques such as photodynamic therapy (PDT) and endoscopic mucosal resection (EMR)(4, 5). Ell and colleagues recently reported on the use of EMR with or without PDT for T1 intramucosal cancer in 100 patients (5). However, in their report, only patients with low-risk criteria were included, and patients with tumor diameter greater than 2 cm, angiolymphatic invasion, or poorly differentiated tumors were all determined to be unsuitable for endoscopic therapy and were excluded. While the emerging endoscopic therapies are of great interest, the adoption of these new endoscopic therapies must take into account an understanding of the pathology and the behavior of the tumors. We analyzed potential prognostic variables in the context of consideration for endoscopic therapies.
The objectives of this study were to a) evaluate the long term results of surgical resection for management of the full spectrum of T1 esophageal cancer and b) identify the prognostic factors, with particular attention to pathological features, to determine the potential suitability of endoscopic therapies.
Methods
We reviewed our experience retrospectively with 100 consecutive, pathologically proven T1 esophageal tumors treated with esophagectomy at the University of Pittsburgh Medical Center from 1995 to 2004. Patients who underwent neoadjuvant therapy or who had distant metastases were excluded. This study was approved by the Institutional Review Board of the University of Pittsburgh. Since this was a retrospective study, individual consent was waived.
Staging and Surgical Procedures
All patients underwent a complete history and physical examination. Other staging studies were: bronchoscopy for mid-esophageal lesions, esophagogastroduodenoscopy (EGD), endoscopic ultrasound (EUS), and computed tomography (CT) scan. Positron emission tomography (PET) scans were performed selectively in this series. The type and approach of esophageal resection was at the discretion of the operating surgeon and comprised the following approaches: (1) transhiatal esophagectomy, (2) thoracic approach combined with a laparotomy or laparoscopy, with a cervical or intrathoracic anastomosis. Our technique for a minimally invasive approach has been detailed previously (6). Patients were monitored during visits to the thoracic surgery clinic. The current follow-up schedule in the clinic is: 2 weeks after discharge, then every 3 months for two years, followed by every 6 months for 2 additional years and then annually.
Pathology Evaluation
The standard procedure for processing esophagectomy specimens at our institution includes immediate assessment of the specimen to measure tumor dimensions and obtain frozen sections of margins, followed by formalin fixation overnight. For T1 tumors, when a mass is seen grossly, the entire mass is submitted for histologic examination along with thorough sampling of any remaining Barrett’s segment. When no mass is seen grossly, the entire Barrett’s segment is submitted for histologic examination. The pathology reports were examined in detail. T1 esophageal cancers were further subdivided as follows: T1a (intramucosal) – no invasion beyond the muscularis mucosae and T1b (submucosal) - carcinoma with invasion into but not beyond the submucosa. Other variables which were examined were tumor type, tumor grade, tumor size/length, nodal status, LVI, and associated BE and HGD.
Quality of Life
We also assessed the quality of life by administering the Gastroesophageal Reflux disease-Health Related Quality of Life (GERD-HRQOL) questionnaire (7). This is a disease-specific instrument consisting of 9 questions (recently expanded to 10) related to heartburn, regurgitation, dysphagia, and bloating, with responses from 0 to 5.The best possible score (no symptoms) is 0 and the worst possible score (most severe symptoms) is 50. We classified HRQOL scores as excellent (0–9), satisfactory (10–15), or poor (16–50) (6).
Statistical Design and Analysis
The primary endpoints of the study were to determine the disease-free survival (DFS) and overall survival (OS). Kaplan-Meier plots were constructed using Greenwood confidence limits for estimation of OS and DFS. Disease-free survival was computed as the time from diagnosis to disease recurrence or death among patients who were rendered disease-free. The log rank test was used to analyze differences between the groups. Log rank p values were adjusted by the Step-down Bonferroni method. Tumor depth (stage T1a or T1b) was cross-classified with other covariates and tested for association with either the chi square test for 2 × 2 tables or the Cochran - Armitage test for trend across categories. In addition, analysis of individual covariates predictive of survival was performed with Cox proportional hazards regression method.
Results
1. Patient Characteristics
Esophagectomy was performed in 100 consecutive patients with a median age of 68 years. There were a total of 79 men and 21 women. The histology was adenocarcinoma in 91% (91/100) and squamous cell carcinoma in 9% (9/100). The tumor was intramucosal (T1a) in 29 patients and submucosal (T1b) in 71 patients. The patient characteristics are summarized in Table 1. There were 12 patients who had a preoperative diagnosis of HGD. Of these patients, 4 patients had T1a (intramucosal) neoplasms, and 8 patients had submucosal lesions in the final pathology specimen
Table 1.
Patient Characteristics
| Gender: Male : Female 79 : 21 | |
| Median Age: 68 years (range: 42 – 83) | |
| T Stage | |
| • T1 a | 29 |
| • T1 b | 71 |
| N Stage | |
| N0: | 79 |
| N1: | 21 |
| Histology | |
| • Adenocarcinoma | 91 |
| • Squamous | 9 |
| Tumor Length: median: 2 cm | |
| Associated Barrett’s Esophagus (BE): 84 | |
| Associated High-grade dysplasia (HGD): 64 | |
| Angiolymphatic Invasion (LVI): 19 | |
2. Staging
All 100 patients were staged with a CT scan (100%); EUS was performed in 91% (91/100) of patients. PET scans were used selectively. In addition, when performing a minimally invasive esophagectomy, our initial step was to perform a laparoscopic exploration, similar to an exploratory laparotomy, prior to esophagectomy. Any suspicious lesions in the liver and peritoneum were biopsied. Both the T and N status were assessed preoperatively primarily by the EUS examination.
3. Surgery and Adjuvant therapy
Esophagectomy with an abdominal and thoracic approach with an intrathoracic or cervical anastomosis was performed in most patients. A McKeown-type, 3-incision esophagectomy was performed in 77 patients, and an intrathoracic anastomosis was performed in 5 patients. A transhiatal esophagectomy was performed in 18 patients. A minimally invasive approach was used in 80 patients (80%). The 30-day operative mortality was zero (0%). The resection margins were microscopically negative (R0) in 99 of the 100 patients (99%). Adjuvant therapy was administered in 15 patients (all with N1 disease).
4. Pathological Evaluation
T1a tumors were noted in 29 patients and T1b tumors were noted in 71 patients. Nodal metastasis (N1) was present in 21 patients (21%). Among patients with T1a tumors, 2/29 (7%) had N1 disease and among patients with T1b tumors, 19/71 (27%) had nodal metastases. The other variables examined were associated HGD, which was present in 64/100(64%), and LVI which was present in 19/100 (19%) of patients. Other prognostic variables examined were tumor length, histology and differentiation. The median tumor length was 2 cm. The pathologic features are summarized in Table 1.
5. Association of depth of invasion with prognostic variables
The association between the depth of invasion (T1a vs. T1b) and nodal status, size, LVI, HGD and degree of differentiation was analyzed. Interestingly, T1a tumors were more likely to be associated with HGD. In addition, T1b lesions were more likely to be N1, more likely to be bigger tumors, and more likely to be associated with angiolymphatic invasion (P<0.05). These results are summarized in Table 2.
Table 2.
Association of Depth with Other Prognostic Factors
| Depth | Total | ||||
|---|---|---|---|---|---|
| T1a | T1b | ||||
| N Stage |
0 | 27 | 52 | 79 | Fisher’s Exact p = .0308 adjusted p = .0616 |
| 1 | 2 | 19 | 21 | ||
| Total | 29 | 71 | 100 | ||
| Depth | Total | ||||
|---|---|---|---|---|---|
| T1a | T1b | ||||
| Tumor Size |
2 cm + | 6 | 44 | 50 | Fisher’s Exact p = .0002 adjusted p = .0010 |
| < 2 cm | 21 | 24 | 45 | ||
| Total | 27 | 68 | 95 | ||
| Depth | |||||
|---|---|---|---|---|---|
| T1a | T1b | Total | |||
| High Grade Dysplasia |
Yes | 25 | 39 | 64 | Fisher’s Exact p = .0030 adjusted p = .0090 |
| No | 4 | 32 | 36 | ||
| Total | 29 | 71 | 100 | ||
| Depth | Total | ||||
|---|---|---|---|---|---|
| T1a | T1b | ||||
| Angiolymphatic Invasion |
Yes | 0 | 19 | 19 | Fisher’s Exact p = .0013 adjusted p = .0052 |
| No | 29 | 52 | 81 | ||
| Total | 29 | 71 | 100 | ||
| Depth | Total | ||||
|---|---|---|---|---|---|
| T1a | T1b | ||||
| Differentiation | Well | 5 | 9 | 14 | Cochran–Armitage Trend Test p = .3263 |
| Moderate | 14 | 29 | 43 | ||
| Poor | 8 | 28 | 36 | ||
| Total | 27 | 66 | 93 | ||
6. Analysis of Overall Survival and Recurrence
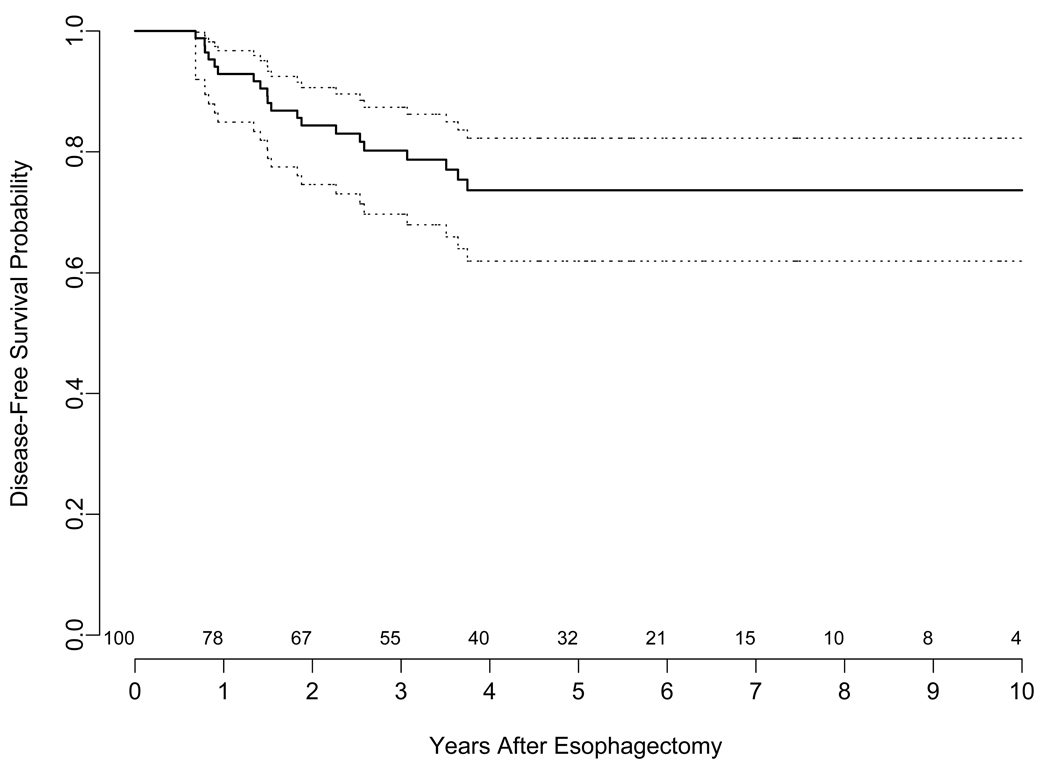
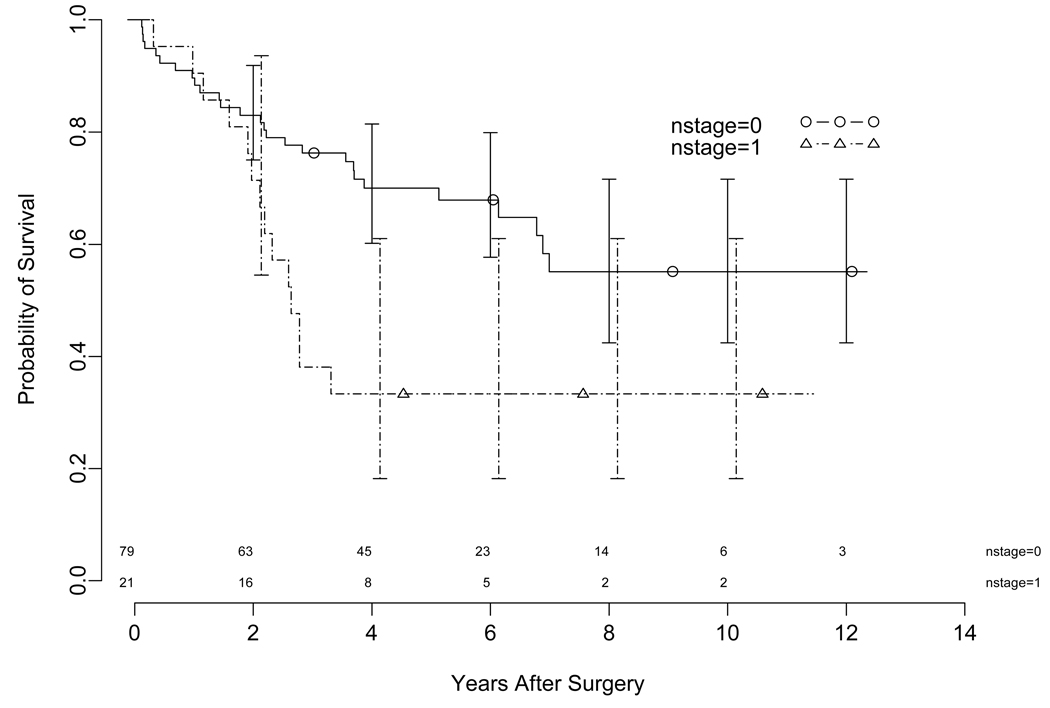
At a median follow-up of 66 months (range 1.2– 144 months), estimated 5-year overall survival (OS) for the entire cohort including N1 patients, was 62 % (95 CI 53%–73%). The median overall survival was 84 months (95% CI 73.2 - NR). There were a total of 20 patients who developed recurrence. The site of recurrence was predominantly distal and only 2 patients had local recurrence only. The estimated 3-year disease free survival (DFS) for all patients (including N1) was 80% (Figure 1). The median DFS has not been reached. The estimated 5-year overall survival for T1a (N0 and N1) and T1b (N0 and N1) were 73% and 60%, respectively. The estimated 5-year overall survival for patients with N0 and N1 were 70% and 35%, respectively (Figure 2).
Figure 1.
Kaplan-Meier Plot illustrating the disease-free survival for the entire group of 100 patients after esophagectomy with confidence limits. The time is shown on the x axis in years. The dotted lines are 95% confidence limits for the probability of disease-free survival. The number of patients at risk at the start of each year is shown above the x-axis.
Figure 2.
Kaplan-Meier Plot illustrating the overall survival stratified by N status with confidence limits. The time is shown on the x axis in years. The error bars are 95% confidence bands for the probability of overall survival (Log Rank P= 0.0057). The number of patients at risk at the start of each year is shown above the x axis.
7. Analysis of Prognostic Factors for Survival
Analysis of various covariates predictive of OS showed significance for nodal status; Tumor size as a continuous variable was a significant predictor of DFS. Tumor length/size were significantly associated with DFS (P<0.05) (Figure 3). Multivariate analysis showed that tumor length (≥2cm vs. <2 cm) was a significant predictor of disease free survival (hazard ratio 5.6 (CI 1.41 to 18.89; P value = 0.013).
Figure 3.
Kaplan-Meier Plot illustrating the disease-free survival with confidence limits stratified by size of the tumor. The time is shown on the x axis in years. The error bars are 95% confidence bands for the probability of disease-free survival (Log Rank P= 0.0012). The number of patients at risk at the start of each year is shown above the x axis.
8. Quality of Life
The GERD-HRQOL questionnaire was administered in 47 patients at a mean of 48.2 months (median 37.6 months). The mean and median scores were 3.66 and 3 respectively (range 0–13). This median score of 3.00 represents a normal score. HRQOL scores were excellent in 42 patients (89.4%; 42/47), and satisfactory in 5 patients (10.6%;5/47). These results suggest that the quality of life was preserved in all patients in whom GERD-related quality of life was studied.
Comment
The incidence of EC has increased dramatically over the past three decades (1). Early stage adenocarcinoma is more frequently encountered in clinical practice (2, 8). In light of emerging endoscopic therapies for early stage cancers, we have analyzed the results of surgical resection for T1 esophageal cancers in 100 consecutive patients. Our data show that esophagectomy is both safe and effective. The perioperative mortality was low and demonstrates the safety of primary surgery for early stage disease. Furthermore, at a median follow-up of 66 months, the estimated three year disease-free survival (including node positive patients; 21%) was 80% , demonstrating the efficacy of this approach. In addition, nodal metastasis was associated with overall survival and tumor size/length was significantly associated with disease free survival.
Liu and colleagues analyzed the results in 90 patients with T1 cancer and reported that LVI was significant in predicting overall survival and recurrence (9). Rice and colleagues have described the association of increased nodal involvement with increasing depth of invasion (2). They reported the results of surgical treatment in 122 patients, of which 38 had HGD, and 76 had T1 cancers (2). In their cohort, which included HGD, the estimated 5-year overall survival was 77%. Our results in the present series of 100 patients, where we have not included HGD, are comparable.
Endoscopic mucosal resection (EMR) has been used in Japan for early gastric neoplasm, and is now being used for the treatment of HGD and early esophageal cancer. In a large series of pathological analyses of EMR specimens from an experienced center, R0 resections were done in only 75% (4). Further, there was a very high incidence of recurrences (30%), even in experienced centers (10). In a very interesting recent updated study by Ell and colleagues, the results of EMR (with PDT in 49%) in100 highly selected patients with T1 intramucosal cancer (out of 667 patients referred for early adenocarcinoma or HGD) were reported (5). The selection criteria for inclusion in this study by Ell and colleagues included diameter less than 2 cm, macroscopic type I, IIa-b, or IIc lesions less than 1 cm, no angiolymphatic invasion, and histologic grades G1 and G2 [well differentiated and moderately differentiated, respectively]arising from BE. The lateral margins of resection were positive in 34% of patients, and could not be assessed in 33% of patients. During follow-up (median 33 months), recurrent or metachronous lesions were detected in 11% of patients. The estimated overall survival at 3 years was 98%. The survival results were encouraging in this highly selected group of patients. However, of concern, is the high rate of positive margins and recurrent or metachronous lesions during follow-up, even in this select group with intramucosal tumors and low risk features.
In another report of EMR, there was a high incidence of incomplete resection, and persistence/recurrence, pointing to the need for further studies with long term follow-up to define the role of EMR in the treatment of early neoplasm (11). In comparison, the current study which includes all patients with T1 disease, including those with adverse prognostic factors, shows that an R0 resection was accomplished in 99% of patients with T1 cancer, and, with a median follow-up of 66 months, the 3-year disease-free survival was 80%, and very few patients had recurrence at the local site only. Although this is not a randomized comparison, and definitive conclusions cannot be made, these results of local control argue in favor of surgery. In addition, some investigators have also shown that genetic abnormalities persist in the mucosa after PDT for dysplasia (12). Therefore, the role of combination of PDT and EMR in the treatment of esophageal neoplasm also needs to be defined.
Staging and Diagnosis
It is generally accepted that the presence of submucosal invasion in T1 cancers is best treated with surgical resection (5, 13). However, even with incorporation of high resolution EUS, the current staging modalities are not accurate in the assessment of submucosal invasion. In a prospective and blinded study, May and colleagues investigated the accuracy of high resolution endoscopy and high resolution EUS (20MHz) in the staging of early esophageal cancer (10). Although the sensitivity to detect intramucosal lesions was high (90%), the sensitivity for detection of submucosal tumors was only 48% with EUS and 56% with endoscopy, and a combination of these resulted in a sensitivity of only 60%. Given the limitations of even high resolution EUS in detection of submucosal lesions, EMR may be a useful staging modality. Recently, Maish, DeMeester and colleagues reported this approach to evaluate the depth of the lesion (11). They point out that analysis of EMR specimens gives a more reliable estimate of the depth of invasion and allows decision-making on the approach and extent of esophagectomy.
There are also significant difficulties with the preoperative diagnosis of HGD (3). In addition, another aspect, which is of concern in the pathological diagnosis, is the duplication of the muscularis mucosa, a characteristic finding in BE, that can pose difficulty in proper staging of T1a cancers (16). In a recent multi-institutional study of 50 T1a adenocarcinoma patients, this phenomenon of duplication of the muscularis mucosa was seen in 92% of patients. Of the 30 patients who had cancer involving the duplicated muscularis mucosa, LVI was present in 17% of patients and nodal metastases were present in 10%. Invasion into the duplicated muscularis mucosa can indicate aggressive properties, despite categorization as an intramucosal T1a adenocarcinoma, and is a cause of concern.
Prognostic Variables in T1 esophageal cancer
It is important to consider several factors before deciding on the appropriate mode of therapy (17). Our analysis of association of depth of invasion (T1a vs. T1b) showed that T1b tumors had a significantly increased incidence of N1, were more likely to be bigger tumors, and were more likely to be associated with LVI (P<0.05). Although it is commonly stated that endoscopic therapies such as EMR and PDT are applicable to T1 lesions in general and intramucosal lesions in particular because of the low risk of lymph node metastases, several important limitations should be emphasized. In addition to lymph node metastases, other factors of adverse prognostic significance include LVI and tumor length. Further, leaving mucosa at risk with BE, particularly with associated HGD, may predispose for future recurrent or metachronous cancer. In fact, early stage esophageal cancer presents the best opportunity for a cure. Surgical resection is the standard wherein the neoplasm along with the mucosa at risk is eliminated. In this study, we have also identified size/ length as an important prognostic variable in resected T1 esophageal cancer. These data may support the initiation of trials of adjuvant therapy protocols for resected early stage esophageal cancer.
Oh, in an analysis of 78 patients with intramucosal carcinoma treated with esophagectomy, pointed out that 32% of patients with intramucosal cancer had no visible lesion to target, the location of the neoplasm did not correspond to the visible lesion on endoscopy in 8%, and multifocal cancer was present in 12% of patients, leading them to conclude that endoscopic therapies are not applicable in nearly one-third of patients with intramucosal cancer (8). Recently Altorki and colleagues reported an analysis in 75 patients with T1 esophageal cancer (18). Nodal metastases were present in 6% of T1a and 17.5% of T1b tumors. Collectively, 10 of 30 (33.3%) patients with T1a and 25 of 45 (55%) with T1b had multifocal neoplasia, LVI, or nodal metastases. These authors concluded that combined high incidence of multifocal neoplasia, LVI, and occult nodal metastases does not support the use of endoscopic therapy in patients with T1 esophageal cancer regardless of depth of invasion, cell type, differentiation or extent of BE and suggested that these therapies be reserved for high risk patients.
Quality of life
In an effort to decrease the morbidity and mortality and preserve the quality of life after esophagectomy, we have adopted a minimally invasive approach. In the current series, the quality of life as measured by the GERD-HRQOL was excellent in a majority of patients. DeMeester and colleagues have proposed a vagal sparing esophagectomy in patients with intramucosal cancer to decrease the morbidity associated with esophagectomy (8). One of the drawbacks of our study is that pre- and postoperative scores were not examined. We are, at present, prospectively evaluating the quality of life in patients who undergo esophagectomy.
Decreasing the risks of esophagectomy
Despite the potential benefits of esophagectomy, one of the main concerns for recommendation of esophagectomy, however, is the mortality associated with the procedure (19). One of the important factors in lowering the risk of esophagectomy is the hospital volume and experience of the surgeon doing the esophagectomy (19, 20). There are now several studies which have addressed mortality in high-volume centers vs. low-volume centers for esophagectomy (19,21,22). However, the reported mortality after esophagectomy in these reports encompasses all stages, including locally advanced cancers and cancers in patients who are malnourished. These results of mortality may not be applicable to patients with early stage esophageal cancers and cannot be the basis for justification of endoscopic therapy with an unknown long-term oncologic efficacy in early stage esophageal cancer patients where there surgery provides the best opportunity for cure. In fact, in this series of 100 consecutive patients, the 30-day mortality was 0%, and similar low mortality figures have been reported from other centers (2, 8, 17, 23, 24).
Conclusion
In summary, we report our experience in 100 consecutive patients who underwent esophagectomy for T1 esophageal carcinoma. T1 cancer represents the best chance for cure and esophagectomy can be performed safely in these patients in experienced centers with good long term results. Other modalities of treatment currently under active investigation are PDT and EMR. Many patients with T1 esophageal cancer may have one or more risk factors, including angiolymphatic invasion, increased length, associated high grade dysplasia, and nodal metastases, that may preclude adequate treatment with endoscopic therapy. These endoscopic techniques are, however, applicable in high-risk medically inoperable patients. While there is a great interest and enthusiasm for emerging endoscopic therapies, the adoption of these new endoscopic therapies must take into account an understanding of the pathology and the biology of these tumors. Surgeons should actively investigate these newer endoscopic modalities and further prospective studies evaluating newer endoscopic therapies are required to define optimal patient selection in this cohort of patients. Esophagectomy should continue to remain the standard treatment in patients with T1 esophageal cancer.
Acknowledgements
This work was supported in part from National Institute of Health NIH grant 5R01CA090665-09. The authors wish to thank Dr. Shannon Wyszomierski for her editorial assistance.
Footnotes
Presented at the Southern Thoracic Surgical Association, Bonita Springs, November 2007
References
- 1.Enzinger PC, Mayer RJ. Esophageal Cancer. N Engl J Med. 2003;349(23):2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 2.Rice TW, Blackstone EH, Goldblum JR, et al. Superficial adenocarcinoma of the esophagus. J Thorac Cardiovasc Surg. 2001 Dec;122(6):1077–1090. doi: 10.1067/mtc.2001.113749. [DOI] [PubMed] [Google Scholar]
- 3.Pennathur A, Landreneau RJ, Luketich JD. Surgical aspects of the patient with high-grade dysplasia. Semin Thorac Cardiovasc Surg. 2005;17(4):326–332. doi: 10.1053/j.semtcvs.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Vieth M, Ell C, Gossner L, May A, Stolte M. Histological analysis of endoscopic resection specimens from 326 patients with Barrett's esophagus and early neoplasia. Endoscopy. 2004 Sep;36(9):776–781. doi: 10.1055/s-2004-825802. [DOI] [PubMed] [Google Scholar]
- 5.Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer) Gastrointest Endosc. 2007 Jan;65(1):3–10. doi: 10.1016/j.gie.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 6.Luketich JD, Alvelo-Rivera M, Buenaventura PO, et al. Minimally invasive esophagectomy: outcomes in 222 patients. Ann Surg. 2003 Oct;238(4):486–494. doi: 10.1097/01.sla.0000089858.40725.68. discussion 494–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velonovich V, Vallance SR, Gusz JR, et al. Quality of life scale for gastroesophageal reflux disease. J Am Coll Surg. 1996;183:217–224. [PubMed] [Google Scholar]
- 8.Oh DS, Hagen JA, Chandrasoma PT, et al. Clinical biology and surgical therapy of intramucosal adenocarcinoma of the esophagus. J Am Coll Surg. 2006 Aug;203(2):152–161. doi: 10.1016/j.jamcollsurg.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Liu L, Hofstetter WL, Rashid A, Swisher SG, et al. Significance of the depth of tumor invasion and lymph node metastasis in superficially invasive (T1) esophageal adenocarcinoma. Am J Surg Pathol. 2005 Aug;29(8):1079–1085. [PubMed] [Google Scholar]
- 10.Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barretts esophagus. Gastroenterology. 2000;118:670–677. doi: 10.1016/s0016-5085(00)70136-3. [DOI] [PubMed] [Google Scholar]
- 11.Mino-Kenudson M, Brugge WR, Puricelli WP, et al. Management of superficial Barrett's epithelium-related neoplasms by endoscopic mucosal resection: clinicopathologic analysis of 27 cases. Am J Surg Pathol. 2005 May;29(5):680–686. doi: 10.1097/01.pas.0000154129.87219.fa. [DOI] [PubMed] [Google Scholar]
- 12.Krishnadath KK, Wang KK, Taniguchi K, Sebo, et al. Persistent genetic abnormalities in Barrett's esophagus after photodynamic therapy. Gastroenterology. 2000 Sep;119(3):624–630. doi: 10.1053/gast.2000.18012. [DOI] [PubMed] [Google Scholar]
- 13.Westerterp M, Koppert LB, Buskens CJ, et al. Outcome of surgical treatment for early adenocarcinoma of the esophagus or gastro-esophageal junction. Virchows Arch. 2005 May;446(5):497–504. doi: 10.1007/s00428-005-1243-1. [DOI] [PubMed] [Google Scholar]
- 14.May A, Günter E, Roth F, Gossner L, Stolte M, Vieth M, Ell C. Accuracy of staging in early oesophageal cancer using high resolution endoscopy and high resolution endosonography: a comparative, prospective, and blinded trial. Gut. 2004 May;53(5):634–640. doi: 10.1136/gut.2003.029421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maish MS, DeMeester SR. Endoscopic mucosal resection as a staging technique to determine the depth of invasion of esophageal adenocarcinoma. Ann Thorac Surg. 2004 Nov;78(5):1777–1782. doi: 10.1016/j.athoracsur.2004.04.064. [DOI] [PubMed] [Google Scholar]
- 16.Abraham SC, Krasinskas AM, Correa AM, et al. Duplication of the Muscularis Mucosae in Barrett Esophagus: An Underrecognized Feature and Its Implication for Staging of Adenocarcinoma. Am J Surg Pathol. 2007 November;31(11):1719–1725. doi: 10.1097/PAS.0b013e318093e3bf. [DOI] [PubMed] [Google Scholar]
- 17.Stein HJ, Feith M, Bruecher BL, Naehrig J, Sarbia M, Siewert JR. Early esophageal cancer: Pattern of lymphatic spread and prognostic factors for long term survival after surgical resection. Ann Surg. 2005 Oct;242(4):566–573. doi: 10.1097/01.sla.0000184211.75970.85. discussion 573–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altorki NK, Lee PC, Liss Y, et al. Multifocal neoplasia and nodal metastases in T1 esophageal carcinoma: implications for endoscopic treatment. Ann Surg. 2008;247(3):434–439. doi: 10.1097/SLA.0b013e318163a2ff. [DOI] [PubMed] [Google Scholar]
- 19.Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 20.Dimick JB, Goodney PP, Orringer MB, et al. Specialty training and mortality after esophageal cancer resection. Ann Thorac Surg. 2005;80:282–286. doi: 10.1016/j.athoracsur.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 21.Swisher SG, DeFord L, Merriman KW, et al. Effects of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg. 2000;119:1126–1134. doi: 10.1067/mtc.2000.105644. [DOI] [PubMed] [Google Scholar]
- 22.Dimick JB, Cattaneo SM, Lipsett PA, et al. Hospital volume is related to clinical and economic outcomes of esophageal resection in Maryland. Ann Thorac Surg. 2001;72:334–341. doi: 10.1016/s0003-4975(01)02781-3. [DOI] [PubMed] [Google Scholar]
- 23.Portale G, Hagen JA, Peters JH, et al. Modern 5-year survival of resectable esophageal adenocarcinoma: single institution experience with 263 patients. J Am Coll Surg. 2006 Apr;202(4):588–596. doi: 10.1016/j.jamcollsurg.2005.12.022. discussion 596–8. [DOI] [PubMed] [Google Scholar]
- 24.Orringer MB, Marshall B, Chang AC, Lee J, Pickens A, Lau CL. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg. 2007 Sep;246(3):363–372. doi: 10.1097/SLA.0b013e31814697f2. discussion 372–4. [DOI] [PMC free article] [PubMed] [Google Scholar]