Abstract
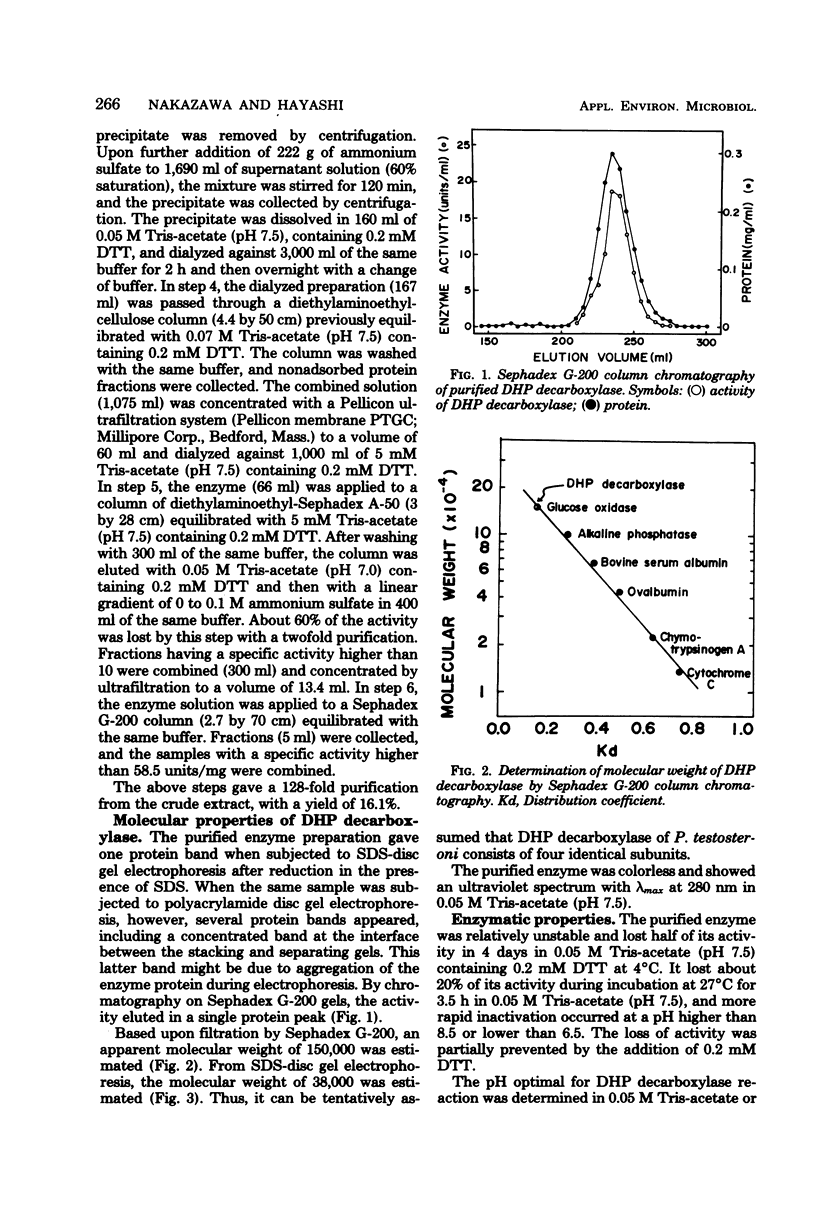
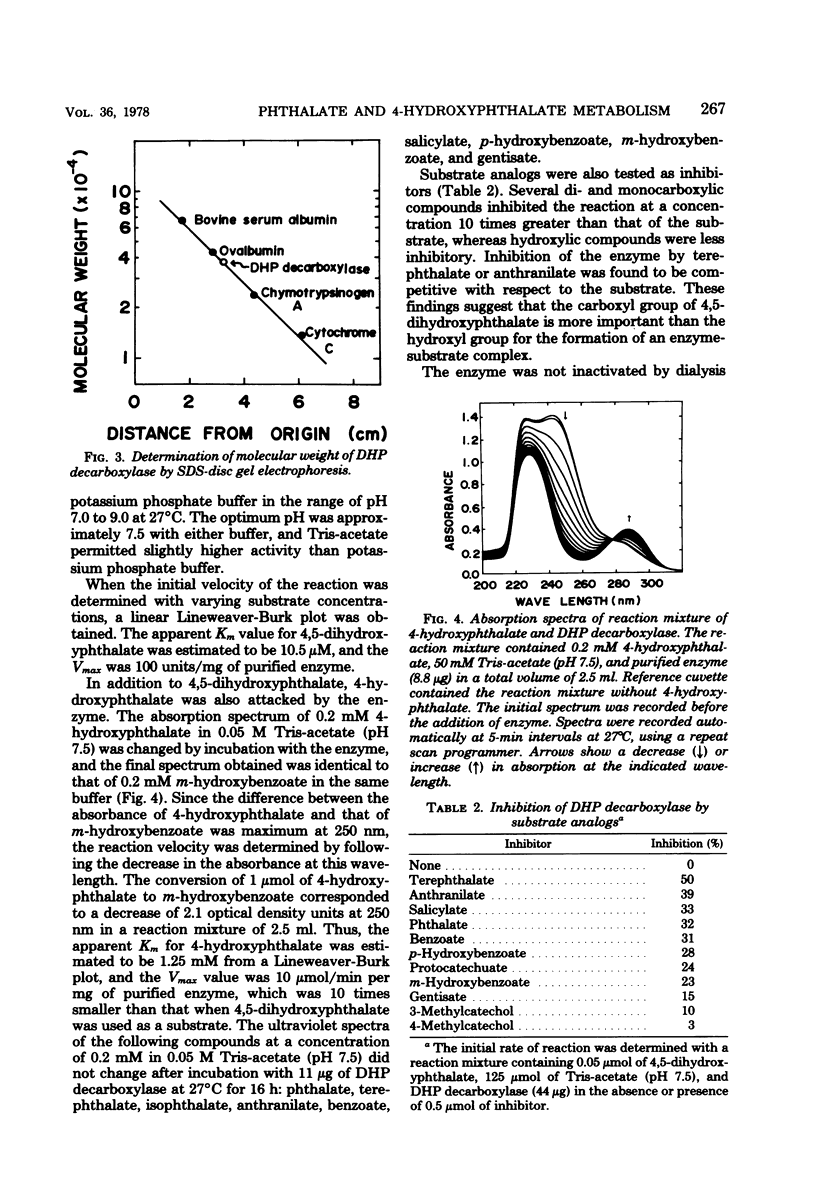
Phthalate is degraded through 4,5-dihydroxyphthalate and protocatechuate in Pseudomonas testosteroni NH1000. The ezyme 4,5-dihydroxyphthalate decarboxylase, catalyzing the conversion of 4,5-dihydroxyphthalate to protocatechuate and carbon dioxide, was purified approximately 130-fold from phthalate-induced cells of a protocatechuate 4,5-dioxygenase-deficient mutant of P. testosteroni. The most purified preparation showed a single protein band on sodium dodecyl sulfate-acrylamide disc gel electrophoresis with a molecular weight of 38,000. The apparent molecular weight of the native enzyme determined by Sephadex G-200 column chromatography was 150,000. Among the substrate analogs tested, only 4-hydroxyphthalate served as a substrate, which was decarboxylated to form m-hydroxybenzoate. The apparent Km values for 4,5-dihydroxyphthalate and 4-hydroxyphthalate were estimated to be 10.5 micrometer and 1.25 mM, respectively, and the Vmax for the former was 10 times larger than that for the latter. Whereas the wild-type strain could utilize 4-hydroxyphthalate as a sole source of carbon, none of the following could grow with the compound: 4,5-dihydroxyphthalate decarboxylase-deficient, m-hydroxybenzoate-nondegradable, and protocatechuate 4,5-dioxygenase-deficient mutants. Since one-step revertants of these mutants could utilize 4-hydroxyphthalate, the compound appears to be metabolized through m-hydroxybenzoate and protocatechuate in P. testosteroni NH1000.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autian J. Toxicity and health threats of phthalate esters: review of the literature. Environ Health Perspect. 1973 Jun;4:3–26. doi: 10.1289/ehp.73043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., EVANS W. C., RIBBONS D. W. New pathways in the oxidative metabolism of aromatic compounds by microorganisms. Nature. 1960 Nov 12;188:560–566. doi: 10.1038/188560a0. [DOI] [PubMed] [Google Scholar]
- Englehardt G., Wallnöfer P. R., Hutzinger O. The microbial metabolism of di-n-butyl phthalate and related dialkyl phthalates. Bull Environ Contam Toxicol. 1975 Mar;13(3):342–347. doi: 10.1007/BF01685348. [DOI] [PubMed] [Google Scholar]
- Keyser P., Pujar B. G., Eaton R. W., Ribbons D. W. Biodegradation of the phthalates and their esters by bacteria. Environ Health Perspect. 1976 Dec;18:159–166. doi: 10.1289/ehp.7618159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K. G., Fish W. W. Protein polypeptide chain molecular weights by gel chromatography in guanidinium chloride. Methods Enzymol. 1972;26:28–42. doi: 10.1016/s0076-6879(72)26004-9. [DOI] [PubMed] [Google Scholar]
- Nakazawa T., Hayashi E. Phthalate metabolism in Pseudomonas testosteroni: accumulation of 4,5-dihydroxyphthalate by a mutant strain. J Bacteriol. 1977 Jul;131(1):42–48. doi: 10.1128/jb.131.1.42-48.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary M. H., Westheimer F. H. Acetoacetate decarboxylase. Selective acetylation of the enzyme. Biochemistry. 1968 Mar;7(3):913–919. doi: 10.1021/bi00843a005. [DOI] [PubMed] [Google Scholar]
- Riley W. D., Snell E. E. Histidine decarboxylase of Lactobacillus 30a. IV. The presence of covalently bound pyruvate as the prosthetic group. Biochemistry. 1968 Oct;7(10):3520–3528. doi: 10.1021/bi00850a029. [DOI] [PubMed] [Google Scholar]
- Saeger V. W., Tucker E. S. Biodegradation of phthalic acid esters in river water and activated sludge. Appl Environ Microbiol. 1976 Jan;31(1):29–34. doi: 10.1128/aem.31.1.29-34.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]



