Abstract
Background/Aims
To explore clinical patterns, predisposing factors, psychosocial aspects and the possible pathogenesis in Functional Vomiting (FV) patients.
Methods
Ten healthy subjects and 19 FV patients participated in this study. Gastrointestinal symptoms and psychological state were evaluated by questionnaires, including Zung self-rating anxiety and depression scale and Eysenck personality questionnaire. Cutaneous electrogastrography, perfusion nutrition load test and intragastric pressure were performed in patients. Perfusion nutrition load test and intragastric pressure were also performed in healthy subjects.
Results
FV involved young female predominantly (4 male, 15 female; age 25.8 ± 8.4 years). Postprandial vomiting soon after meal without self-induced maneuver was the most common pattern of FV. Prevalence for overlaps between FV and functional dyspepsia was high (84.2%). Emotional changes and stress contributed to the development of FV. Prevalence of abnormal psychological status and personality in patients with FV was high (83.3% and 47.1%). Patients with FV had significant postprandial gastric dysrhythmia, impaired gastric accommodation and enhanced gastric sensitivity. There were significant correlations between clinical presentations, gastric function and psychological states.
Conclusions
Patients with FV had abnormal psychological status, gastric dysmotility and hypersensitivity, which indicated that both of peripheral and central abnormalities could contribute to the pathogenesis of FV.
Keywords: Vomiting, Pathophysiology, Psychosocial factors
Introduction
Functional gastrointestinal disorders (FGIDs) are highly prevalent throughout the world.1 The absence of anatomic or biochemical markers for FGIDs and the limited number of clinical trials that have been performed, make them a challenge to diagnose. Functional gastroduodenal disorders, one of FGIDs, are classified into 4 categories: functional dyspepsia, belching disorders, functional nausea and vomiting disorders (comprising chronic idiopathic nausea, functional vomiting [FV] and cyclic vomiting syndrome) and the rumination syndrome.2
According to the Rome III criteria, FV is defined as recurrent, unexplained vomiting at least once per week that is not cyclical and lacks an organic basis.3 Although FV is a fairly rare disorder and has been under-investigated, it has been increasingly recognized that this condition can be highly disabling.4 Additional studies that investigate the epidemiology and clinical presentation, as well as treatment trials and most importantly mechanistic studies are desperately needed. Psychogenic vomiting was used to describe chronic nausea and vomiting for which there was no apparent cause.5 Currently there is no evidence to support an association between any psychiatric disorder and chronic, unexplained nausea and vomiting.4 However, stress and psychosocial factors can act as modulators via the brain-gut axis to influence clinical presentation and outcome, which suggests that the association between FV and psychosocial aspects needs to be further recognized and investigated.6
We present a study of consecutive patients admitted to gastrointestinal (GI) clinic in Peking Union Medical College Hospital with chronic vomiting of unknown etiology. The study aims to explore clinical patterns, predisposing factors, psychosocial aspects and gastric sensorimotor function in FV patients, and to improve recognition of pathogenesis, diagnosis and management of this disorder.
Materials and Methods
1. Study subjects
A total of 10 healthy subjects (HS) (2 male, 8 female; age 30.2 ± 7.7 years) and 19 FV patients (4 male, 15 female; 25.8 ± 8.4 years) participated in this 8-month (2008.10-2009.6) study consecutively. None of the HS reported symptoms or a history of GI disease or drug allergies, nor were they taking any medication. All patients fulfilled the Rome III criteria2 that must include all of the following: (1) on average, 1 or more episodes of vomiting per week; (2) absence of criteria for an eating disorder, rumination or major psychiatric disease according to Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) and (3) absence of self-induced vomiting and chronic cannabinoid use, and absence of abnormalities in the central nervous system or metabolic diseases to explain the recurrent vomiting. Criteria fulfilled for the past 3 months, with symptom onset at least 6 months before diagnosis. All patients underwent a structured, psychiatric interview as well as a psychological and behavioral history to rule out overt eating disorders, such as anorexia nervosa or bulimia. All patients had undergone previously basic diagnostic testing including complete blood count, electrolyte, fasting glucose, liver and renal function, upper GI barium study and esophagogastroduodenoscopy to exclude structural disorders. The study protocol was approved by the Institutional Ethics committee. Informed consent was obtained from all patients and HS.
2. Methods
1) Questionnaires
Demographic and clinical data were recorded by questionnaires which included vomiting symptoms, overlapping symptoms, psychological states (Zung self-rating anxiety and depression scale [SAS/SDS]),7,8 social stress (stressful life events or abuse history), and Eysenck personality questionnaire (EPQ).9
2) Cutaneous electrogastrography
Measurements were made using a DIGITRAPPER EGG™ and the accompanying computerized data analysis package (Synectics Medical Inc, Stockholm, Sweden). Electrogastrography (EGG) was recorded for 30 minutes in fasting state in the morning after an overnight fast and thereafter for another 60 minutes after the test meal. Subjects were requested to intake the test meal within 10 minutes. The test meal consisted of 80 g of instant noodles, 50 g of ham and 400 mL of water. The total energy was 470 kcal. Obtained EGG parameters included dominant frequency (DF), dominant power (DP), % normal rhythm (%N), % bradygastria (%B), % tachygastria (%T) and power ratio (PR). DF was defined as the frequency appearing with peak power value of spectra, whereas DP was the power value observed at DF. Defined range of normal rhythm was 2.4-3.7 cpm. PR was the ratio of the postprandial DP divided by the fasting DP. PR < 1 was considered as abnormal and likely a poor motor response to the meal. The data of HS were from our previous tudy.10
3) Perfusion nutrition load test and intragastric pressure
Subjects were received perfusion nutrition load test (P-NLT, through nasal-gastric tube) with a constant rate of 50 mL/min (0.75 kcal/mL) in the morning after an overnight fast.11 Percentages of protein, carbohydrate and lipid in nutrition liquid were 13%, 48% and 39%, respectively. Meanwhile, intragastric pressure (IGP) was recorded to assess the sensitivity to gastric distention.11 Visual analogue scale (0-10) was used to evaluate satiety during NLT.
3. Statistical methods
All data were analyzed by SPSS17.0 software packet. The differences were considered statistically significant when a p-value < 0.05 was obtained. All measurement data were reported as mean ± SD. T tests were used to compare differences between 2 groups. Categorical data were analyzed using χ2 test. The correlation of symptoms and gastric function was analyzed by χ2 or Fisher's exact test. The Pearson's correlation analysis was used to evaluate associations among psychological factors and gastric function.
Results
1. Clinical characteristics of FV
Age
In FV patients (4 male, 15 female), the age at onset of the first episode ranged from 15 to 44 years (mean, 24 years; median 21 years).
Duration
The duration of the FV at the time of consultation ranged from 6 to 109 months (mean, 25 months; median, 13 months).
Severity
The severity of FV at the time of the consultation was deemed mild if patients did not have major impairment in daily life function; moderate, if patients had intermittent disruptions in activity; and severe if patients had chronically impaired daily functioning.1 Of 19 patients, 1 had mild, 8 had moderate and 10 had severe FV. One patient required nasogastric intubation and enteral nutrition. Six (31.6%) were disabled and could not maintain their jobs or study.
Patterns of FV
All 19 patients had 1 or more episodes of vomiting per week and 78.9% of them vomited every day. Postprandial vomiting occurred in 94.7% of these patients. The length of emesis often ranged from minutes to hours. Vomiting occurred without self-induced maneuver. Some patients reported oro-pharyngeal discomfort soon after meals, which could be lessened by vomiting. The vomitus consisted of food initially, sometimes changing to gastric juice and bile. Only 1 patient reported blood streaks and none of them reported vomiting with retained food after overnight fasting.
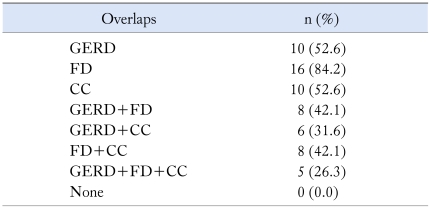
Overlaps
Those between FV and other GI diseases occurred in all patients. The prevalences of overlaps with functional dyspepsia (FD), gastroesophageal reflux disease and chronic constipation were 84.2%, 52.6% and 52.6% respectively. More than 50% of patients reported 2 or more overlaps (Table 1).
Table 1.
The Prevalence of Overlaps in 19 Functional Vomiting Patients
GERD, gastroesophageal reflux disease; FD, functional dyspepsia; CC, chronic constipation.
Complications
Weight loss occurred in 94.7% of patients and almost half of women reported menstrual disturbance. Other complications included esophagitis (21.0%), electrolytes disturbance (15.8%) and dental disease (5.3%).
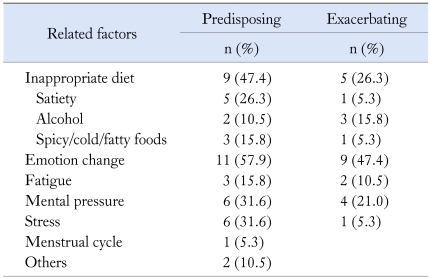
Predisposing factors
Sixteen patients (84.2%) identified conditions that seemed to trigger the vomiting episodes. The most frequent triggers reported were: emotional change in 11, inappropriate diet in 9, mental pressure in 6, noxious stress in 6 and fatigue in 3. Only 1 patient contributed vomiting to menstrual cycle.
Almost half the number of patients reported that emotional change was also a contributory factor for their deterioration of vomiting; in turn, relaxation and distraction could help some patients improve their symptoms (Table 2).
Table 2.
Related Factors of Vomiting Spells in 19 Functional Vomiting Patients
The relieving factors reported were: multiple small meals in 5 (26.3%), relaxation and distraction in 5 (26.3%) and rest in 1 (5.3%).
2. Psychosocial aspects of FV
Environmental stress was common in FV patients. Negative life events were elicited in 11 (57.9%) of our patients and 2 (10.5%) of them reported physical or emotional abuse history.
Prevalence of abnormal psychological states was high in FV patients (83.3%). Ten (55.6%) of our patients displayed significant anxiety and 13 (72.2%) with depression.
As for personality factors, 17 patients completed EPQ and 8 (47.1%) of them had high scores on neuroticism scale.
3. Gastric sensorimotor function
1) EGG
Sixteen FV patients received both pre- and post-prandial EGG. One FV patient completed fasting study only because of severe postprandial vomiting. Compared to previous data of HS in our center,10 FV patients had significantly decreased %N and postprandial DF, increased DP and 50% with abnormal PR, which indicated postprandial dysrhythmia in FV patients (Table 3).
Table 3.
The Electrogastrography of Functional Vomiting Patients
%N, % normal rhythm; %B, % bradygastria; %T, % tachygastria; DF, dominant frequency; cpm, counts per minute; DP, dominant power; dB, decibel.
The data of HS were from previous study in our center.10
ap < 0.05, bp < 0.01 compared to healthy subjects.
2) P-NLT and IGP
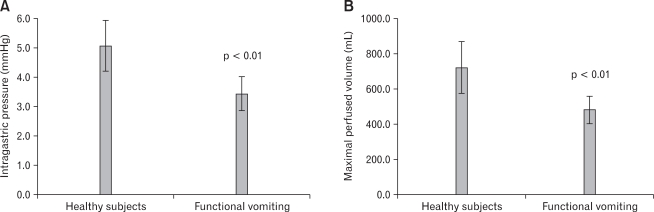
Ten HS and 13 FV patients received both P-NLT and IGP test. Two FV patients completed P-NLT only. Compared to HS, the maximal perfused volume and the IGP of FV patients was significantly decreased, indicating impaired accommodation and hypersensitivity in FV patients (Fig. 1).11,12
Figure 1.
The intragastric pressure (IGP) and the perfused volume of healthy subjects (HS) and functional vomiting (FV) patients at the maximal satiety. (A) Compared to HS, FV patients had significantly decreased IGP at the maximal satiety (5.1 ± 1.2 vs 3.4 ± 1.0 mmHg), indicating enhanced hypersensitivity. (B) Compared to HS, maximal perfused volume in FV patients was significantly decreased (722.0 ± 206.2 vs 481.3 ± 140.0 mL), which indicated that impaired gastric accommodation could involve in the pathogenesis of FV.
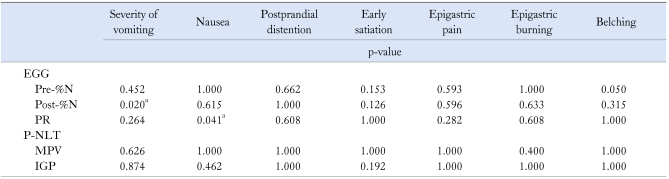
4. Relationships among the symptoms, gastric function and psychological factors
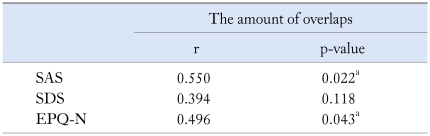
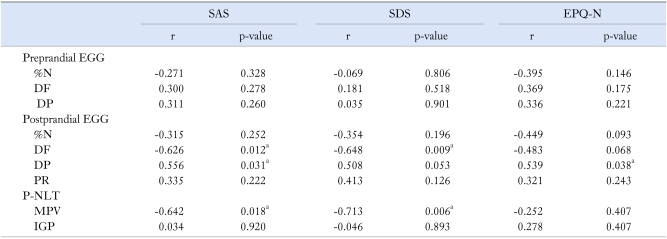
We found significant correlation between the severity of vomiting and % N, as well as the prevalence of nausea and PR. Anxiety (SAS) and neuroticism (EPQ-N) could predict the amount of overlaps. The postprandial DF, DP and the MPV were related to the scores of SAS, SDS and EPQ-N (Table 4-6).
Table 4.
Relationships Between Psychiatric States, Personality and Overlaps in Functional Vomiting Patients
r, contingency coefficient; SAS and SDS, self-rating anxiety and depression scale; EPQ-N, neuroticism scale in Eysenck personality questionnaire.
ap < 0.05.
Table 6.
Relationships Between Psychiatric States, Personality and Gastric Sensorimotor Function in Functional Vomiting Patients
SAS and SDS, self-rating anxiety and depression scale; EPQ-N, neuroticism scale in Eysenck personality questionnaire; r, contingency coefficient; EGG, electrogastrography; %N, % normal rhythm; DF, dominant frequency; DP, dominant power; PR, power ratio; MPV, maximal perfused volume; IGP, intragastric pressure.
ap < 0.05.
Discussion
Vomiting was a common complaint and a study from USA showed a total of 2% reported vomiting monthly or more frequently.13 However, FV was not common after an extensive evaluation.14 Muraoka et al15 reported that 59 patients with vomiting of unclear etiology were divided into five arbitrary patterns of vomiting (continuous, postprandial, irregular, nausea and self-induced). The pattern of vomiting in FV was just like that in the postprandial group described by Muraoka et al and was unmistakably different from other vomiting disorders as rumination syndrome, bulimia, gastroparesis and cyclic vomiting syndrome. In our study, the majority of FV patients could stop vomiting themselves, indicating that behavior therapy could be beneficial for the FV patients.
Olden and Crowell4 found that FV patients were much more likely to have a FGID than patients with vomiting of organic diseases. Recent studies have revealed that overlaps are common in patients with FGIDs and about two thirds have symptoms of multiple disorders at the same time.16,17 There were significant correlation between overlaps and psychological factors.18,19 Our study also found that anxiety and neuroticism could predict the prevalence of overlaps.
It is now widely accepted that dysfunction of the bidirectional neural pathways between the brain and the gut ('brain-gut axis') at any level can contribute to the various symptoms experienced by FGID patients.20 From the brain level, psychological disturbances, for example acute or chronic stress reaction, psychiatric disorders, especially emotional disorders, anxiety disorders and somatoform disorders, may be associated with alterations in the processing of visceral sensation and gut-related autonomic nervous system function, affecting gut motility and sensation. As shown in our study, 11/16 patients cited emotional changes as a triggering factor of the occurrence of their symptom, and almost half the number of patients reported that emotional change was also a contributory factor for their deterioration of vomiting, while relaxation and distraction could help some patients improve their symptoms. Negative life events and abuse history were also found in patients in our study. This was observed in many other studies.6 Some researchers suggested that personality traits may explain the association between abuse and FGIDs. Abuse may induce the expression of abnormal personality traits that in turn leads to FGIDs.21 There was also an increased prevalence of abnormal psychological states in clinic patients with FGIDs (in our study, 83.3%) compared with HS (20%).6 One study reported higher levels of depression in FV patients.15 Another study showed that neuroticism was increased in patients with FGIDs.22 Our study found that about half FV patients showed high neuroticism, which meant that they were more likely than the average to experience feelings such as anxiety and depression and cope poorly with environmental stress.9 Neuroticism may explain the association between abuse and IBS22 and predict treatment outcome in FGID patients.23 Our findings pointed to the possibility that psychosocial factors could play an important role in pathogenesis of FV. Accordingly, psychosocial factors and psychiatric disorders should be considered in the evaluation and treatment of FV patients. It may also be a direction to improve further study, that to include professional psychiatrists/psychologists to thoroughly evaluate life events and abuse and do possible interventions.
Patients with unexplained nausea and vomiting had abnormal EGG and the improvement of EGG was related to the relief of the symptoms.14,24 The characteristic abnormality observed in EGG pattern in FV patients was postprandial gastric dysrhythmia, such as decreased %N and DF, an absence of the normal postprandial power increase after the test meal. The observed correlation of decreased %N with severity of vomiting and abnormal PR with nausea revealed that the decreased postprandial electrical response activity was related to the symptoms in FV patients. Our study found that decreased DF was associated with the scores of SAS, SDS and EPQ-N, which suggested that psychological factors could involve in gastric dysrhythmia. Our findings also showed correlations between impaired gastric accommodation and the level of SAS/SDS, which was also supported by previous studies.25,26 However, our study did not reveal the relationship between gastric hypersensitivity and psychological status. The relationship between psychological status and gastric dysfunction now is still controversial. Although we assumed that P-NLT was more objective than drink nutrition load test and more physiological than barostat,11 it has not been recognized as golden standard for assessment of gastric accommodation and sensation. Further studies are necessary to confirm the validation of this method. It is unclear if psychosocial factors mainly determine healthcare seeking or have a more direct influence on GI sensorimotor function in FGIDs. Recent epidemiological and neurobiological studies have provided increasing evidence for the second hypothesis.6 Central nervous system structures, which integrate sensory information or regulate autonomic output to the viscera, largely overlap with regions involved in emotional regulation in the brain.20 This might provide an explanation for the positive relationship between abnormal psychological status and gastric function in FV patients.
In summary, this is, to our knowledge, the first study to describe the clinical pattern, pathophysiology, psychosocial factors and their relationships in patients with FV. Gastric dysmotility and hypersensitivity may have a key role in the causation of FV. Gender, age, stress and psychosocial factors can act as modulators via the brain-gut axis or other approaches to influence clinical presentation and outcome of FV. Both of peripheral and central abnormalities could contribute to the pathogenesis of FV. Further studies are warranted to gain more insight into the complex interaction of peripheral abnormalities and brain centers.
Table 5.
Relationships Between Symptoms and Gastric Sensorimotor Function in Functional Vomiting Patients
EGG, electrogastrography; Pre- and Post-%N, pre- and post-prandial % normal rhythm; PR, power ratio; P-NLT, perfusion nutrition load test; MPV, maximal perfused volume; IGP, intragastric pressure.
ap < 0.05, p-value is for r (contingency coefficient).
Acknowledgements
The authors would like to thank staffs from the Department of Gastroenterology in the Peking Union Medical College Hospital for their assistance.
Footnotes
Financial support: None.
Conflicts of interest: None.
References
- 1.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 3.Talley NJ. Functional nausea and vomiting. Aust Fam Physician. 2007;36:694–697. [PubMed] [Google Scholar]
- 4.Olden KW, Crowell MD. Chronic nausea and vomiting: new insights and approach to treatment. Curr Treat Gastroenterol. 2005;8:305–310. doi: 10.1007/s11938-005-0023-y. [DOI] [PubMed] [Google Scholar]
- 5.Hederos CA. Psychogenic vomiting. Lancet. 1992;339:1228. doi: 10.1016/0140-6736(92)91163-3. [DOI] [PubMed] [Google Scholar]
- 6.Levy RL, Olden KW, Naliboff BD, et al. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology. 2006;130:1447–1458. doi: 10.1053/j.gastro.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 7.Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371–379. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]
- 8.Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70. doi: 10.1001/archpsyc.1965.01720310065008. [DOI] [PubMed] [Google Scholar]
- 9.Eysenck H, Eysenck S. The manual of the Eysenck Personality Questionnaire. London: Hodder & Stoughton; 1975. [Google Scholar]
- 10.Chen YM, Ke MY, Wang ZF, Zhang XL, Pan GZ. Electrogastrogrephy in fifty healthy subjects. Chin J Dig. 2000;20:227–229. [Google Scholar]
- 11.Zhang J, Sun XH, Ke MY, Wang ZF. Perfusion nutrition load test in assessment of gastric accommodation and sensitivity in functional dyspepsia patients - a preliminary study [abstract] Korean J Neurogastroenterology Motil. 2009;15:29. [Google Scholar]
- 12.Tack J, Caenepeel P, Piessevaux H, Cuomo R, Janssens J. Assessment of meal induced gastric accommodation by a satiety drinking test in health and in severe functional dyspepsia. Gut. 2003;52:1271–1277. doi: 10.1136/gut.52.9.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talley NJ, Zinsmeister AR, Schleck CD, Melton LJ., 3rd Dyspepsia and dyspepsia subgroups: a population-based study. Gastroenterology. 1992;102:1259–1268. [PubMed] [Google Scholar]
- 14.Abell TL, Familoni B, Voeller G, et al. Electrophysiologic, morphologic, and serologic features of chronic unexplained nausea and vomiting: lessons learned from 121 consecutive patients. Surgery. 2009;145:476–485. doi: 10.1016/j.surg.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Muraoka M, Mine K, Matsumoto K, Nakai Y, Nakagawa T. Psychogenic vomiting: the relation between patterns of vomiting and psychiatric diagnoses. Gut. 1990;31:526–528. doi: 10.1136/gut.31.5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corazziari E. Definition and epidemiology of functional gastrointestinal disorders. Best Pract Res Clin Gastroenterol. 2004;18:613–631. doi: 10.1016/j.bpg.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 18.Lee SY, Lee KJ, Kim SJ, Cho SW. Prevalence and risk factors for overlaps between gastroesophageal reflux disease, dyspepsia, and irritable bowel syndrome: a population-based study. Digestion. 2009;79:196–201. doi: 10.1159/000211715. [DOI] [PubMed] [Google Scholar]
- 19.Song ZQ, Ke MY, Wang ZF, Liu XH, Fang XC. Dyspeptic symptomatology and pathogenesis of functional dyspepsia with and without overlapping symptoms: a comparative study. Chin J Gastroenterol. 2006;11:458–461. [Google Scholar]
- 20.Van Oudenhove L, Demyttenaere K, Tack J, Aziz Q. Central nervous system involvement in functional gastrointestinal disorders. Best Pract Res Clin Gastroenterol. 2004;18:663–680. doi: 10.1016/j.bpg.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Talley NJ, Boyce PM, Jones M. Is the association between irritable bowel syndrome and abuse explained by neuroticism? A population based study. Gut. 1998;42:47–53. doi: 10.1136/gut.42.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanum L, Malt UF. Personality and physical symptoms in non-psychiatric patients with functional gastrointestinal disorder. J Psychosom Res. 2001;50:139–146. doi: 10.1016/s0022-3999(00)00219-1. [DOI] [PubMed] [Google Scholar]
- 23.Tanum L, Malt UF. Personality traits predict treatment outcome with an antidepressant in patients with functional gastrointestinal disorder. Scand J Gastroenterol. 2000;35:935–941. doi: 10.1080/003655200750022986. [DOI] [PubMed] [Google Scholar]
- 24.Geldof H, van der Schee EJ, van Blankenstein M, Grashuis JL. Electrogastrographic study of gastric myoelectrical activity in patients with unexplained nausea and vomiting. Gut. 1986;27:799–808. doi: 10.1136/gut.27.7.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geeraerts B, Van denberghe J, Van Oudenhove L, et al. Influence of experimentally induced anxiety on gastric sensorimotor function in humans. Gastroenterology. 2005;129:1437–1444. doi: 10.1053/j.gastro.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Van Oudenhove L, Vandenberghe J, Geeraerts B, et al. Relationship between anxiety and gastric sensorimotor function in functional dyspepsia. Psychosom Med. 2007;69:455–463. doi: 10.1097/PSY.0b013e3180600a4a. [DOI] [PubMed] [Google Scholar]