Abstract
Background/Aims
Irritable bowel syndrome (IBS) is a common disorder with significant morbidity and impairment of quality of life. Most patients (26%-83%) with IBS from Asia reported bloating. Bloating may result from increased amount or distribution of gas in the gut or exaggerated perception of distension. To evaluate whether patients with IBS produce more hydrogen even in fasting state, we conducted a study with the following aims: (1) to estimate fasting breath hydrogen levels among patients with IBS as compared with healthy controls (HC) and (2) to study relationship between symptoms of IBS and stool frequency and fasting breath hydrogen levels.
Methods
Eighty-one patients with IBS (Rome III criteria) and 123 HC were included. Hydrogen breath test was performed using a gas analyzer after an overnight (12 hours) fast. Both patients with IBS and HC had similar preparation before breath hydrogen estimation.
Results
Of 93 patients with symptoms of functional gastrointestinal disorders, 81 (87.1%) met Rome III criteria and 12 (12.9%) were negative and hence, excluded from the study. Patients with IBS were comparable in age (35 ± 11.8 years vs 37.5 ± 13.1 years, p = NS) and gender (male 61/81 [75.3%] vs 77/123 [62.6%], p=0.67) with HC. Average fasting breath hydrogen was higher in patients with IBS as compared to HC (mean 10.1 ± 6.5 ppm vs 5.5 ± 6.2 ppm, p < 0.0001). Number of stools per week correlated with average fasting breath hydrogen excretion in patients with IBS (r = 0.26, p = 0.02).
Conclusions
Inspite of similar preparation for the test, fasting breath hydrogen was higher in patients with IBS as compared to HC. Number of stools per week correlated with fasting breath hydrogen levels among patients with IBS.
Keywords: Breath tests, Bloating, Abdominal discomfort, Diarrhea, Small intestine
Introduction
Irritable bowel syndrome (IBS) is a common gastrointestinal disorder with significant morbidity,1 impairment of quality of life2 and work absenteeism.3 Most patients (26%-83%) with IBS from Asia reported bloating.4 Bloating may result from increased amount of gas or its abnormal movement in the gut or increased perception of the distension.5 A few studies using computerized tomography or radiographs of abdomen revealed higher amount of gas in patients with IBS.6,7 A study using infusion of radioactive xenon gas into small bowel revealed that patient with IBS tend to trap the gas in small bowel, which has low ability to distend compared to colon whereas, in healthy subjects the gas moved down to the colon.8 In another study from our center, patients with IBS complained of more symptoms than healthy subjects to a comparable level of hydrogen production following lactose load, which was likely to be related to visceral hypersensitivity.9 In a recent study, it was found that patients with IBS were less often methane producers compared with the healthy subjects.10 Since 4 atoms of hydrogen combine to produce 1 molecule of methane, the authors suggested that increased frequency of bloating among patients with IBS might be explained by reduced methane production among them.10 However, there is no study directly comparing fasting breath hydrogen among patients with IBS and healthy subjects and relationship between various symptoms of IBS and fasting breath hydrogen levels.
Hwang et al11 suggested that methane production among patients with IBS was associated with constipation. Methane slows the gut transit, therefore, may cause constipation.11 Hence, it is logical to propose that excess hydrogen production should be associated with higher stool frequency. In a recent study from our center, patients with chronic non-specific diarrhea produced more hydrogen than those with IBS and healthy subjects in response to glucose load.12 Hence, it is interesting to evaluate whether there is any relationship between fasting breath hydrogen levels and stool frequency. Accordingly, we aimed to study: (1) fasting breath hydrogen levels among patients with IBS as compared to healthy subjects with same preparation and (2) relationship between symptoms of IBS and stool frequency and fasting breath hydrogen levels.
Materials and Methods
1. Study subjects
From December 2008 to February 2010, 93 patients with functional lower gastrointestinal disorder referred to Gastrointestinal Pathophysiology and Motility Laboratory of the Department of Gastroenterology of a tertiary referral center in the northern India for breath hydrogen testing and 123 healthy controls (HC) were included. IBS was diagnosed using Rome III criteria.13 IBS was classified into diarrhea predominant (D-IBS) and constipation predominant (C-IBS) on subjective feeling of bowel movement. Also, on Bristol stool form scale, stool type 1, 2 and 3 were taken as C-IBS and 5, 6 and 7 were taken as D-IBS; those who reported alternating type of stool were taken as I-IBS (indeterminate type-IBS) (Asian IBS consensus).14 HC were selected from the community or staff members of the institute excluding those fulfilling criteria for IBS on enquiry. The study protocol was approved by the Institutional Ethics committee. Informed consent was obtained from all the patients and HC. Patients who received antibiotics, proton pump inhibitors and promotility or antimotility drugs within 4 weeks before inclusion into the study were excluded.
2. Hydrogen breath test
Hydrogen breath test was performed using a breath gas analyzer (Bedfont gastrolyzer, Bedfont Scientific Ltd, ME13QX, England) after an overnight (12 hours) fast.15
3. Patient preparation
Both the patients with IBS and HC were asked to avoid slowly absorbed carbohydrates (like bread and potato) and fiber during the previous 48 hours before the test as this might lead to high basal levels of hydrogen in the breath.16 Subjects were not allowed to take fruits, vegetables, and legumes. Also, they were asked not to ingest any beans, nuts, soy or large meals, and to limit dairy intake the day before the test. Patients who were on lactulose were asked to stop it 72 hours before the test. Cigarette smoking and exercise were avoided 2 hours before and during the test, as hyperventilation can cause changes in breath hydrogen content. The subjects were then asked to brush their teeth and rinse their mouth with antiseptic mouth wash (chlorhexidine) and tap water before the test. Three values of breath hydrogen were recorded from each subject every 5-minute in fasting state and an average of these values was also taken.16
4. Clinical evaluation
Subjects were given a bowel symptom questionnaire. Symptoms included bloating, excess gas, diarrhea, constipation and abdominal pain, passage of mucus, feeling of incomplete evacuation, straining, and urgency. The patients were asked how they feel about their bowel movements (diarrhea or constipation). Western definitions of constipation (less than 3 stools per week)17 and diarrhea (more than 3 stools per day)17 were also applied. Bristol Stool Form Scale was also used to define bowel pattern.18
5. Statistical methods
Categorical and continuous data were analyzed using chi-square and Mann-Whitney U test, respectively. The time by group interaction was analyzed using repeated measures ANOVA (General linear model). Spearman rank correlation was used to evaluate the relationship between each pair of continuous variables. The p-values less than 0.05 were considered significance.
Results
1. Demographic and clinical parameters
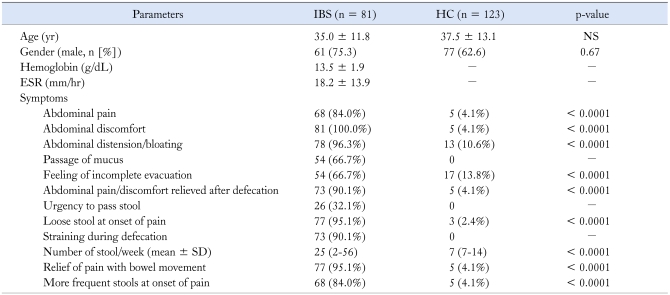
Of the 93 patients with functional lower gastrointestinal symptoms, 81 (87.1%) fulfilled Rome III criteria for IBS and 12 (12.9%) were negative and hence, excluded from this study. Patients with IBS were comparable in age (mean 35 ± 11.8 years vs 37.5 ± 13.1 years, p = NS) and gender (male 61/81 [75.3%] vs 77/123 (62.6%), p = 0.67) with HC (Table 1). On subjective feeling of bowel movement, of 81 patients with IBS 50 (61.7%) and 29 (35.8%) were constipation and diarrhea predominant, respectively while 2 (2.5%) patients felt neither constipation nor diarrhea. Of 81 patients with IBS, 3 (3.7%), 4 (4.9%), 18 (22.2%), 13 (16%), 15 (18.5%) and 28 (34.6%) were passing Bristol stool type 1, type 2, type 3, type 4, type 5 and type 6, respectively. Of 128 HC initially screened, 5 were excluded from the study as they fulfilled Rome III criteria for IBS.
Table 1.
Demographic and Clinical and Laboratory Parameters of Patients With IBS and Healthy Controls
IBS, irritable bowel syndrome; HC, healthy control; ESR, erythrocyte sedimentation rate.
Values within parenthesis indicate percentage. Continuous data is presented as mean and SD. The categorical and continuous data were compared using chi-square test, and Student's t test, respectively.
2. Fasting breath hydrogen in IBS and HC
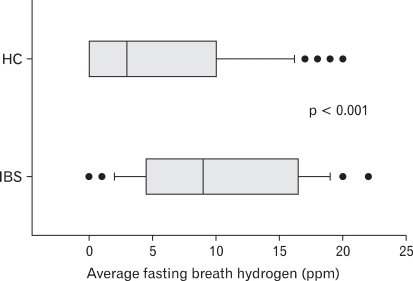
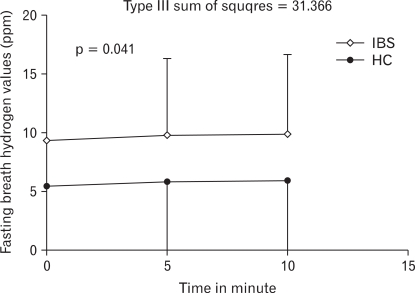
Average fasting breath hydrogen concentration was higher in patients with IBS as compared to HC (mean 10.1 ± 6.5 ppm vs 5.5 ± 6.2 ppm, p < 0.0001; Fig. 1). There was significant difference in fasting breath hydrogen excretion at different time in patients with IBS as compared to HC (Type III sum of squares = 31.366, p = 0.041; Fig. 2). 12/123 (10%) HC and 20/81 (25%) patients with IBS had fasting breath hydrogen value more than 16 ppm. There was no difference in excretion of fasting breath hydrogen among patients with D-IBS and C-IBS (either by subjective feeling or by Bristol stool form).
Figure 1.
Comparison of fasting breath hydrogen level in patients with irritable bowel syndrome and healthy controls. IBS, irritable bowel syndrome; HC, healthy controls.
Figure 2.
Comparison of fasting breath hydrogen level in patients with irritable bowel syndrome and healthy controls at different time interval using repeated measures of ANOVA. IBS, irritable bowel syndrome; HC, healthy controls.
3. Correlation between stool frequency and fasting breath hydrogen excretion
There was a positive correlation between number of stools per week and average fasting breath hydrogen excretion in patients with IBS (r = 0.26, p = 0.02). There was positive correlation between frequency of stools per week and average fasting breath hydrogen excretion in patients with IBS who reported subjective feeling of diarrhea (r = 0.45, p = 0.01) and D-IBS (according to Bristol Stool Form Scale, r = 0.35; p = 0.02). In contrast, there was no correlation between frequency of stools per week and average fasting breath hydrogen excretion in patients with IBS who reported subjective feeling of constipation and C-IBS (according to Bristol stool type).
Discussion
The present study showed that inspite of similar preparation for the test, fasting breath hydrogen excretion was higher in patients with IBS as compared to HC. Also, number of stools per week correlated with fasting breath hydrogen levels among patients with IBS. But, there was no difference in fasting breath hydrogen excretion in patients with D-IBS and C-IBS.
Chami et al19 reported that comparison between the quantity of bowel gas of patients with IBS and HC using plain abdominal radiographs resulted in a marked difference in measured values. However, our study is perhaps the first study documenting increased breath hydrogen in patients with IBS compared to HC. In a recent study, methane production was found commoner among HC (34.6%) than patients with IBS (14.5%).10 Since 4 atoms of hydrogen combine to produce 1 molecule of methane, the authors suggested that increased frequency of bloating among patients with IBS might be explained by reduced methane production among them.10 In another study, Peled et al20 also reported methane production to be commoner among controls (50.0%) than patients with IBS (34.4%). Since methane production is expected to have an inverse relationship with hydrogen levels, these studies support our finding of increased breath hydrogen among patients with IBS than HC. Since as high as 96% of our patients had abdominal bloating, increased hydrogen gas production, at least partly might explain abdominal bloating in IBS.
Fasting breath hydrogen levels were comparable among patients with IBS and controls in a study by Bratten et al21 from United States of America (5 vs 7 ppm, respectively). However, the 2 groups were not comparable as control population was 10-year younger than the IBS population.21 Furthermore, in that study, baseline hydrogen level was significantly lower among methane producing IBS than those without methane production.21 Interestingly, in this study, patients with IBS (20%)21 were more often methane producer than control population (15%),21 though not significant statistically; this in contrast to a large study that found methane production to be less common among IBS than controls.10 Hence, contradictory result in the study by Bratten et al21 as compared with the present study might be related to non-comparable IBS and control group and bias in selection of IBS patients.
In another study by Riordan et al22 from Australia, median fasting breath hydrogen among 45 controls was 5 ppm (range, 1-19). This is in accordance with the results in our control population. In that study, the upper 95% confidence limit (mean + 2 standard deviation) of square root of fasting breath hydrogen value was 4 ppm, corresponding to a measured value of fasting breath hydrogen of 16 ppm. Hence, the authors considered > 16 ppm as abnormal and considered it to be diagnostic of small intestinal bacterial overgrowth (SIBO) with a sensitivity and specificity of 22% and 75%, respectively considering the result of quantitative bacterial culture of distal duodenal aspirate as the gold standard. 10% HC and 25% patients with IBS had fasting breath hydrogen value more than 16 ppm in our study. This is in discordance with our previous studies that showed 8.5% to 13% IBS had SIBO compared with 2% HC using glucose hydrogen breath test9,12,23; these data, therefore, further substantiate that fasting breath hydrogen alone should not be used as a criterion for diagnosis of SIBO as reported by some authors previously.24,25 Poor performance of fasting breath hydrogen in diagnosis of SIBO has been shown by Riordan et al22 too.
Chatterjee et al26 showed that methane production correlated with constipation in patients with IBS. This is related to slowing of gut transit by methane.26 Our finding of correlation of stool frequency per week and fasting breath hydrogen level, albeit weakly, suggests fasting breath hydrogen levels might correlate with diarrhea. In another study, we found that patients with chronic non-specific diarrhea produced more breath hydrogen than those with IBS and HC.12 Lack of significant difference in fasting breath hydrogen among patients with D-IBS and C-IBS might be related to criteria used to subtype, as it was based on Bristol stool type or subjective feeling rather than stool frequency. Also, patients with IBS with incomplete evacuation might visit toilet repeatedly resulting in spuriously increased stool frequency.
Other explanation of abdominal bloating in patients with IBS may be related to abnormal movement of gas within gut27 and visceral hypersensitivity.5,28 Indeed, earlier studies have shown that patients with IBS had abnormal handling and tolerance of intestinal gas; when challenged with an exogenous gas load, these patients developed gas retention and symptoms.29,30 Further, studies have shown that this abnormality might be related to impaired transit of gas in the small bowel, rather than in the colon.31,32 Healthy subjects were able to propel gas from the gut when it was infused into the proximal jejunum at a rate as high as 30 mL/min.33 Patients complaining of bloating retain gas infused into the proximal jejunum, but the clearance of gas infused into the distal ileum and the cecum was normal.32 Furthermore, scintigraphic studies measuring transit of labeled gas showed that impaired clearance of gas from the gut was due to delayed gas transit in the small bowel.8,31 Also, we have shown that patients with IBS more often reported symptoms (bloating, distention, and diarrhea) following 50 g lactose load than HC inspite of a similar frequency of lactose malabsorption in the 2 groups and comparable amount of hydrogen production.9 In a previous study, patients with IBS more often reported symptoms following ingestion of dairy products (60% vs 27%) inspite of the same frequency of lactose malabsorption as HC.34 This might be related to visceral hypersensitivity or a decreased pain threshold in patients with IBS, which is common.35, 36
What could be the reason for higher hydrogen production in patients with IBS? Since the preparation before the test was similar among the 2 groups, it is unlikely to be related to difference in the dietary intake. Gut flora in patients with IBS may differ from that in HC.37 In a study on 20 patients with IBS, Balsari et al37 showed that there was considerable homogeneity in the fecal flora, and that there was a decrease of Coliforms, Lactobacilli and Bifidobacteria in patients with IBS compared with HC. Lactobacilli are less gas producing than some other bacteria, such as Clostridia and Enterobacteriaceae.38 Administration and colonization of the gut with Lactobacilli of patients with IBS have been associated with reduced gas related symptoms.39 Thus, the abnormal gut flora in patients with IBS might cause higher excretion of fasting breath hydrogen. Small intestinal bacterial overgrowth is commoner among patients with IBS than HC.40 Since SIBO can be associated with higher amount of hydrogen production in fasting state,41-43 this can be another explanation for our observation.
In conclusion, the present study showed that inspite of similar preparation for the test, fasting breath hydrogen excretion was higher in patients with IBS as compared to HC. Also, number of stools per week correlated with fasting breath hydrogen levels among patients with IBS. Our finding of correlation of stool frequency and fasting breath hydrogen level, albeit weakly, suggests fasting breath hydrogen levels might correlate with diarrhea.
Acknowledgements
Authors thank Mr. Raghunath of the Gastrointestinal Pathophysiology and Motility Laboratory for his technical support.
Footnotes
Financial support: Mr. Sunil Kumar thanks Indian Council of Medical Research, New Delhi, towards financial support via the grant no. 3/1/2(3)/GAS/2009-NCD-II for Senior research fellowship. UCG also, thanks Indian Council of Medical Research towards the financial support to the Gastrointestinal Pathophysiology and Motility Laboratory of Department of Gastroenterology through Grant No. no. 5/4/3-2/2008-NCD-II.
Conflicts of interest: None.
References
- 1.Whitehead WE, Palsson OS, Levy RR, Feld AD, Turner M, Von Korff M. Comorbidity in irritable bowel syndrome. Am J Gastroenterol. 2007;102:2767–2776. doi: 10.1111/j.1572-0241.2007.01540.x. [DOI] [PubMed] [Google Scholar]
- 2.Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400–411. doi: 10.1023/a:1018831127942. [DOI] [PubMed] [Google Scholar]
- 3.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 4.Gwee KA, Lu CL, Ghoshal UC. Epidemiology of irritable bowel syndrome in Asia: something old, something new, something borrowed. J Gastroenterol Hepatol. 2009;24:1601–1607. doi: 10.1111/j.1440-1746.2009.05984.x. [DOI] [PubMed] [Google Scholar]
- 5.Quigley EM. Germs, gas and the gut; the evolving role of the enteric flora in IBS. Am J Gastroenterol. 2006;101:334–335. doi: 10.1111/j.1572-0241.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 6.Koide A, Yamaguchi T, Odaka T, et al. Quantitative analysis of bowel gas using plain abdominal radiograph in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1735–1741. doi: 10.1111/j.1572-0241.2000.02189.x. [DOI] [PubMed] [Google Scholar]
- 7.Maxton DG, Martin DF, Whorwell PJ, Godfrey M. Abdominal distension in female patients with irritable bowel syndrome: exploration of possible mechanisms. Gut. 1991;32:662–664. doi: 10.1136/gut.32.6.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernando-Harder AC, Serra J, Azpiroz F, et al. Colonic responses to gas loads in subgroups of patients with abdominal bloating. Am J Gastroenterol. 2010;105:876–882. doi: 10.1038/ajg.2010.75. [DOI] [PubMed] [Google Scholar]
- 9.Gupta D, Ghoshal UC, Misra A, Choudhuri G, Singh K. Lactose intolerance in patients with irritable bowel syndrome from northern India: a case-control study. J Gastroenterol Hepatol. 2007;22:2261–2265. doi: 10.1111/j.1440-1746.2007.04986.x. [DOI] [PubMed] [Google Scholar]
- 10.Rana SV, Sharma S, Sinha SK, Kaur H, Sikander A, Singh K. Incidence of predominant methanogenic flora in irritable bowel syndrome patients and apparently healthy controls from North India. Dig Dis Sci. 2009;54:132–135. doi: 10.1007/s10620-008-0315-x. [DOI] [PubMed] [Google Scholar]
- 11.Hwang L, Low K, Khoshini R, et al. Evaluating breath methane as a diagnostic test for constipation-predominant IBS. Dig Dis Sci. 2010;55:398–403. doi: 10.1007/s10620-009-0778-4. [DOI] [PubMed] [Google Scholar]
- 12.Ghoshal UC, Kumar S, Mehrotra M, Lakshmi CP, Misra A. Frequency of small intestinal bacterial overgrowth in patients with irritable bowel syndrome and chronic non-specific diarrhea. J Neurogastroenterol Motil. 2010;16:40–46. doi: 10.5056/jnm.2010.16.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hattori T, Fukudo S. Use of Rome III criteria for diagnosing irritable bowel syndrome. Nippon Rinsho. 2006;64:1425–1428. [PubMed] [Google Scholar]
- 14.Gwee KA, Bak YT, Ghoshal UC, et al. The Asian consensus on the irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1189–1205. doi: 10.1111/j.1440-1746.2010.06353.x. [DOI] [PubMed] [Google Scholar]
- 15.Babu J, Kumar S, Babu P, Prasad JH, Ghoshal UC. Frequency of lactose malabsorption among healthy southern and northern Indian populations by genetic analysis and lactose hydrogen breath and tolerance tests. Am J Clin Nutr. 2010;91:140–146. doi: 10.3945/ajcn.2009.27946. [DOI] [PubMed] [Google Scholar]
- 16.Ghoshal UC, Ghoshal U, Das K, Misra A. Utility of hydrogen breath tests in diagnosis of small intestinal bacterial overgrowth in malabsorption syndrome and its relationship with oro-cecal transit time. Indian J Gastroenterol. 2006;25:6–10. [PubMed] [Google Scholar]
- 17.Ghoshal UC, Abraham P, Bhatt C, et al. Epidemiological and clinical profile of irritable bowel syndrome in India: report of the Indian Society of Gastroenterology Task Force. Indian J Gastroenterol. 2008;27:22–28. [PubMed] [Google Scholar]
- 18.Ersryd A, Posserud I, Abrahamsson H, Simrén M. Subtyping the irritable bowel syndrome by predominant bowel habit: Rome II versus Rome III. Aliment Pharmacol Ther. 2007;26:953–961. doi: 10.1111/j.1365-2036.2007.03422.x. [DOI] [PubMed] [Google Scholar]
- 19.Chami TN, Schuster MM, Bohlman ME, Pulliam TJ, Kamal N, Whitehead WE. A simple radiologic method to estimate the quantity of bowel gas. Am J Gastroenterol. 1991;86:599–602. [PubMed] [Google Scholar]
- 20.Peled Y, Weinberg D, Hallak A, Gilat T. Factors affecting methane production in humans. Gastrointestinal diseases and alterations of colonic flora. Dig Dis Sci. 1987;32:267–271. doi: 10.1007/BF01297052. [DOI] [PubMed] [Google Scholar]
- 21.Bratten JR, Spanier J, Jones MP. Lactulose breath testing does not discriminate patients with irritable bowel syndrome from healthy controls. Am J Gastroenterol. 2008;103:958–963. doi: 10.1111/j.1572-0241.2008.01785.x. [DOI] [PubMed] [Google Scholar]
- 22.Riordan SM, Mciver CJ, Bolin TD, Duncombe VM. Fasting breath hydrogen concentrations in gastric and small intestinal bacterial overgrowth. Scand J Gastroenterol. 1995;30:252–257. doi: 10.3109/00365529509093273. [DOI] [PubMed] [Google Scholar]
- 23.Lakshmi CP, Ghoshal UC, Kumar S, et al. Frequency and factors associated with small intestinal bacterial overgrowth in patients with cirrhosis of the liver and extra hepatic portal venous obstruction. Dig Dis Sci. 2010;55:1142–1148. doi: 10.1007/s10620-009-0826-0. [DOI] [PubMed] [Google Scholar]
- 24.Pande C, Kumar A, Sarin SK. Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease. Aliment Pharmacol Ther. 2009;29:1273–1281. doi: 10.1111/j.1365-2036.2009.03994.x. [DOI] [PubMed] [Google Scholar]
- 25.Park JH, Park DI, Kim HJ, et al. The relationship between small-intestinal bacterial overgrowth and intestinal permeability in patients with irritable bowel syndrome. Gut Liver. 2009;3:174–179. doi: 10.5009/gnl.2009.3.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatterjee S, Park S, Low K, Kong Y, Pimentel M. The degree of breath methane production in IBS correlates with the severity of constipation. Am J Gastroenterol. 2007;102:837–841. doi: 10.1111/j.1572-0241.2007.01072.x. [DOI] [PubMed] [Google Scholar]
- 27.Chang L, Lee OY, Naliboff B, Schmulson M, Mayer EA. Sensation of bloating and visible abdominal distension in patients with irritable bowel syndrome. Am J Gastroenterol. 2001;96:3341–3347. doi: 10.1111/j.1572-0241.2001.05336.x. [DOI] [PubMed] [Google Scholar]
- 28.Lembo T, Naliboff B, Munakata J, et al. Symptoms and visceral perception in patients with pain-predominant irritable bowel syndrome. Am J Gastroenterol. 1999;94:1320–1326. doi: 10.1111/j.1572-0241.1999.01009.x. [DOI] [PubMed] [Google Scholar]
- 29.Serra J, Salvioli B, Azpiroz F, Malagelada JR. Lipid-induced intestinal gas retention in irritable bowel syndrome. Gastroenterology. 2002;123:700–706. doi: 10.1053/gast.2002.35394. [DOI] [PubMed] [Google Scholar]
- 30.Serra J, Azpiroz F, Malagelada JR. Impaired transit and tolerance of intestinal gas in the irritable bowel syndrome. Gut. 2001;48:14–19. doi: 10.1136/gut.48.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salvioli B, Serra J, Azpiroz F, et al. Origin of gas retention and symptoms in patients with bloating. Gastroenterology. 2005;128:574–579. doi: 10.1053/j.gastro.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 32.Salvioli B, Serra J, Azpiroz F, Malagelada JR. Impaired small bowel gas propulsion in patients with bloating during intestinal lipid infusion. Am J Gastroenterol. 2006;101:1853–1857. doi: 10.1111/j.1572-0241.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- 33.Serra J, Azpiroz F, Malagelada JR. Intestinal gas dynamics and tolerance in humans. Gastroenterology. 1998;115:542–550. doi: 10.1016/s0016-5085(98)70133-7. [DOI] [PubMed] [Google Scholar]
- 34.Vernia P, Di Camillo M, Marinaro V. Lactose malabsorption, irritable bowel syndrome and self-reported milk intolerance. Dig Liver Dis. 2001;33:234–239. doi: 10.1016/s1590-8658(01)80713-1. [DOI] [PubMed] [Google Scholar]
- 35.Dong WZ, Zou DW, Li ZS, et al. Study of visceral hypersensitivity in irritable bowel syndrome. Chin J Dig Dis. 2004;5:103–109. doi: 10.1111/j.1443-9573.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- 36.Iovino P, Tremolaterra F, Boccia G, Miele E, Ruju FM, Staiano A. Irritable bowel syndrome in childhood: visceral hypersensitivity and psychosocial aspects. Neurogastroenterol Motil. 2009;21:e940–e974. doi: 10.1111/j.1365-2982.2009.01303.x. [DOI] [PubMed] [Google Scholar]
- 37.Balsari A, Ceccarelli A, Dubini F, Fesce E, Poli G. The fecal microbial population in the irritable bowel syndrome. Microbiologica. 1982;5:185–194. [PubMed] [Google Scholar]
- 38.Nobaek S, Johansson ML, Molin G, Ahrné S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1231–1238. doi: 10.1111/j.1572-0241.2000.02015.x. [DOI] [PubMed] [Google Scholar]
- 39.O'Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses sand relationship to cytokine profiles. Gastroenterology. 2005;128:541–551. doi: 10.1053/j.gastro.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 40.Ghoshal UC, Park H, Gwee KA. Bugs and irritable bowel syndrome: The good, the bad and the ugly. J Gastroenterol Hepatol. 2010;25:244–251. doi: 10.1111/j.1440-1746.2009.06133.x. [DOI] [PubMed] [Google Scholar]
- 41.Kerlin P, Wong L. Breath hydrogen testing in bacterial overgrowth of the small intestine. Gastroenterology. 1988;95:982–988. doi: 10.1016/0016-5085(88)90173-4. [DOI] [PubMed] [Google Scholar]
- 42.Corazza GR, Strocchi A, Gasbarrini G. Fasting breath hydrogen in celiac disease. Gastroenterology. 1987;93:53–58. doi: 10.1016/0016-5085(87)90313-1. [DOI] [PubMed] [Google Scholar]
- 43.Perman JA, Modler S, Barr RG, Rosenthal P. Fasting breath hydrogen concentration: normal values and clinical application. Gastroenterology. 1984;87:1358–1363. [PubMed] [Google Scholar]