Abstract
We studied the impact of MAOA genotype, childhood sexual assault, and harsh discipline on clinical externalizing symptoms (substance problems, adult antisocial behavior, and conduct disorder). Participants were 841 individual twins from the Minnesota Twin Family Study assessed through age 25. MAOA genotype was not associated with differences in any phenotype, nor was there a significant interaction between MAOA and harsh discipline for any phenotype or a significant interaction between MAOA and childhood sexual assault for substance problems. We found evidence that childhood sexual assault interacted with MAOA genotype to predict antisocial behavior and conduct disorder symptoms. Individuals with the low MAOA activity genotype who reported childhood sexual assault had more symptoms than individuals with either the high MAOA activity genotype and/or no history of childhood sexual assault. These findings suggest that the previously reported interaction between MAOA and childhood maltreatment may be specific to the antisocial subset of externalizing disorders.
Keywords: gene-environment interaction, substance use disorders, antisocial behavior, conduct disorder, MAOA, childhood sexual assault, harsh discipline
Childhood sexual assault, harsh discipline, and MAOA genotype: An investigation of main and interactive effects on diverse clinical externalizing outcomes
The gene that codes for the enzyme monoamine oxidase A (MAOA), which is involved in the degradation of biogenic amines including the neurotransmitters serotonin, norepinephrine and dopamine, is located in humans on the X chromosome. A functional variable number of tandem repeats (VNTR) polymorphism in the MAOA promoter region has been widely investigated. Number of repeats of the 30bp sequence is typically dichotomized into those that result in either high or low MAOA activity in vitro (Sabol et al. 1998; although the classification of the 5-repeat allele has been disputed, cf. Deckert et al. 1999). Low MAOA activity has been implicated in increased antisocial, aggressive, and violent behavior (in males: Alia-Klein et al. 2008; Beitchman et al. 2004; Kim-Cohen et al. 2006; Manuck et al. 2000; Reif et al. 2007; in males, with non-significant results for females: Guo et al. 2008; Huang et al. 2004; and in females: Prom-Wormley et al. 2009), though null results of this main effect have also been reported (in males: Caspi et al. 2002; Huizinga et al. 2006; Koller et al. 2003).
The relationship between MAOA and antisocial, aggressive, and violent behavior has been reported to be moderated by childhood maltreatment, such that individuals with low MAOA activity who experienced childhood maltreatment are at increased risk for these externalizing behaviors during childhood (in males: Foley et al. 2004; Kim-Cohen et al. 2006; Nilsson et al. 2006) and as adults (in males: Caspi et al. 2002; in males, with non-significant results for females: Frazzetto et al. 2007; Huang et al. 2004; and in females: Ducci et al. 2008;). This gene-by-environment (GxE) interaction does not seem to be due to either passive or evocative gene-environment correlation (e.g. in males: Caspi et al. 2002; Kim-Cohen et al. 2006; in females: Prom-Wormley et al. 2009; and in both males and females, using twin modeling: Shulz-Heik et al. 2009).
Evidence suggests that this moderating relationship may be specific to those of European descent (Widom & Brzustowicz 2006), and the interaction is not universally detected (in males: Huizinga et al. 2006; Reif et al. 2007; van der Vegt et al. 2009; Young et al. 2006; and in females: Prom-Wormley et al. 2009; Sjöberg et al. 2007), even in predominantly Caucasian samples. A potential source of heterogeneity within this literature may be differences in MAOA expression between males and females. As MAOA is located on the X-chromosome, females each have two copies of the MAOA gene, while males have one copy each. Typically in females, random X-inactivation serves a dosage compensation function to ensure that, for example, a female with two high MAOA activity alleles would not produce twice as much MAOA as a male with one high MAOA allele. However, the process of X-inactivation and expression specifically at the site of MAOA is not yet fully understood, and appears to be less complete and more subject to additional factors than for genes located elsewhere on the X-chromosome (e.g. Carrel & Willard 2005; Pinsonneault et al. 2006).
While most studies collapse across a broad range of maltreatment types (e.g. neglect, emotional, physical, and sexual, Caspi et al. 2002; Foley et al. 2004; Huang et al. 2004; Nilsson et al. 2006), two studies of males examined childhood physical maltreatment alone, with one replicating the moderation effect (Kim-Cohen et al. 2006) and one not (Huizinga et al. 2006). Of those studies examining childhood sexual assault alone, one succeeded at replicating the MAOA-abuse interaction as previously observed (Ducci et al. 2008), while another found a significant interaction but in the opposite direction (i.e. the high activity genotype was a risk factor when sexual assault had been reported, Sjöberg et al. 2007), though both studies included only female participants. An investigation of four types of maltreatment (physical, sexual, verbal, and neglect) found no evidence of any type interacting with MAOA to explain conduct disorder symptoms in a male clinical sample (Young et al. 2006).
Though the mechanism of interaction is still unclear, Buckholtz and Meyer-Lindenberg (2008) suggest that a reduction in social evaluation and emotional regulation abilities in individuals low in MAOA activity may result in an amplification of adverse environmental effects. An experimental study demonstrated that low MAOA individuals display greater aggression in high provocation situations (but they did not differ from high MAOA individuals in low provocation situations), suggesting that we may see GxE effects only in significantly threatening environments (McDermott et al. 2009). However, a replication of the moderating effect of maltreatment on MAOA showed the opposite effect of severity stratification, where genotype was only influential on aggression in children who had experienced moderate trauma. Those experiencing extreme abuse displayed increased aggression regardless of genotype (Weder et al. 2009).
Previous research on the structure of antisocial behavior and substance problems support the exploration of whether this GxE interactive effect extends to substance problems. Twin studies indicate that antisocial disorders such as conduct disorder and substance use disorders are part of a coherent externalizing or disinhibitory spectrum of disorders (e.g. Kendler et al. 2003; Krueger et al. 2002; Young et al. 2000). Genetic influences account for a greater proportion of variance (approximately 80%) in the externalizing factor connecting these phenotypes, when compared with any of its individual indicators (e.g. Krueger et al. 2002; Young et al. 2000). These common genetic influences are largely specific to externalizing behaviors, and do not account for a substantial proportion of variance (i.e. less than 10%) in internalizing disorders (such as major depression, generalized anxiety disorder, and specific phobias, e.g. Kendler et al. 2003). Compared to the common genetic influences shared among externalizing behaviors, there may be environmental influences on externalizing that are disorder-specific (e.g. Kendler et al. 2003; Krueger et al. 2002; Young et al. 2000).
The consistently observed presence of a coherent externalizing spectrum raises the question of whether previous findings of the interactive effect between MAOA genotype and childhood maltreatment extend beyond antisocial behavior and aggression to other externalizing behaviors. We are aware of only three previous studies of the interaction between MAOA genotype and childhood adversity that examined substance problems, all of which examined only alcohol problems. Ducci et al. (2008) demonstrated a significant interaction between MAOA genotype and childhood sexual abuse on alcoholism in American Indian females, although the effect was strongest for those individuals with more overall externalizing problems (i.e., both alcoholism and antisocial personality disorder). Nilsson et al. (2007) reported evidence for the typically-observed interaction between MAOA and family relations or childhood maltreatment/abuse on male adolescent alcohol-related problems (i.e. low activity genotype combined with an adverse environment predicted increased alcohol problems). In a complementary adolescent female sample, Nilsson et al. (2008) demonstrated an interaction between family relations and MAOA genotype on alcohol-related problems in the opposite direction of what has been typically observed in studies of aggressive/antisocial behavior (i.e., the high MAOA activity genotype was a risk factor for alcohol-related problems when paired with poor quality family relations), although the evidence for an interaction between MAOA and abuse/maltreatment in their female sample was more equivocal than the evidence for interaction between MAOA and family relations in those same females.
The current study
Previous studies of GxE interactions between MAOA and childhood maltreatment on externalizing behaviors tend to collapse across the broad range of childhood maltreatment (e.g. Caspi et al. 2002; Foley et al. 2004; Huang et al. 2004; Nilsson et al. 2006) or focus on either physical maltreatment (Huizinga et al. 2006; Kim-Cohen et al. 2006) or childhood sexual assault (CSA; Ducci et al. 2007; Sjöberg et al. 2007) without statistically controlling for the other maltreatment type. These studies of MAOA, childhood maltreatment, and externalizing behaviors also rarely include measures of substance problems, despite evidence that substance problems and antisocial behavior share common genetic influences (e.g. Kendler et al. 2003; Krueger et al. 2002; Young et al. 2000). We therefore explored moderation of the effect of MAOA genotype on a broad range of adult clinical externalizing symptoms (substance problems, adult antisocial behavior, and conduct disorder) by physical harsh discipline (HD) and/or childhood sexual abuse (CSA) in a community sample.
Methods
Participants
The Minnesota Twin Family Study (MTFS) includes a state-wide sample of same-sex twins recruited in several waves with birth years ranging from 1971 to 1985, who were initially assessed at either age 11 (younger cohort) or age 17 (older cohort) and followed up regularly thereafter (as described by Iacono and McGue 2002). Our sample included 841 MTFS twin participants (29.3% female, 98.4% Caucasian) with available data on lifetime substance problems and adult antisocial behavior symptoms through age 25, lifetime conduct disorder symptoms assessed at age 17, HD, CSA, and MAOA genotype. The relatively low number of females reflects the fact that females who were heterozygous for the High-Low activity genotype were not included in our sample due to uncertain MAOA activity level (e.g. Caspi et al. 2002 note 30).
Measures
Substance problems
Lifetime data on DSM-IV criteria for substance abuse and dependence symptoms through approximately age 25 were assembled from longitudinal assessments. Substance abuse and dependence symptoms were not separated as previous research has shown them to be well conceptualized as single, underlying dimensions of problem use (e.g. Langenbucher et al. 2004; Martin et al. 2006; Gillespie et al. 2007; Harford et al. 2009). Alcohol, tobacco (dependence only, with six possible symptoms as tolerance was not assessed), and cannabis were the individual substances with the greatest number of symptoms endorsed in the current sample.
All participants were assessed for substance use disorder symptoms via the expanded Substance Abuse Module (SAM) of the Composite International Diagnostic Interview (CIDI, Robins et al. 1987) at approximately ages 17, 21 and 25. In addition, the cohort who began the study at age 11 was assessed via a slightly modified version of the Diagnostic Interview for Children and Adolescents-Revised (DICA-R, Reich & Welner 1988) at ages 11 and 14. Symptoms were assessed lifetime at intake (age 11 or 17) and since the last assessment at each follow-up visit. At ages 11, 14, and 17, mothers of the twins were also asked about their children's substance use disorder symptoms. A symptom was considered present if either participants or their mothers endorsed it (and a consensus committee of at least two graduate-level diagnosticians agreed that it had definitely been endorsed) at any assessment. The consensus process and the rationale for combining both child and mother reports of externalizing behavior in the MTFS are discussed in Burt et al. (2001). Each individual received a count of symptoms that had definitely been met for each specific substance, which was then log transformed [ln(symptoms+1)] to adjust for the skewed nature of the data.
Antisocial behavior and conduct disorder
Lifetime prevalence of the DSM-IV criterion A symptoms of antisocial personality disorder was assessed at ages 21 and 25, and lifetime prevalence of DSM-III-R conduct disorder symptoms that onset prior to age 15 was assessed at age 17. A structured interview developed by the MTFS staff was used (Krueger et al. 2002) and a symptom was considered present if a consensus committee of at least two graduate-level diagnosticians agreed that the criteria had definitely been met. Each individual received log-transformed [ln(symptoms+1)] counts of the total number of symptoms ever endorsed for each of antisocial behavior and conduct disorder.
Harsh discipline
Harsh discipline (HD) exposure was assessed via retrospective self-report at age 21. As part of an assessment of social adjustment, participants were asked a question about typical discipline while growing up. Endorsement of experiencing either “hit in the face” or “hit with an object” as methods of typical discipline was considered to be indicative of HD exposure. HD exposure was coded as ‘1’, and non-exposure (i.e. responding to both items in the negative) was coded as ‘0’.
Childhood sexual assault
Childhood sexual assault (CSA) experience was assessed via retrospective self-report at ages 21 and 25. Participants were asked two questions about CSA as part of a pre-diagnostic interview screen for Post-Traumatic Stress Disorder (based on the Trauma Assessment of Adults – Self-Report Version, Resnick et al. 1993): “Did you ever have sexual contact with anyone five years or more older than you before you reached the age of 13?” and “Has anyone, including a friend or relative, ever used pressure, force, or physical threats to make you have some type of unwanted sexual contact?”, as well as the age at which they first experienced it. Any CSA experience through age 16 was coded as ‘1’, and non-exposure (i.e. responding to both items in the negative at either assessment) was coded as ‘0’.
Genotyping
MAOA was assessed from participants’ peripheral blood samples (84.1%) or buccal cell swabs (15.9%) using primers and PCR protocol as described in Haberstick et al. (2005). Individuals were dichotomized for MAOA activity level, with high activity indicated by 3.5 or 4 repeats for the MAOA gene and low activity indicated by 2, 3, or 5 repeats (as established in Caspi et al. 2002). MAOA was coded ‘0’ for high activity, ‘1’ for low activity (the previously implicated high-risk genotype, e.g. Caspi et al. 2002). Females heterozygous for high-low activity number of repeats on the MAOA functional VNTR polymorphism were not included in our analyses, due to uncertain X-inactivation and resulting in vivo MAOA activity levels.
Analyses
Data on all predictor and outcome variables were available from 841 individuals. Gene-environment correlations between MAOA and HD or CSA were modeled as logistic regressions with MAOA regressed on HD and CSA, and sex included as a covariate (with females coded 0, males coded 1). GxE interactions were modeled as regressions, including sex as a covariate; main effects of HD, CSA, and MAOA genotype; and two-way interactions between MAOA and each of HD and CSA, as well as between HD and CSA. The three-way interaction between MAOA, HD, and CSA was not modeled due to small cell counts, e.g. only two people in our sample had low MAOA and experienced both HD and CSA; see Table 1 for an accounting of participant distribution among all possible MAOA genotype-by-HD/CSA exposure groups.
Table 1.
Participants in each potential harsh discipline/childhood sexual assault-by-MAOA group.
| MAOA Genotype |
||
|---|---|---|
| Reported experience | High | Low |
| Neither | 463 (55.1%; 33.5% female) | 220 (26.2%; 24.5% female) |
| Harsh Discipline | 91 (10.8%; 18.7% female) | 41 (4.9%; 9.8% female) |
| Sexual Assault | 13 (1.5%; 84.6% female) | 6 (0.7%; 33.3% female) |
| Both | 5 (0.6%; 40.0% female) | 2 (0.2%; 50.0% female) |
Effects on each substance (alcohol, tobacco, and cannabis), adult antisocial behavior, and conduct disorder symptoms were modeled as independent regressions, and sex was included as a covariate in each model. All analyses were conducted in Mplus (Muthén & Muthén 1998-2007) using the robust maximum likelihood estimator (MLR), taking into account the non-independent nature of the twin data by including family membership as a clustering variable.
Results
Descriptive statistics
HD was reported by 16.5% of the sample (19.3% males, 9.8% females). CSA was reported by 3.1% of the sample (1.7% males, 6.5% females). Genotypes coding for low MAOA activity were less frequent than those coding for high activity, accounting for 32.0% of the sample (35.0% males, 24.8% of homozygous females), which is similar to previous reports (e.g. Guo et al. 2008). Cell counts for all possible HD/CSA-by-MAOA groups are reported in Table 1. Descriptive statistics for the outcome measures (alcohol, tobacco, cannabis, adult antisocial behavior, and conduct disorder) are reported in Table 2. Skewness and kurtosis values for all outcome measures suggested no substantial deviations from normality following the log-transformation of symptom counts (i.e., |skewness| < 2 for all, |kurtosis| < 2 for all, e.g. Kline 2005).
Table 2.
Descriptive statistics for clinical outcome measures.
| Alcohol | Tobacco | Cannabis | Antisocial Behavior | Conduct Disorder | |
|---|---|---|---|---|---|
| Symptom counts | |||||
| Mean | 1.842 | 1.423 | 0.936 | 1.467 | 0.824 |
| St. Dev. | 2.307 | 1.897 | 2.172 | 1.434 | 1.356 |
| Range | 0 - 11 | 0 - 6 | 0 - 11 | 0 - 7 | 0 - 10 |
| Inter-Quartile Range | 1 - 3 | 0 - 3 | 0 - 0 | 1 - 2 | 0 - 1 |
| Log-transformed symptom counts | |||||
| Mean | 0.753 | 0.600 | 0.340 | 0.748 | 0.418 |
| St. Dev. | 0.750 | 0.734 | 0.672 | 0.557 | 0.557 |
Zero-order correlations among all model variables, taking into account the non-independent nature of the twin data, are reported in Table 3. Sex was significantly correlated with all variables at the p<0.05 level, which highlights the importance of including sex as a covariate. HD and CSA were not significantly correlated (r=0.050, p=0.216), suggesting the potential utility of modeling their separate effects. Log-transformed alcohol, tobacco, cannabis, antisocial behavior, and conduct disorder symptoms were all significantly inter-correlated (r=0.281-0.626, p<0.001).
Table 3.
Correlations among all model variables.
| Sex | 1. | 2. | 3. | 4. | 5. | 6. | 7. | |
|---|---|---|---|---|---|---|---|---|
| 1. Harsh discipline | 0.117*** | |||||||
| 2. Sexual assault | -0.127** | 0.050 | ||||||
| 3. MAOA genotype | 0.099* | -0.010 | -0.005 | |||||
| 4. Alcohol | 0.300*** | 0.138** | 0.016 | 0.054 | ||||
| 5. Tobacco | 0.097** | 0.111** | 0.112** | -0.056 | 0.476*** | |||
| 6. Cannabis | 0.089* | 0.131** | 0.093 | -0.026 | 0.512*** | 0.467*** | ||
| 7. Antisocial | 0.288*** | 0.170*** | 0.039 | 0.077* | 0.626*** | 0.479*** | 0.514*** | |
| 8. Conduct disorder | 0.313*** | 0.224*** | 0.045 | 0.007 | 0.348*** | 0.281*** | 0.328*** | 0.441*** |
Note. Sex and variables 1-3 are coded 0/1 with the "high risk" group (i.e. males, HD/CSA exposure, low MAOA) coded 1.
p < 0.05
p < 0.01
p < 0.001
Gene-environment correlation
Cell counts for MAOA genotype by HD/CSA status are presented in Table 1. When modeled as a logistic regression of MAOA activity level on HD and CSA exposure, with sex included as a covariate, low MAOA genotype was not significantly associated with either HD (b=-0.080, OR=0.923, p=0.553) or CSA (b=0.069, OR=1.071, p=0.817).
Effects of harsh discipline, childhood sexual assault, and MAOA genotype
Regression analysis results for the effects of HD, CSA, and MAOA are reported in Table 4. Sex was a significant (p<0.02) covariate for all outcome phenotypes, with males reporting a greater number of symptoms than females. HD was significantly associated with an increase in reported symptoms (p<0.04) for all outcomes except cannabis. CSA was not associated with a significant difference in any outcome measure (p>0.21 for all), though positive coefficients for all outcomes except alcohol suggested that the tendency was for greater symptom reports when CSA was present. In these regression models, there was no main effect of MAOA genotype on any outcome variable (p>0.09 for all), nor were there interactions observed between HD and CSA (p>0.07 for all) or MAOA and HD (p>0.33 for all).
Table 4.
Regression results from GxE models of harsh discipline (HD), childhood sexual assault (CSA), and MAOA genotype influences on externalizing disorder symptoms.
| Alcohol |
Tobacco |
Cannabis |
Antisocial Behavior |
Conduct Disorder |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b | Z | p | b | Z | p | b | Z | p | b | Z | p | b | Z | p | |
| Intercept | 0.368 | 7.842 | <0.001 | 0.473 | 9.514 | <0.001 | 0.231 | 4.940 | <0.001 | 0.456 | 12.509 | <0.001 | 0.120 | 4.443 | <0.001 |
| Sex | 0.474 | 8.373 | <0.001 | 0.168 | 2.825 | 0.005 | 0.132 | 2.456 | 0.014 | 0.333 | 7.827 | <0.001 | 0.365 | 10.395 | <0.001 |
| HD | 0.208 | 2.189 | 0.029 | 0.183 | 2.087 | 0.037 | 0.135 | 1.400 | 0.161 | 0.210 | 3.208 | 0.001 | 0.274 | 3.718 | <0.001 |
| CSA | -0.020 | -0.123 | 0.902 | 0.238 | 1.241 | 0.215 | 0.136 | 0.641 | 0.521 | 0.020 | 0.122 | 0.903 | 0.055 | 0.423 | 0.673 |
| MAOA genotype | 0.033 | 0.526 | 0.599 | -0.108 | -1.687 | 0.092 | -0.082 | -1.542 | 0.123 | 0.056 | 1.187 | 0.235 | -0.044 | -1.087 | 0.277 |
| HD*CSA | 0.232 | 0.643 | 0.520 | 0.423 | 1.768 | 0.077 | 0.586 | 1.469 | 0.142 | 0.194 | 0.920 | 0.358 | 0.011 | 0.055 | 0.956 |
| HD*MAOA | -0.036 | -0.200 | 0.842 | -0.058 | -0.364 | 0.716 | 0.156 | 0.969 | 0.332 | -0.051 | -0.391 | 0.696 | 0.013 | 0.106 | 0.916 |
| CSA*MAOA | 0.531 | 1.551 | 0.121 | 0.523 | 1.936 | 0.053 | 0.295 | 0.715 | 0.475 | 0.470 | 2.467 | 0.014 | 0.576 | 2.697 | 0.007 |
Note. Predictor variables are coded 0/1 with the "high risk" group (i.e. males, HD/CSA exposure, low MAOA) coded 1. Significant terms (p < 0.05) are indicated in bold.
Model: DV = b0 + b1sex + b2HD+ b3CSA + b4MAOA + b5HD*CSA+ b6HD*MAOA + b7CSA*MAOA
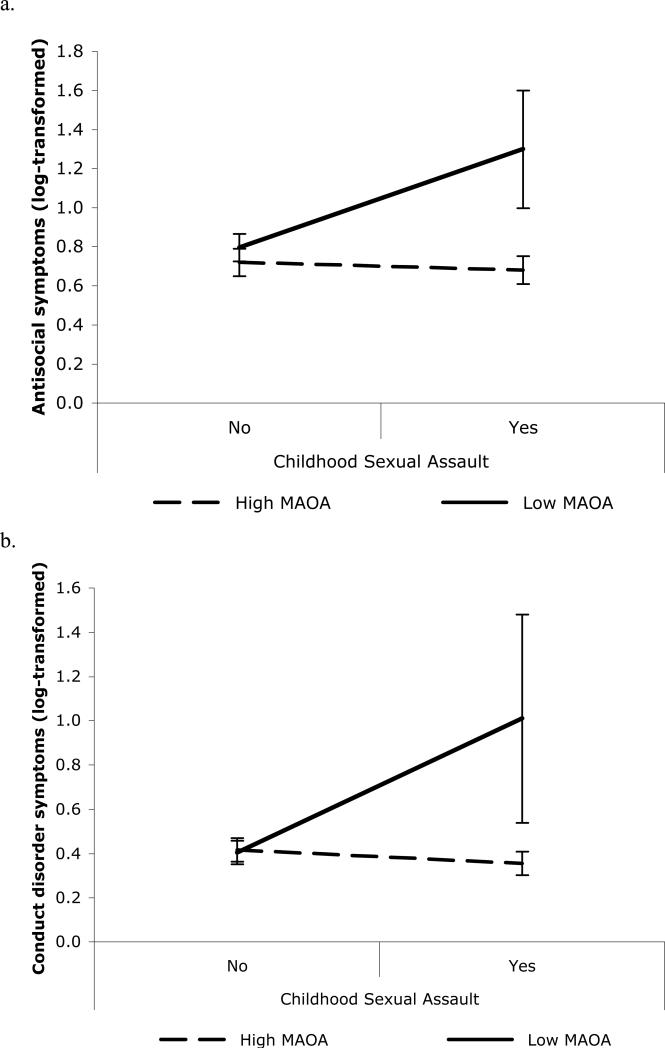
There was a significant interaction between CSA exposure and MAOA genotype on adult antisocial behavior (p=0.014; for this significant interaction alone, ΔR2=0.4%) and conduct disorder symptoms (p=0.007, ΔR2=0.7%). Figure 11 shows that individuals with the low MAOA genotype and a history of CSA reported more symptoms of adult antisocial behavior (Figure 1a) and conduct disorder (Figure 1b) than individuals who had no reported history of CSA and/or the high MAOA genotype. The interaction between CSA and MAOA was not significant for any substance problem (p>0.05). The interpretation of a gene-by-environment interaction assumes that the environmental exposure (in this case, CSA) preceded the emergence of or a change in the outcome(s) of interest. In our sample, the 26 individuals who experienced CSA reported an average age at first CSA exposure of 8.46 years (SD = 4.38, range = 3-16, median = 7). There was no significant difference in average age at first CSA exposure between high MAOA (N = 18, M = 7.78, SD = 4.54) and low MAOA (N = 8, M = 10.00, SD = 3.78) genotype groups [t(24) = -1.21, p = 0.24].
Figure 1.
Individuals with the low MAOA activity genotype and a history of childhood sexual assault reported more a) adult symptoms of antisocial personality disorder (p=0.014); and b) conduct disorder symptoms (p=0.007). (Note. Error bars represent 95% confidence intervals. Cell sizes for each group were as follows: high MAOA, no CSA = 554; low MAOA, no CSA = 261; high MAOA, CSA = 18; low MAOA, CSA = 8.)
Power estimation
We undertook analyses to ensure that we had adequate power to detect GxE in our sample. Power to detect effects on our outcome measures given our sample size was calculated in the program Quanto (Gauderman & Morrison 2007). Of our total sample of 841 individuals, 608 were members of twin pairs for whom complete data was available and 233 were “singletons” (participants for whom complete data from their corresponding twin was not available). To correct for the non-independent nature of data obtained from twins, each member of a complete twin pair was counted as half of an observation for the purposes of our power analyses (representing a conservative adjustment of our sample size). This resulted in a corrected sample size of 537. Working from the additional information provided by our findings, we simulated several assumptions, using the minor-allele recessive inheritance model (i.e., comparing two forms of a gene, in our case, high and low MAOA activity), minor allele frequency (Plow=32.0%), probability of HD or CSA exposure (PHD=16.5%, PCSA=3.1%), and a standard normal phenotypic distribution. We estimated a range of effects that we would have the power to detect, based on our conservatively adjusted sample size of 537 and our unadjusted sample size of 841. In terms of GxE interactions between MAOA and either HD or CSA, we had 80% power to detect effects accounting for 0.92-1.41% of the variance in outcomes, and 90% power to detect effects of 1.22-1.87%.
Discussion
Our regression analyses showed that retrospective reports of HD were associated with having more clinical symptoms across phenotypes (except for cannabis, where the effect was not significant). There was a non-significant trend of CSA exposure being associated with elevated symptom counts (except for alcohol). We found no evidence of a gene-environment correlation between HD or CSA exposure and MAOA genotype, nor for gene-environment interaction between MAOA genotype and HD on externalizing outcomes through young adulthood. There was evidence for a significant interactive effect between MAOA genotype and CSA exposure, in which individuals with the low MAOA activity genotype who reported a history of CSA had more symptoms of the antisocial subset of externalizing disorders (antisocial behavior, p=0.014; and conduct disorder symptoms, p=0.007). The interaction between MAOA genotype and CSA was not significant for any substance problem.
These findings should be interpreted in light of a number of limitations. Our sample consisted of young adult twins born in Minnesota, and so may not generalize to other populations. Due to the low rate of CSA in our community-based sample, we were unable to model the potential three-way interaction between MAOA, HD, and CSA, nor were we able to model differential effects by sex (owing to considerably reduced cell sizes), though the current literature is mixed as to the effect of sex on this GxE interaction (e.g. Ducci et al. 2008; Frazzetto et al. 2007; Nilsson et al. 2007; Nilsson et al. 2008; Sjöberg et al. 2007). Our lifetime symptom counts for each externalizing disorder represent the aggregation of longitudinal data from clinical interviews, with all symptoms agreed upon by a diagnostic consensus committee, and so are likely to be more valid than a single lifetime retrospective self-report of symptoms. However, our self-report measures of both HD and CSA were necessarily retrospective (to avoid mandatory reporting requirements) and limited in scope due to time constraints implicit in including a broad range of assessments in a single-day study visit. In addition, our HD measure was unlikely to have captured only severe physical maltreatment; some punishments encompassed by our HD measure may fall within what some would consider the normal range of parental discipline appropriate for children. An additional limitation of our retrospective HD measure was that we would be unable to disentangle with certainty the temporal ordering of the HD experience and the onset of pre-adulthood externalizing symptoms. These limitations of our HD and CSA measures are typical of the GxE literature on this topic (e.g. Ducci et al. 2008; Huang et al. 2004), and so should not affect our ability to replicate and extend the findings of previous research.
The categorical nature of our HD and CSA measures and the low prevalence of individuals who reported exposure to both HD and CSA (0.8%) in our community sample resulted in us being unable to examine the potentially important effects of HD or CSA severity (e.g. McDermott et al. 2009; Weder et al. 2009), and GxE studies of stress and candidate genes tend to be underpowered in general (Munafò et al. 2009). While we were likely underpowered to detect a small main effect of MAOA on externalizing behavior, power estimation suggested that our sample size provided at least 80% power to detect an effect size accounting for around 1% of the variance in clinical externalizing indicators. We did not find evidence for a GxE interactive effect on substance problems through age 25. Our analyses indicated that MAOA genotype interacted with exposure to CSA specifically on antisocial behavior (ΔR2=0.4%) and conduct disorder symptoms (ΔR2=0.7%), where individuals at risk from both predictors (i.e. low MAOA activity genotype coupled with CSA exposure) reported a greater number of symptoms.
This evidence for an interactive effect between MAOA genotype and CSA exposure on antisocial clinical outcomes (see Figure 1) is consistent with previous findings of GxE interactions on antisocial and aggressive behavior, where the presence of both risk factors (i.e. low MAOA and CSA) confers additional risk (e.g. Caspi et al. 2002). This effect was not observed in the interaction between HD and MAOA genotype, suggesting potential further specificity of the interaction between MAOA genotype and early life stress to CSA exposure (when statistically controlling for HD). However, since the low prevalence of CSA in our community sample resulted in only eight individuals being in the low MAOA and CSA risk group (which is the expected cell count given our sample size and prevalences), these results may be capitalizing on chance. Nevertheless, our study provides the first evidence we are aware of regarding the specificity of this GxE effect between MAOA genotype and CSA to the antisocial subset of externalizing disorders (despite the highly inter-correlated nature of antisocial behavior and conduct disorder with substance problems).
This observed pattern of effects fits with recent research on the hierarchical structure of externalizing behaviors. The comorbidity among substance problems, antisocial personality disorder, and conduct disorder corresponds with the highest-order factor in the hierarchy, general externalizing tendencies (e.g. Kendler et al. 2003; Krueger et al. 2002; Young et al. 2000). However, moving down one level in the structural hierarchy, two narrower, lower-order factors emerge, loading on substance-related and aggressive/antisocial indices, respectively (Krueger et al. 2007, Krueger et al. 2009). The pattern of influence in the MAOA-by-maltreatment interaction detected here and elsewhere suggests that the interaction may be operating specifically at this lower level of the externalizing spectrum hierarchy, where the distinction between antisocial and substance-related problems is seen phenotypically.
In sum, we report here the first evidence for potential specificity of the interaction between MAOA and maltreatment to antisocial aspects of the broader externalizing spectrum. These findings may fit the conceptualization of MAOA as a gene for plasticity because the “risk allele” (i.e., those alleles coding for low MAOA activity) only increased antisocial behavior in conjunction with a “risk environment.” To more fully test a plasticity account of the impact of MAOA polymorphisms on behavior, it would be helpful to assess the effects of the gene in the context of a profoundly positive environment (Belsky et al. 2009; Tabery 2009). The plasticity conceptualization implies a cross-over effect, indicating that both positive and negative features of an environment may shape the effect of a gene on a phenotype. That is, plasticity suggests that a certain genotype conveys added risk in a negative environment but protective effects in a positive environment (e.g. Kim-Cohen et al. 2006). Evaluating the plasticity hypothesis represents an intriguing direction for future gene-by-environment interaction research. Nevertheless, given the small effect size of the specific interaction we observed in our sample (ΔR2=0.4-0.7%), the absence of this interaction influencing substance problems, and the corresponding specificity to antisocial behavior, this interaction may be a chance observation that warrants replication before it is regarded as a reliable effect.
Acknowledgements
NIH grants DA05147 and AA09367.
Footnotes
Cell sizes for each group were as follows: high MAOA, no CSA = 554; low MAOA, no CSA = 261; high MAOA, CSA = 18; low MAOA, CSA = 8. While power considerations prevented us from analyzing males and females separately, the results shown in Figure 1 were consistent when males and females are plotted separately. Heterozygous females, who have been removed from our analyses due to uncertain MAOA activity levels, appear at an intermediate symptom level between females with high and low MAOA genotypes. These additional Figures are available from the first author upon request.
References
- Alia-Klein N, Goldstein RZ, Kriplani A, Logan J, Tomasi D, Williams B, Telang F, Shumay E, Biegon A, Craig IW, Henn F, Wang GJ, Volkow ND, Fowler JS. Brain monoamine oxidase A activity predicts trait aggression. J Neurosci. 2008;28:5099–5104. doi: 10.1523/JNEUROSCI.0925-08.2008. doi:10.1523/jneuroSCI.0925-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitchman JH, Mik HM, Ehtesham S, Douglas L, Kennedy JL. MAOA and persistent, pervasive childhood aggression. Mol Psychiatry. 2004;9:546–547. doi: 10.1038/sj.mp.4001492. doi:10.1038/sj.mp.4001492. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. doi:10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends Neurosci. 2008;31:120–129. doi: 10.1016/j.tins.2007.12.006. doi:10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Burt SA, Krueger RF, McGue M, Iacono WG. Sources of covariation among Attention-Deficit/Hyperactivity Disorder, Oppositional Defiant Disorder, and Conduct Disorder: The importance of shared environment. Journal of Abnormal Psychology. 2001;110:516–525. doi: 10.1037/0021-843X.110.4.516. doi: 10.1037//0021-843X.110.4.516. [DOI] [PubMed] [Google Scholar]
- Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. doi:10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. doi:10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Choi IG, Kee BS, Son HG, Ham BJ, Yang BH, Kim SH, Lee JS, Son BK, Lee BY, Lee SY, Chai YG, Shin HD. Genetic polymorphisms of alcohol and aldehyde dehydrogenase, dopamine and serotonin transporters in familial and non-familial alcoholism. Eur Neuropsychopharmacol. 2006;16:123–128. doi: 10.1016/j.euroneuro.2005.07.006. doi:10.1016/j.euroneuro.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Deckert J, Catalano M, Syagailo YV, Bosi M, Okladnova O, Di Bella D, Nöthen MM, Maffei P, Franke P, Fritze J, Maier W, Propping P, Beckmann H, Bellodi L, Lesch KP. Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet. 1999;8:621–624. doi: 10.1093/hmg/8.4.621. [DOI] [PubMed] [Google Scholar]
- Ducci F, Enoch MA, Hodgkinson C, Xu K, Cantena M, Robin RW, Goldman D. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Mol Psychiatry. 2008;13:334–347. doi: 10.1038/sj.mp.4002034. doi:10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- Foley DL, Eaves LJ, Wormley B, Silberg JL, Maes HH, Kuhn J, Riley B. Childhood adversity, monoamine oxidase A genotype, and risk for conduct disorder. Arch Gen Psychiatry. 2004;61:738–744. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- Frazzetto G, Di Lorenzo G, Carola V, Proietti L, Sokolowska E, Siracusano A, Gross C, Troisi A. Early trauma and increased risk for physical aggression during adulthood: The moderating role of MAOA genotype. PLoS ONE. 2007;5:e486. doi: 10.1371/journal.pone.0000486. doi:10.1371/journal.pone.0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauderman WJ, Morrison JM. QUANTO version 1.2.3: A computer program for power and sample size calculations for genetic-epidemiology studies. 2007 http://hydra.usc.edu/gxe.
- Gillespie NA, Neale MC, Prescott CA, Aggen SH, Kendler KS. Factor and item-response analysis DSM-IV criteria for abuse of and dependence on cannabis, cocaine, hallucinogens, sedatives, stimulants and opioids. Addiction. 2007;102:920–930. doi: 10.1111/j.1360-0443.2007.01804.x. doi:10.1111/j.1360-0443.2007.01804.x. [DOI] [PubMed] [Google Scholar]
- Guo G, Ou XM, Roettger M, Shih JC. The VNTR 2 repeat in MAOA and delinquent behavior in adolescence and young adulthood: Associations and MAOA promoter activity. Eur J Hum Genet. 2008;16:626–634. doi: 10.1038/sj.ejhg.5201999. doi:10.1038/sj.ejhg.5201999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberstick BC, Lessem JM, Hopfer CJ, Smolen A, Ehringer MA, Timberlake D, Hewitt JK. Monoamine oxidase A (MAOA) and antisocial behaviors in the presence of childhood and adolescent maltreatment. Am J Med Genet B Neuropsychiatr Genet. 2005;135B:59–64. doi: 10.1002/ajmg.b.30176. doi:10.1002/ajmg.b.30176. [DOI] [PubMed] [Google Scholar]
- Harford TC, Yi H, Faden VB, Chen CM. The dimensionality of DSM-IV alcohol use disorders among adolescent and adult drinkers and symptom patterns by age, gender, and race/ethnicity. Alcohol Clin Exp Res. 2009;33:1–11. doi: 10.1111/j.1530-0277.2009.00910.x. doi:10.1111/j.1530-0277.2009.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Cate SP, Battistuzzi C, Oquendo MA, Brent D, Mann JJ. An association between a functional polymorphism in the monoamine oxidase A gene promoter, impulsive traits, and early abuse experiences. Neuropsychopharmacology. 2004;29:1498–1505. doi: 10.1038/sj.npp.1300455. doi:10.1038/sj.npp.1300455. [DOI] [PubMed] [Google Scholar]
- Huizinga D, Haberstick BC, Smolen A, Menard S, Young SE, Corley RP, Stallings MC, Grotpeter J, Hewitt JK. Childhood maltreatment, subsequent antisocial behavior, and the role of monoamine oxidase A genotype. Biol Psychiatry. 2006;60:677–683. doi: 10.1016/j.biopsych.2005.12.022. doi:10.1016/j.biopsych.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Iacono WG, McGue M. Minnesota Twin Family Study. Twin Res. 2002;5:482–487. doi: 10.1375/136905202320906327. doi:10.1375/twin.5.5.482. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children's mental health: New evidence and a meta-analysis. Mol Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. doi:10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 2nd ed. Guilford Press; New York: 2005. [Google Scholar]
- Koller G, Bondy B, Preuss UW, Bottlender M, Soyka M. No association between a polymorphism in the promoter region of the MAOA gene with antisocial personality traits in alcoholics. Alcohol Alcohol. 2003;38:31–34. doi: 10.1093/alcalc/agg003. doi:10.1093/alcalc/agg003. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. doi:10.1037//0021-843X.111.3.411. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer M. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. J Abnorm Psychol. 2007;116:645–666. doi: 10.1037/0021-843X.116.4.645. doi:10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, South SC. Externalizing disorders: Cluster 5 of the proposed meta-structure for DSM-V and ICD-11. Psych Med. 2009;39:2061–2070. doi: 10.1017/S0033291709990328. doi:10.1017/S003329170999032. [DOI] [PubMed] [Google Scholar]
- Langenbucher JW, Labouvie E, Martin CS, Sanjuan PM, Bavly L, Kirisci L, Chung T. An application of item response theory analysis to alcohol, cannabis, and cocaine criteria in DSM-IV. J Abnorm Psychol. 2004;113:72–80. doi: 10.1037/0021-843X.113.1.72. doi:10.1037/0021-843X.113.1.72. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Mann JJ, Muldoon MF. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res. 2000;95:9–23. doi: 10.1016/s0165-1781(00)00162-1. doi:10.1016/S0165-1781(00)00162-1. [DOI] [PubMed] [Google Scholar]
- Martin CS, Chung T, Kirisci L, Langenbucher JW. Item response theory analysis of diagnostic criteria for alcohol and cannabis use disorders in adolescents: Implications for DSM-V. J Abnorm Psychol. 2006;115:807–814. doi: 10.1037/0021-843X.115.4.807. doi:10.1037/0021-843X.115.4.807. [DOI] [PubMed] [Google Scholar]
- McDermott R, Tingley D, Cowden J, Frazzetto G, Johnson DDP. Monoamine oxidase A gene (MAOA) predicts behavioral aggression following provocation. Proc Natl Acad Sci U S A. 2009;106:2118–2123. doi: 10.1073/pnas.0808376106. doi:10.1073/pnas.0808376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Durrant C, Lewis G, Flint J. Gene x environment interactions at the serotonin transporter locus. Biol Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. doi:10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide. Fifth Edition Muthén & Muthén; Los Angeles, CA: 1998-2007. [Google Scholar]
- Nilsson KW, Sjöberg RL, Damberg M, Leppert J, Öhrvik J, Alm PO, Lindström L, Oreland L. Role of monoamine oxidase A genotype and psychosocial factors in male adolescent criminal activity. Biol Psychiatry. 2006;59:121–127. doi: 10.1016/j.biopsych.2005.06.024. doi:10.1016/j.biopsych.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Sjöberg RL, Wargelius HL, Leppert J, Lindström L, Oreland L. The monoamine oxidase A (MAO-A) gene, family function and maltreatment as predictors of destructive behaviour during male adolescent alcohol consumption. Addiction. 2007;102:389–398. doi: 10.1111/j.1360-0443.2006.01702.x. doi: 10.1111/j.1360-0443.2006.01702.x. [DOI] [PubMed] [Google Scholar]
- Nilsson KW, Wargelius HL, Sjöberg RL, Leppert J, Oreland L. The MAO-A gene, platelet MAO-B activity and psychosocial environment in adolescent female alcohol-related problem behaviour. Drug Alcohol Depend. 2008;93:51–62. doi: 10.1016/j.drugalcdep.2007.08.022. doi: 10.1016/j.drugalcdep.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Pinsonneault JK, Papp AC, Sadée W. Allelic mRNA expression of X-linked monoamine oxidase a (MAOA) in human brain: Dissection of epigenetic and genetic factors. Hum Mol Genet. 2006;15:2636–2649. doi: 10.1093/hmg/ddl192. doi: 10.1093/hmg/ddl192. [DOI] [PubMed] [Google Scholar]
- Prom-Wormley EC, Eaves LJ, Foley DL, Gardner CO, Archer KJ, Wormley BK, Maes HH, Riley BP, Silberg JL. Monoamine oxidase A and childhood adversity as risk factors for conduct disorder in females. Psychol Med. 2009;39:579–590. doi: 10.1017/S0033291708004170. doi:10.1017/S0033291708004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W, Welner Z. Diagnostic Interview for Children and Adolescents – Revised: DSMIII-R version (DICA-R) Washington University; St. Louis: 1988. [Google Scholar]
- Reif A, Rosler M, Freitag CM, Schneider M, Eujen A, Kissling C, Wenzler D, Jacob CP, Retz-Junginger P, Thome J, Lesch K, Retz W. Nature and nurture predispose to violent behavior: Serotonergic genes and adverse childhood environment. Neuropsychopharmacology. 2007;32:2375–2383. doi: 10.1038/sj.npp.1301359. doi:10.1038/sj.npp.1301359. [DOI] [PubMed] [Google Scholar]
- Resnick HS, Best CL, Kilpatrick DG, Freedy JR, Falsetti SA. Trauma Assessment for Adults—Self-Report Version. National Crime Victims Research and Treatment Center, Medical University of South Carolina; Charleston, SC: 1993. Unpublished Scale. [Google Scholar]
- Robins LM, Babor T, Cottler LB. Composite International Diagnostic Interview: Expanded Substance Abuse Module. Authors; St. Louis: 1987. [Google Scholar]
- Sabol SZ, Hu S, Hamer S. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet. 1998;103:273–279. doi: 10.1007/s004390050816. doi:10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Shulz-Heik RJ, Rhee SH, Silvern L, Lessem JM, Haberstick BC, Hopfer C, Hewitt JK. Investigation of genetically mediated child effects on maltreatment. Behav Genet. 2009;39:265–276. doi: 10.1007/s10519-009-9261-4. doi:10.1007/s10519-009-9261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg RL, Nilsson KW, Wargelius HL, Leppert J, Lindström L, Oreland L. Adolescent girls and criminal activity: Role of MAOA-LPR genotype and psychosocial factors. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:159–164. doi: 10.1002/ajmg.b.30360. doi:10.1002/ajmg.b.30360. [DOI] [PubMed] [Google Scholar]
- Tabery J. From a genetic predisposition to an interactive predisposition: rethinking the ethical implications of screening for gene-environment interactions. J Med Philos. 2009;34:27–48. doi: 10.1093/jmp/jhn039. doi:10.1093/jmp/jhn039. [DOI] [PubMed] [Google Scholar]
- van der Vegt EJM, Oostra BA, Arias-Vasquez A, van der Ende J, Verhulst FC, Tiemeier H. High activity Monoamine oxidase A is associated with externalizing behaviour in maltreated and nonmaltreated adoptees. Psychiatr Genet. 2009;19:209–211. doi: 10.1097/YPG.0b013e32832a5084. doi: 10.1097/YPG.0b013e32832a5084. [DOI] [PubMed] [Google Scholar]
- Weder N, Yang BZ, Douglas-Palumberi H, Massey J, Krystal JH, Gelernter J, Kaufman J. MAOA genotype, maltreatment, and aggressive behavior: The changing impact of genotype at varying levels of trauma. Biol Psychiatry. 2009;65:417–424. doi: 10.1016/j.biopsych.2008.09.013. doi:10.1016/j.biopsych.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widom CS, Bzustowicz LM. MAOA and the “Cycle of Violence”: Childhood abuse and neglect, MAOA genotype, and risk for violent and antisocial behavior. Biol Psychiatry. 2006;60:684–689. doi: 10.1016/j.biopsych.2006.03.039. doi:10.1016/j.biopsych.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Young SE, Smolen A, Hewitt JK, Haberstick BC, Stallings MC, Corley RP, Crowley TJ. Interaction between MAO-A genotype and maltreatment in the risk for conduct disorder: Failure to confirm in adolescent patients. Am J Psychiatry. 2006;163:1019–1025. doi: 10.1176/ajp.2006.163.6.1019. doi:10.1176/appi.ajp.163.6.1019. [DOI] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. Am J med Genet (Neuropsychiatr Genet) 2000;96:684–695. doi:10.1002/1096-8628(20001009)96:5<684::AID-AJMG16>3.0.CO;2-G. [PubMed] [Google Scholar]