Abstract
The Mechanisms by which chronic nicotine self-administration augments hypothalamo-pituitary-adrenal (HPA) responses to stress are only partially understood. Nicotine self-administration alters neuropeptide expression in corticotropin-releasing factor (CRF) neurons within paraventricular nucleus (PVN) and increases PVN responsiveness to norepinephrine during mild footshock stress. Glutamate and GABA also modulate CRF neurons, but their roles in enhanced HPA responsiveness to footshock during chronic self-administration are unknown. We show that nicotine self-administration augmented footshock-induced PVN glutamate release, but further decreased GABA release. In these rats, intra-PVN kynurenic acid, a glutamate receptor antagonist, blocked enhanced adrenocorticotropic hormone and corticosterone responses to footshock. In contrast, peri-PVN kynurenic acid, which decreases activity of GABA afferents to PVN, enhanced footshock-induced corticosterone secretion only in control rats self-administering saline. Additionally, in rats self-administering nicotine, footshock-induced elevation of corticosterone was significantly less than in controls after intra-PVN saclofen (GABA-B receptor antagonist). Therefore, the exaggerated reduction in GABA release by footshock during nicotine self-administration disinhibits CRF neurons. This disinhibition combined with enhanced glutamate input provides a new mechanism for HPA sensitization to stress by chronic nicotine self-administration. This mechanism, which does not preserve homeostatic plasticity, supports the concept that smoking functions as a chronic stressor that sensitizes the HPA to stress.
Keywords: nicotine self-administration, adrenocorticotropic hormone, corticosterone, footshock stress, homeostatic plasticity, rat
Introduction
Nicotine, the principal psychoactive component of tobacco, acutely stimulates secretion of the stress-responsive hypothalamo-pituitary-adrenal (HPA) axis hormones, adrenocorticotropic hormone (ACTH) and corticosterone (Matta et al. 1987). In addition, chronic nicotine self-administration (Chen et al. 2008) and other stressors (Aguilera 1994) augment ACTH and corticosterone responses to a novel stressor, such as mild footshock stress. However, the neuroplastic changes underlying this effect of chronic nicotine exposure on the stress response are only partially understood.
In the hypothalamic paraventricular nucleus (PVN), the critical output nucleus controlling the HPA axis, corticotropin-releasing factor (CRF) neurons in the parvocellular division (pcPVN), integrate the HPA responsiveness to diverse stressors and stimulate plasma ACTH secretion (Herman et al. 2005). We have shown that acutely injected nicotine and chronically self-administered nicotine are stressors that activate CRF neurons and elevate plasma ACTH levels (Valentine et al. 1996, Yu et al. 2008, Chen et al. 2008). Chronic nicotine self-administration also alters the phenotype of pcPVN CRF neurons by inducing the co-expression of arginine vasopressin (AVP). Stressor stimulation of these CRF+/AVP+ neurons would potentiate CRF-dependent ACTH secretion by co-releasing AVP, another modulator of pituitary corticotrophs (Rivier and Vale 1983, Yu et al. 2008). We have reported that PVN responsiveness to norepinephrine, a primary regulator of CRF neurons, is selectively enhanced in rats self-administering nicotine, but only during stress (Yu and Sharp 2010). Neuroplasticity in the phenotype of CRF neurons and their responsiveness to norepinephrine during stress would both contribute to the enhanced secretion of ACTH and corticosterone during mild footshock stress. However, the contribution of other neurotransmitters to this interaction between nicotine self-administration and a stressor is unknown.
From a neuroanatomical perspective, pcPVN CRF neurons receive both glutamatergic and GABAergic afferents (van den Pol et al. 1990, Decavel and Van den Pol 1990), with CRF neurons juxtaposed to glutamatergic or GABAergic terminals (Ziegler and Herman 2000, Miklos and Kovacs 2002). Additionally, ionotropic glutamate receptor and GABA receptor subunits are highly expressed on pcPVN CRF neurons (Cullinan 2000, Herman et al. 2000). Pharmacological studies have shown that an intra-PVN injection of either glutamate or a GABA receptor antagonist elicited ACTH and corticosterone release (Feldman and Weidenfeld 1997, Cole and Sawchenko 2002). Blockade of PVN glutamate receptors inhibited the corticosterone response to restraint stress (Ziegler and Herman 2000), whereas GABA receptor blockade enhanced restraint stress-induced corticosterone release (Cullinan et al. 2008). Therefore, both glutamate and GABA inputs to PVN CRF neurons modulate basal activity and stress responsiveness, and could potentially affect responsiveness to norepinephrine during stress.
We have proposed that nicotine self-administration is a chronic stressor, sensitizing the HPA to novel stressors (Chen et al. 2008, Yu et al. 2008). We have shown that increased c-Fos expression in CRF+/AVP+ neurons after stress is a distal component of the HPA sensitization induced by chronic nicotine self-administration. However, apart from norepinephrine (Yu and Sharp 2010), the effect of nicotine self-administration on neurotransmitters modulating these critical neurons is unknown. We hypothesized that nicotine self-administration would alter the balance of glutamatergic stimulation and GABAergic inhibition during mlid footshock stress, thereby augmenting the response of CRF neurons to the stressor. We determined the effects of nicotine self-administration on footshock-induced release of (1) PVN glutamate and GABA, and (2) plasma ACTH and corticosterone after blockade of glutamate or GABA receptors in the PVN. These experiments demonstrate that chronic nicotine self-administration both enhanced the PVN glutamate release and magnified the reduction in PVN GABA levels by footshock, providing new evidence of plasticity in neurotransmission that mediates enhanced HPA responsiveness.
Materials and methods
Materials
(−)-Nicotine hydrogen tartrate (dose expressed as free base), xylazine, ketamine, kynurenic acid, and saclofen were purchased from Sigma–Aldrich (St. Louis, MO). Dual channel swivels, polyethylene buttons and metal springs for intravenous infusion were purchased from Instech Laboratories (Plymouth Meeting, PA). Operant chambers, circuit boards, interface modules and software for nicotine self-administration and grid floors for electrical footshock were purchased from Coulbourn Instruments (Allentown, PA). Sodium dihydrogen phosphate monohydrate, EDTA, methanol, acetonitrile for the mobile phase were obtained from Fisher Scientific (Fair Lawn, NJ). Cellulose fiber tubing and silica tubing for microdialysis probes were purchased from Spectrum (Laguna Hills, CA), and Polymicron Technologies (Phoenix, AZ), respectively.
Animal and surgeries
Adult male Sprague-Dawley rats (300-350 g, Harlan, Madison, WI) were given access ad libitum to standard rat chow and water. After acclimation to a reversed 12:12 hours light/dark cycle for 7 days, rats were anesthetized with xylazine-ketamine anesthesia (13:87 mg/kg, respectively, i.m.). Guide cannulae (20 gauge for microdialysis or 23 gauge for microinjection) were stereotaxically implanted bilaterally into the PVN with a 10° angle; coordinates from bregma with a flat skull were: anteroposterior, − 2.0 mm; dorsoventral, −7.5 mm; mediolateral, ±0.3 mm (Paxinos and Watson 1986). After 7 days of recovery, rats received jugular or both jugular and femoral catheters under xylazine/ketamine anesthesia and were then placed into an operant chamber located inside a sound-attenuating environmental chamber. All procedures conformed to NIH guidelines and were approved by the Institutional Animal Care and Use Committee at the University of Tennessee Health Science Center.
Nicotine self-administration
The nicotine self-administration procedure was conducted using our published protocol (Valentine et al. 1997). Briefly, after recovery (3 days) from jugular surgery, rats were given access to nicotine or saline self-administration 23 hours/day for 12-15 days, without prior training, priming or food deprivation. The final hour of the 12 hours lights-on cycle (i.e., 9:00-10:00 AM) was reserved for housekeeping tasks. The operant chamber contained 2 horizontal levers, and a green cue light above each lever was illuminated when nicotine was available. Lever presses were recorded and syringe pumps controlled by computers and interfaces, using L2T2 or Graphic State software. Pressing the active lever, randomly designated, elicited a computer-driven injection (i.v., 50 μl/0.81 sec) of nicotine (0.03 mg/kg) or saline via jugular vein. Each injection was followed by a 7 sec period during which the green cue light was extinguished but nicotine was unavailable. Pressing the alternate (inactive) lever had no programmed consequence.
Mild footshock stress
Electric footshocks were delivered 4 hours after the beginning of a 23 hours self-administration session. A total of 5 shocks (0.6 mA, 0.5 sec per shock) were randomly delivered over 5 min through the grid floor. As previously reported, lever press behavior is unaffected during the hour immediately after mild footshock (Chen et al. 2008).
Experimental protocols
First, the effects of nicotine self-administration on footshock-induced glutamate and GABA release in the PVN were determined. On day 12 of nicotine (n = 7) or saline (n = 10) self-administration, footshock was administered and in vivo microdialysis was used to measure PVN glutamate and GABA release. Second, the effects of intra-PVN microinjections of the ionotropic glutamate receptor antagonist, kynurenic acid (1.5nmol/side) vs. vehicle (artificial CSF; 100 nl/side) on plasma ACTH and corticosterone responses to footshock were measured in nicotine and saline self-administration groups. Kynurenic acid also has been shown to inhibit α7-containing nicotinic cholinergic receptors (Hilmas et al. 2001), but the known effects of nicotine on the PVN and HPA axis are indirect, mediated through nicotinic receptors in the nucleus tractus solitarius (Fu et al. 1997, Zhao et al. 2007). Therefore, intra-PVN kynurenic acid would not be expected to modulate the HPA response to stress due to inhibition of PVN nicotinic receptors. The dose of kynurenic acid was selected on the basis of previous reports showing the IC50 for intraparenchymal kynurenic acid injection was approximately 1.0 nmol (Soltis and DiMicco 1992, Soltis et al. 1998). Third, the effects of the GABA-B receptor antagonist, saclofen (10 pmol/side) vs. vehicle were evaluated. PVN neurons express GABA-B receptors (Margeta-Mitrovic et al. 1999) and intra-PVN phaclofen (0.5-50 pmol), a less potent antagonist than saclofen, is known to enhance the HPA response to ether stress (Marques de Souza and Franci 2008). In both the second and third studies, rats self-administering nicotine (n = 16) or saline (n = 15) for 15 days, were used to determine the effects of drug or vehicle on self-administration day 12 and day 15; the order of drug vs. vehicle treatment alternated between animals. To diminish the stress of microinjection, bilateral PVN injection cannulae (30 gauge) were inserted through guide cannulae 1-2 hour before the self-administration session. Microinjections were delivered, using a microsyringe infusion pump, 4 hours after the self-administration session was initiated. Animals remained in their home operant chambers and were not handled during the microinjection. Each drug was bilaterally microinjected into the PVN (100 nl over 1 min) 10 min prior to footshock. Two baseline blood samples (0.2 ml at -15 and 0 min) were withdrawn from the femoral vein prior to footshock and then 3 consecutive samples (at +15, +35, +55 min) were collected. An equivalent volume of saline, containing heparin (100U/ml) for maintenance of catheter patency, was injected immediately after each sampling. Experiment 4 (Table 1) was performed to further evaluate the effects of kynurenic acid or saclofen on basal corticosterone levels in unstressed rats self-administering saline. On self-administration day 12 and day 15, a rat received two of the following treatments: kynurenic acid (1.5nmol/side), saclofen (10 pmol/side) or vehicle (aCSF; 100 nl/side). Drug treatments were randomized between rats. Sham footshock was administered 10 min, thereafter.
Table 1.
The effects of kynurenic acid and saclofen on corticosterone secretion in unstressed rats self-administering saline.
| Time (min) | −15 | 0 | 15 | 35 | 55 |
|---|---|---|---|---|---|
| Vehicle, n = 5 | 41.5 ± 8.9 | 62.3 ± 13.8 | 52.4 ± 16.9 | 83.9 ± 19.6 | 66.5 ± 12.6 |
| KYN, n = 5 | 43.7 ± 13.4 | 54.4 ± 17.7 | 64.7 ± 13.1 | 60.9 ± 11.1 | 58.9 ± 10.5 |
| Saclofen, n = 5 | 46.8 ± 16.1 | 48.5 ± 17.9 | 147.7 ± 23.7* | 100.4 ± 29.2 | 63.8 ± 14.1 |
Kynurenic acid (KYN, 1.5 nmol), saclofen (10 pmol), or vehicle were bilaterally microinjected intra-PVN. Vehicle and kynurenic acid had no effects on corticosterone release (one-way ANOVA: F(4,24) = 1.2, and 0.4, respectively, p > 0.05). Saclofen increased corticosterone levels only at 15 min (one-way ANOVA: F(4,24) = 4.2, p < 0.05).
, p < 0.05, vs. baseline values at −15 and 0 min (Scheffé).
In vivo microdialysis
The microdialysis procedure was performed as described previously (Fu et al. 2000) with concentric microdialysis probes (1.5 mm dialysis membrane) constructed in our laboratory. The recovery efficiency for glutamate was 7.5 ± 0.6 % (n = 5) and GABA was 8.7 ± 0.4 % (n = 5). In the morning of self-administration day 12, 1-2 hour before the self-administration session began, probes were randomly inserted into the right or left PVN and perfused at 2.0 μl/min with a solution of artificial CSF (140 mM NaCl, 2.5 mM KCl, 1.2 mM MgCl2, 1.4 mM CaCl2, 5 mM glucose, pH 7.2-7.4). Microdialysis sample collections were begun 4 hours after the self-administration session was initiated. Three baseline samples (10 min/sample) were collected, footshock was administered, and then 7 consecutive samples were obtained. All samples were stored at -80 °C. At the end of each experiment, probe positions were verified by histological examination. Only data obtained from animals with probes in the correct position were used for HPLC analysis.
HPLC-electrochemical detection of glutamate and GABA
For HPLC, as previously reported (Fu et al. 2000), the mobile phase (100 mM sodium dihydrogen phosphate monohydrate in 10% methanol and 10% acetonitrile v/v in polished water, pH 4.4) was perfused through a column (MCM C18 150 × 4.6 mm; ESA Inc., Chelmsford, MA) at 1.5 ml/min, using an ESA model 580 HPLC pump. O-phthalaldehyde (OPA)/sulfite stock solution was made by dissolving 22 mg of OPA in 0.5 ml of ethanol, and then adding 0.5 ml of 1 M sodium sulfite and 9 ml of 0.1 M sodium tetraborate, pH 10.0. The working OPA/sulfite solution was prepared daily by diluting the stock OPA/sulfite solution with polished water. Microdialysis samples were maintained at 12°C; to derivatize glutamate and GABA, 20 μl samples were mixed with 20 μl OPA/sulfite working solution and automatically injected by an ESA 542 autosampler. Glutamate and GABA levels were measured by electrochemical detection, using a VT-03 electrochemical flow cell and an INTRO detector (Antec, Netherlands) at a potential of 750 mV with gain at 50 nA. Serially diluted standards containing known quantities of glutamate and GABA were included in each assay (R2>0.98). The limits of detection for glutamate and GABA were 3.9 pg/injection and 0.98 pg/injection, respectively.
Plasma corticosterone and ACTH radioimmunoassays
Blood samples were collected into ice-cold tubes containing EDTA (20 mg/ml, 20 μl), centrifuged at 830 g, 4°C, 10 min, and plasma was stored at −80°C. Corticosterone and ACTH were measured by using the ImmuChemTM Double Antibody Corticosterone-125I and ACTH-125I RIA kits (MP Biomedicals, Orangeburg, NY), and minor modifications of published methods (Chen et al. 2008).
Data analysis and statistics
Chromatographic data were collected and analyzed with the PowerChrom system (ADInstruments, Castle Hill, Australia). Glutamate and GABA levels were expressed as a percentage of the pre-shock baseline, defined as the average concentration of the 3 consecutive samples collected before footshock. The incremental or decremental change in cumulative glutamate and GABA levels from baseline (i.e., average of 3 samples) to peak post-shock levels was calculated by applying the following formula to the data from each animal: (transmitter level10min – baseline) + (transmitter level20min – baseline) + --- + (transmitter level70min –baseline). Similarly, incremental or decremental change was calculated for corticosterone. Data were expressed as mean ± SEM.
ACTH and corticosterone data, after intra-PVN vs. peri-PVN microinjection of kynurenic acid in rats self-administering nicotine vs. saline, were analyzed using factorial analysis of variance (ANOVA). Unless otherwise specified, glutamate and GABA levels, compiled lever press data from experiments 1-3, and ACTH and corticosterone levels throughout this study were analyzed using two-way ANOVA. Time course of shock, self-administration group (nicotine or saline) and PVN treatment (drug or vehicle) were treated as fixed factors. One-way ANOVA with Scheffé’s post hoc comparison was used to compare the corticosterone levels, shown in Table 1. Statistical significance was assigned at p < 0.05.
Results
Nicotine self-administration
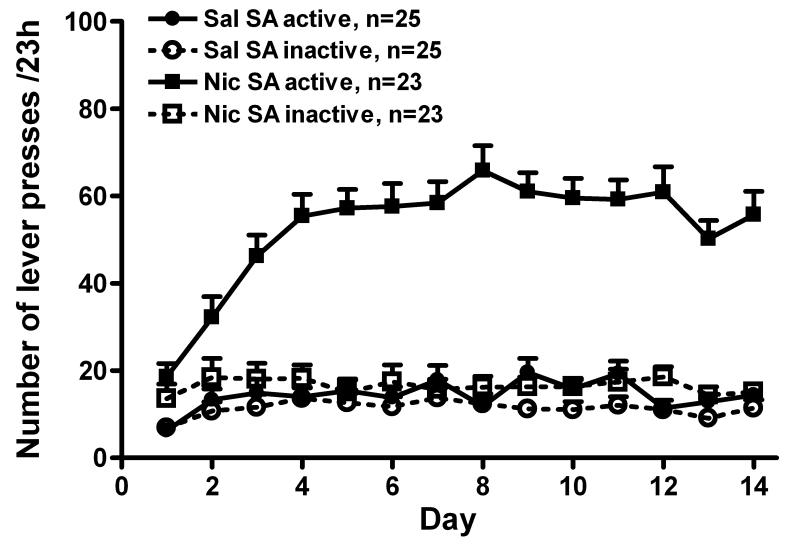
Adult male Sprague-Dawley rats were given ad lib access to nicotine for 23 hours/day and acquired nicotine self-administration without prior training, priming or food deprivation. In figure 1, the lever press data were compiled from all rats in experiments 1-3. The number of active lever presses was approximately 3-4-fold greater than inactive lever presses in rats self-administering nicotine (F(1, 596) = 534.5, p < 0.001), and significantly more than observed in the saline group (F(1, 615) = 714.8, p < 0.001). Active and inactive lever presses were not different in the saline group. The combination of footshock and venous blood sampling did not affect active lever presses in the nicotine group on self-administration day 12 (59.2 ± 4.5 vs. 60.9 ± 5.8, self-administration day 11 vs. day 12, respectively; p > 0.05, t-test). Total daily nicotine intake during the last 3 days of self-administration (d12-14) was: 1.13 ± 0.1, 1.10 ± 0.11, and 1.14 ± 0.1 mg/kg, respectively; these intakes were attributable to approximately 38 infusions per day.
Fig. 1.
The acquisition and maintenance of chronic nicotine (Nic) self-administration (SA). Adult male Sprague-Dawley rats acquired Nic self-administration (30 μg/kg/injection, iv) without prior training, priming or food deprivation. Nicotine was available 23 hours/day for 12-15 days; self-administration terminated on the 15th day with a microinjection experiment. Rats self-administering nicotine had significantly more active than inactive lever presses [two way ANOVA: time, p < 0.001; SA (active vs. inactive) p < 0.001; time × SA, p < 0.001] and more active presses than the saline (Sal) SA group (two way ANOVA: time, p < 0.001; SA group, p < 0.001; time × SA group, p < 0.001). Active and inactive presses were not different in the Sal SA group [time, p > 0.05, SA (active vs. inactive; p > 0.05). All data are expressed as mean ± SEM.
PVN glutamate and GABA responses to mild footshock stress
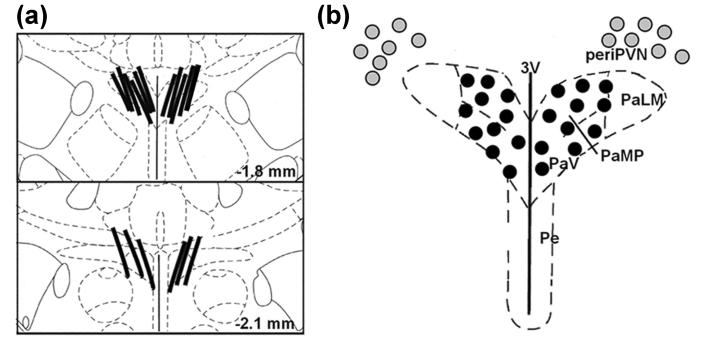
In experiment 1, the effect of footshock on PVN glutamate and GABA levels was determined by in vivo microdialysis on day 12 of nicotine self-administration. Figure 2A shows the location of the membrane segment of microdialysis probes situated within the PVN of all rats that were included in the data analysis. Based on these probe placements relative to the dimensions of PVN, individual dialysates reflect analyte changes throughout the PVN.
Fig. 2.
The location of microdialysis probes (panel a) and microinjection cannulae (b) in the hypothalamic paraventricular nucleus (PVN). Panel (a): In two coronal schematics of the hypothalamus, the membrane segments of microdialysis probes from all experiments are shown relative to the PVN (adapted from Paxinos and Watson 1986). Panel (b): Microinjection sites for bilateral administration of kynurenic acid (KYN, 1.5 nmol in 100 nl) are located intra-PVN (dark circles) or peri-PVN (light circles). Abbreviations: 3V, 3rd ventricle; PaLM, lateral magnocellular PVN; PaMP, medial parvocellular PVN; PaV, ventral PVN; Pe, periventricular hypothalamic nucleus.
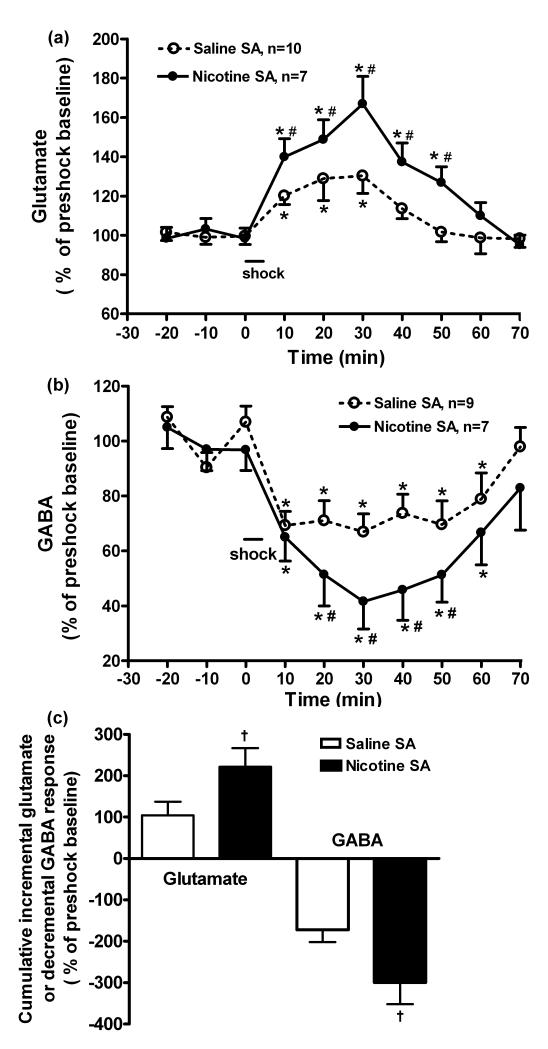
In rats self-administering nicotine, the elevation of glutamate induced by footshock was greater than in rats self-administering saline (Figure 3A: time, F(9,150) = 14.9, p < 0.001; self-administration group, F(1,150) = 12.9, p < 0.001, time × self-administration group, F(9,150) = 2.5, p < 0.05). Furthermore, in rats self-administering nicotine, glutamate levels were elevated for 50 min, whereas the elevation persisted for only 30 min in rats self-administering saline (p < 0.05). Following footshock, GABA levels, in rats self-administering nicotine, decreased to a greater extent than in rats self-administering saline (Figure 3B: time, F(9,140) = 11.1, p < 0.001; self-administration group, F(1,140) = 20.3, p < 0.001); from 20-50 min post-shock, GABA levels were significantly lower in the nicotine group (p < 0.05). Figure 3C shows these data expressed as cumulative incremental glutamate and decremental GABA responses to footshock (across time post-shock). In rats self-administering nicotine, the incremental glutamate and decremental GABA responses were approximately 2-fold greater than in rats self-administering saline (nicotine vs. saline self-administration: 221.5 ± 45.1% vs. 104.7 ± 22.6% for glutamate; −299.2 ± 52.9 % vs. −172.1 ± 29.9 % for GABA; p < 0.05, respectively). Nicotine self-administration did not affect basal PVN glutamate levels (2331 ± 357 vs. 2046 ± 510 pg/20 μl for the nicotine and saline groups, respectively; p > 0.05) or GABA levels (17.3 ± 1.4 vs. 18.4 ± 1.6 pg/20 μl, respectively; p > 0.05). Therefore, mild stress (e.g., mild footshock) induces a prolonged release of glutamate and a concomitant reduction of GABA levels in the PVN. The effects on both glutamate (elevation and duration) and GABA (reduction) are augmented in rats self-administering nicotine.
Fig. 3.
The effects of nicotine self-administration (SA) on PVN glutamate (panel a) and GABA release (b) and their incremental or decremental responses (c) induced by mild footshock stress. Glutamate and GABA levels were measured by in vivo microdialysis and HPLC with electrochemical detection, and expressed as a percentage of the pre-footshock baseline levels. Panel (a): Nicotine SA significantly augmented the PVN glutamate response to footshock compared to saline SA (two way ANOVA: time, p < 0.001; SA group, p < 0.01; time × SA group, p < 0.05). Basal glutamate levels for saline vs. nicotine SA: 2331.3 ± 357 vs. 2046.0 ± 510.6 pg/20 μl, respectively (p > 0.05, t-test). *, p < 0.05, vs. 3 pre-shock baseline levels, respectively (t-test); #, p < 0.05, nicotine vs. saline SA at the same time interval, respectively, (t-test). Panel (b): Nicotine SA significantly decreased PVN GABA release in response to footshock compared to saline SA (two way ANOVA: time, p < 0.001; SA group, p < 0.001; time × SA group, p > 0.05). Basal GABA levels for saline vs. nicotine SA: 17.3 ± 1.4 vs 18.4 ± 1.6 pg/20 μl, respectively (p > 0.05, t-test). Panel (c): The cumulative increment in glutamate and decrement in GABA after footshock (across time) were greater in nicotine SA than saline SA groups. , †, p < 0.05, nicotine vs. saline SA, respectively (t-test). All data are expressed as mean ± SEM.
Blockade of intra- or peri- PVN ionotropic glutamate receptors affects ACTH and corticosterone release induced by mild footshock stress
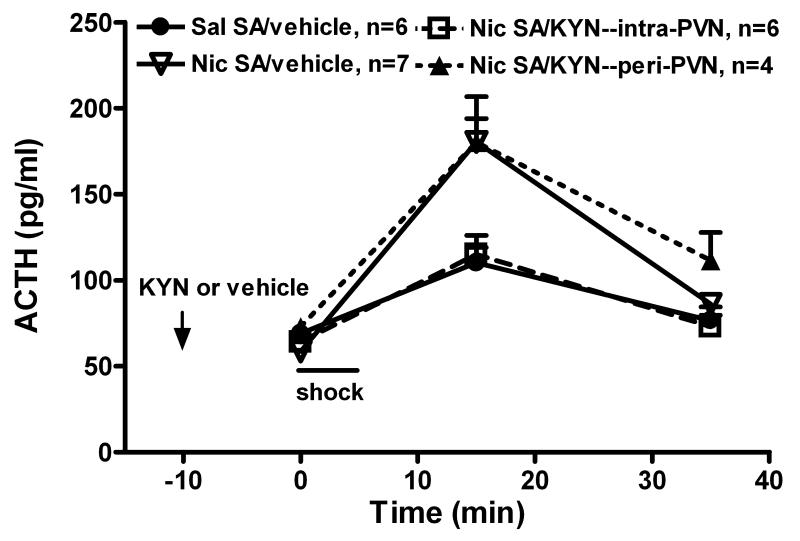
In experiment 2, the ionotropic glutamate receptor antagonist, kynurenic acid (1.5 nmol/side), was bilaterally microinjected into the PVN 10 min before footshock to evaluate the potential role of PVN glutamate in the corticosterone response to footshock during nicotine self-administration. Histological evaluation (Figure 2B) demonstrated the microinjection sites of kynurenic acid injections. While many injection sites were located within the PVN, others were located in the peri-PVN of the hypothalamus.
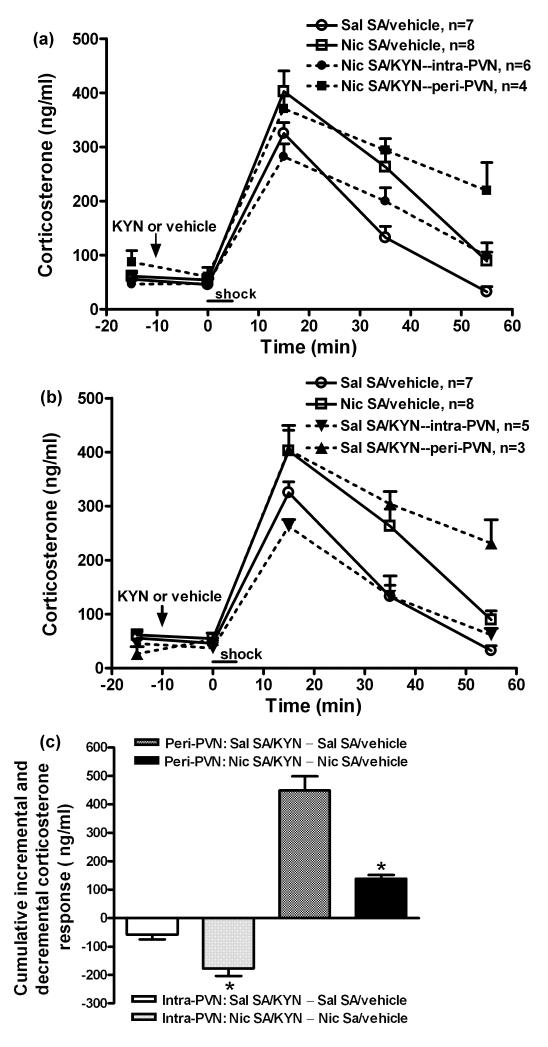
Initially, we determined the effect of kynurenic acid on corticosterone levels in unstressed rats self-administering saline. Table 1 shows that kynurenic acid did not affect corticosterone levels. The effects of kynurenic acid on corticosterone responses to footshock during nicotine or saline self-administration are shown in figure 4. The differential effects of the intra- vs. peri-PVN microinjection of kynurenic acid on corticosterone release by the nicotine (panel A) vs. saline (panel B) groups were delineated by graphing them separately. In all experiments, corticosterone results were combined from the groups receiving vehicle intra-PVN or peri-PVN, as the site of vehicle microinjection did not affect corticosterone levels in either the saline (F(1,25) = 0.1, p > 0.05) or nicotine groups (F(1,30) = 1.6, p > 0.05). The general findings (factorial ANOVA) were: footshock stimulated corticosterone release in all groups (time, F(4,135) = 148, p < 0.001); the corticosterone elevation was greater in rats self-administering nicotine (self-administration group, F(1,135) = 8.2, p < 0.01); and kynurenic acid affected the corticosterone response (PVN treatment, F(1,135) = 6.5, p < 0.01; time × PVN treatment F(4,135) = 3.7, p < 0.01) depending on the sites of injection (site, F(1,135) = 51.8, p < 0.001; time × site F(4,135) = 6.6, p < 0.001). Figure 4A shows that intra-PVN kynurenic acid prevented the enhanced corticosterone response in rats self-administering nicotine (intra-PVN kynurenic acid vs. vehicle, F(1,60) = 7.1, p < 0.05), but peri-PVN kynurenic acid was ineffective.
Fig. 4.
The effects of intra- and peri-PVN kynurenic acid (KYN) on overall corticosterone responses (panels a, b) and incremental or decremental changes (c) in corticosterone secretion induced by mild footshock stress during nicotine (Nic) and saline (Sal) self-administration (SA). Kynurenic acid, an ionotropic glutamate receptors antagonist, (1.5 nmol/side) was bilaterally microinjected into PVN 10 min prior to footshock. Corticosterone levels were increased by footshock (factorial ANOVA: time, p < 0.001); the increase was greater in rats self-administering nicotine (SA group, p < 0.01), and also was affected by kynurenic acid (PVN treatment, p < 0.01; time × PVN treatment, p < 0.01) depending on the injection site (site, p < 0.001; time × site, p < 0.001). The differential effects of intra- vs. peri-PVN microinjections of kynurenic acid in the Nic and Sal SA groups were delineated by graphing them separately. Both panels contain the same data from the groups pretreated with vehicle. Panel (a): Intra-PVN kynurenic acid completely blocked the enhanced corticosterone response in the nicotine group (two-way ANOVA: Nic SA/vehicle vs. Nic SA/KYN--intra-PVN, p < 0.05). In contrast, peri-PVN kynurenic acid was ineffective (p > 0.05). Panel (b): peri-PVN kynurenic acid augmented the corticosterone response in the saline group (two-way ANOVA: Sal SA/vehicle vs. Sal SA/KYN--peri-PVN, p < 0.001). A main effect of intra-PVN kynurenic acid was not detected (p > 0.05). Analysis of peak corticosterone levels (15 min) did indicate a 20% reduction in the Sal SA/KYN--intra-PVN vs. Sal SA/vehicle (p < 0.05, t-test). Panel (c): Intra-PVN kynurenic acid induced a significantly greater cumulative (across time) decrement in footshock-induced corticosterone secretion in the nicotine SA group compared to saline controls; conversely, peri-PVN kynurenic acid induced a significantly greater cumulative increment in footshock-induced corticosterone secretion in saline SA group compared to the nicotine group. *, p <0.05, for cumulative change in nicotine vs. saline groups after intra- or peri-PVN kynurenic acid (t-test).
In rats self-administering saline, peri-PVN kynurenic acid enhanced corticosterone release (Figure 4B: peri-PVN kynurenic acid vs. vehicle, F(1,40) = 39.5, p < 0.001); a main effect of intra-PVN kynurenic acid was not, however, detected across the time course, although peak corticosterone levels (+15 min) were reduced by 20% (intra-PVN kynurenic acid vs. vehicle, p < 0.05). As shown in figure 4C, the effect of intra-PVN kynurenic acid on the cumulative decrement in corticosterone was 3-fold greater in the nicotine than saline group (−176 ± 26.3 vs. −57 ± 17.2, p < 0.05). In contrast, the incremental effect of peri-PVN kynurenic acid was 3-fold less in rats self-administering nicotine than saline (138 ± 13.1 vs 448.5 ± 49.4, p < 0.05). Therefore, in rats self-administering nicotine, the enhanced secretion of corticosterone induced by footshock depends on amplified glutamate neurotransmission within the PVN. Additionally, corticosterone release appears to depend on intra-PVN glutamate neurotransmission in rats self-administering saline. In this group, glutamate neurotransmission primarily affects the peri-PVN region, restraining the corticosterone response to footshock.
ACTH was measured on a subset of samples to determine whether hypophysiotropic CRF neurons in the PVN, which control the release of ACTH, were activated by glutamate in the nicotine self-administration group. Plasma ACTH levels were increased by footshock, delivered immediately following the basal blood sampling in rats self-administering nicotine and saline (Figure 5: time, F(2,57) = 33, p < 0.001). The ACTH response, greater in the nicotine group (factorial ANOVA: self-administration group, F(1,57) = 5.8, p < 0.05; time × self-administration group, F(2,57) = 6.3, p < 0.01), was reduced by PVN kynurenic acid (factorial ANOVA: PVN treatment, F(1,57) = 6.5, p < 0.05; time × PVN treatment F(2,57) = 4.9, p < 0.05). Indeed, intra-PVN kynurenic acid completely prevented this enhanced ACTH response in rats self-administering nicotine (intra-PVN kynurenic acid vs. vehicle, F(1,33) = 4.8, p < 0.05). In contrast, peri-PVN kynurenic acid was ineffective. Therefore, the enhanced ACTH response to footshock in rats self-administering nicotine depends on glutamate neurotransmission within the hypophysiotropic region of PVN.
Fig. 5.
The effects of intra- and peri-PVN kynurenic acid (KYN) on ACTH responses to mild footshock stress during chronic nicotine (Nic) or saline (Sal) self-administration (SA). Based on studies showing that kynurenic acid alone had no effect on corticosterone levels (Table 1), kynurenic acid (1.5 nmol/side) or vehicle was bilaterally microinjected into PVN 10 min prior to the first blood sample, and then rats received footshock. ACTH measurements were obtained on a subset of the same animals shown in Fig. 4A. Footshock increased plasma ACTH levels (factorial ANOVA: time, p < 0.001) to a greater degree in rats self-administering nicotine (SA group, p < 0.05; time × SA group, p < 0.01), and this was attenuated by kynurenic acid (PVN treatment, p < 0.05; time × PVN treatment, p < 0.05). In fact, intra-PVN kynurenic acid completely blocked the enhanced ACTH response (two-way ANOVA: Nic SA/vehicle vs. Nic SA/KYN--intra-PVN, p < 0.05). In contrast, peri-PVN kynurenic acid was ineffective (p > 0.05).
Blockade of PVN GABA-B receptors affects corticosterone release induced by mild footshock stress
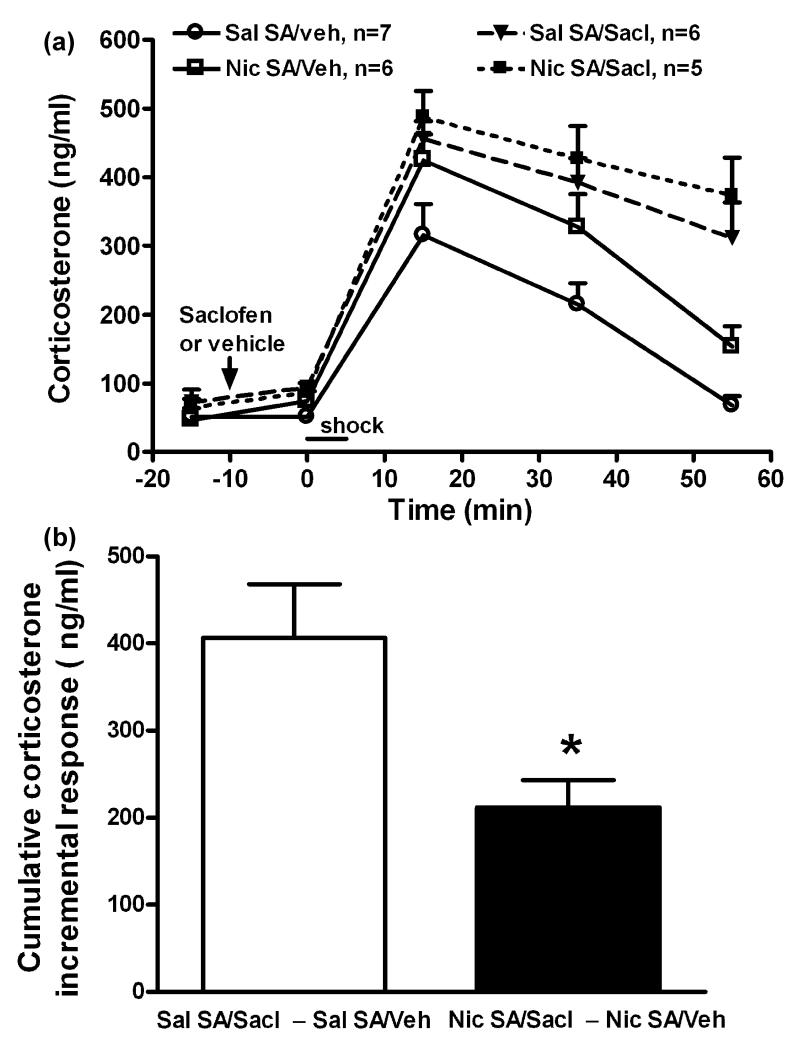
To evaluate the potential role of PVN GABA in augmentation of the corticosterone response to footshock during nicotine self-administration, the GABA-B receptor antagonist, saclofen (10 pmol/side), was bilaterally microinjected intra-PVN 10 min before footshock. The PVN microinjection sites (not shown) were all located within the PVN in a distribution similar to that shown for intra-PVN kynurenic acid. As shown in figure 6A, corticosterone release was increased by footshock (time, F(4, 99) = 96.7, p < 0.001), the increase was greater in rats self-administering nicotine (self-administration group, F(1,99) = 12, p < 0.01), and also greater in saclofen vs. vehicle in both nicotine and saline self-administration groups (PVN treatment, F(1,99) = 58, p < 0.001; time × PVN treatment, F(4,99) =7.1, p < 0.001). We further analyzed this data by calculating the cumulative incremental corticosterone response to footshock during nicotine vs. saline self-administration (Figure 6B). In rats that self-administered saline and received saclofen, the incremental change in footshock-induced corticosterone release was approximately 2-fold greater than in the nicotine group (406.4 ± 61.4 vs. 211.9 ± 31.3 ng/ml, p < 0.05). Therefore, in rats self-administering saline compared to nicotine, the reduction in PVN GABA after footshock was significantly less (Figure 3C). This was associated with greater sensitivity to the inhibitory effect of saclofen on footshock-induced corticosterone release.
Fig. 6.
The effects of saclofen on overall corticosterone responses (panel a) and the increment in corticosterone secretion (b) induced by mild footshock stress during chronic nicotine (Nic) vs. saline (Sal) self-administration (SA). Saclofen (Sacl, GABA-B receptor antagonist, 10 pmol/side) was bilaterally microinjected intra-PVN. Panel (a): Corticosterone levels were increased by footshock (factorial ANOVA: time, p < 0.001), and the elevation was greater in rats self-administering nicotine (SA group, p < 0.01; time × SA group, p > 0.05), and also greater in all saclofen pretreated rats in both the nicotine and saline SA groups (PVN treatment, p < 0.001; time × PVN treatment, p < 0.001). Panel (b): In saline SA groups, saclofen enabled a significantly greater cumulative incremental corticosterone response to footshock than in the nicotine SA groups. *, p < 0.05 (t-test).
The effect of saclofen on corticosterone levels in unstressed rats self-administering saline was also determined over a longer time course (Table 1). Saclofen briefly increased corticosterone levels; the maximum increase above vehicle was approximately 100 ng/ml at 15 min (time, F(4,24) = 4.2, p < 0.05). By 35 and 55 min post-saclofen, corticosterone was no longer significantly elevated and the increments above vehicle were approximately 15 and 0 ng/ml, respectively. Therefore, the effects of saclofen on basal corticosterone, which are brief and limited in magnitude, cannot account for either the degree or the prolonged duration of the saclofen-enhanced corticosterone response to footshock.
Discussion
We have previously shown that chronic nicotine self-administration augments the HPA axis response to mild footshock stress (Chen et al. 2008). To our knowledge, the current studies are the first to characterize concomitant changes in glutamate and GABA within the PVN during stress in animals self-administering drug and control. These experiments demonstrate that chronic nicotine self-administration altered both excitatory and inhibitory neurotransmission within the PVN during mild stress by augmenting glutamate and diminishing GABA release, in each case approximately two-fold more than in controls. The significance of these changes for HPA function was determined in pharmacological studies that showed blockade of PVN glutamate receptors prevented the augmented ACTH and corticosterone responses to footshock during nicotine self-administration. Additionally, blockade of inhibitory GABA-B receptors in the PVN enhanced the corticosterone response to footshock to a greater extent in rats self-administering saline compared to nicotine, indicating that CRF neurons are relatively disinhibited during mild footshock stress in rats self-administering nicotine. These findings indicate that both enhanced PVN glutamate and reduced GABA neurotransmission participate in augmenting HPA responsiveness to footshock during nicotine self-administration.
Stimulation of the HPA by stressors depends, in part, on glutamatergic inputs to pcPVN (Herman et al. 2004). Forced swim stress, restraint, and immobilization have been reported to increase glutamate release in medial prefrontal cortex, hippocampus, and central amygdala (Moghaddam 2002, Ebner et al. 2005, Pung et al. 2006, Zhu et al. 2008). To our knowledge, the effect of stress on PVN glutamate levels has not been reported. Mild footshock stress significantly increased PVN glutamate release in rats self-administering saline and to a greater extent in those receiving nicotine. Intra-PVN kynurenic acid prevented the nicotine self-administration-enhanced release of corticosterone induced by footshock, but did not attenuate the overall corticosterone response to stress in rats self-administering saline. The peak corticosterone response was, however, reduced by 20% in the saline group. The failure of kynurenic acid to block the overall corticosterone response, which differs from a report showing that intra-PVN microinjection of kynurenic acid significantly reduced restraint stress (30 min)-induced corticosterone release by 24%, may reflect the magnitude of both the stressor stimulus and the corticosterone response to restraint stress vs. mild footshock stress (Ziegler and Herman 2000). Corticosterone was elevated to a greater degree and for a much longer duration (150 min) after acute restraint compared to footshock (duration: 35 min) (Ziegler and Herman 2000). Although kynurenic acid induced a small but significant reduction in the peak corticosterone level of rats self-administering saline (20%), the rapidly declining corticosterone levels make it difficult to detect a difference at the later time points (i.e., 35 and 55 min). Nevertheless, a higher dose of kynurenic acid might be more efficacious. Therefore, it is likely that footshock-induced glutamate release contributes to the elevation of corticosterone in rats self-administering saline. In the nicotine self-administration group, the exaggerated release of PVN glutamate is necessary for the enhanced corticosterone response to footshock, which depends on the augmented release of ACTH (see figure 5). The efficacy of intra-PVN kynurenic acid in blocking the augmentation of ACTH implicates pcPVN hypophysiotropic CRF neurons in the action of this enhanced glutamate release.
The activity of GABAergic inputs to the PVN, which restrain HPA responses to stressors, diminishes during stress (Cullinan et al. 2008). Restraint stress suppressed inhibitory postsynaptic potentials in the PVN, and cold stress decreased PVN GABA levels (Ohtani et al. 1999, Verkuyl et al. 2005). Moreover, intra-PVN microinjection of muscimol, a GABA-A receptor agonist, blunted the rise in plasma corticosterone induced by restraint (Cullinan et al. 2008). Lastly, blockade of PVN GABA-A and especially GABA-B receptors increased ether-induced corticosterone secretion (Marques de Souza and Franci 2008). These findings accord with the current studies, in which footshock significantly reduced PVN GABA levels in the saline group; this reduction was magnified two-fold in rats self-administering nicotine. Studies with saclofen, which blocks GABA-B receptors expressed on pcPVN neurons (Margeta-Mitrovic et al. 1999), clarified the functional significance of these differences in PVN GABA levels. The disinhibition of CRF neurons by intra-PVN saclofen caused significantly less augmentation of the corticosterone response to footshock in rats self-administering nicotine compared to saline. Lower PVN GABA levels were, therefore, associated with less effect of saclofen in the nicotine group. In summary, the additional decrement in PVN GABA levels reduced the inhibition of pcPVN CRF neurons, contributing to the augmented HPA response to footshock in rats self-administering nicotine.
The GABAergic input to the PVN is primarily from the peri-PVN region and the bed nucleus of stria terminals. Glutamatergic projections from the medial prefrontal cortex and hippocampus innervate the peri-PVN and the bed nucleus of stria terminals GABAergic neurons, increasing the inhibitory control of PVN CRF neurons (Herman et al. 2005). Similar to a previous report showing that peri-PVN kynurenic acid increased the corticosterone response to restraint stress (Ziegler and Herman 2000), we found that peri-PVN kynurenic acid augmented the corticosterone response to footshock in the saline group. Although the activity of GABAergic inputs to the PVN is reduced by stress, kynurenic acid blockade of glutamate receptors on peri-PVN GABA neurons would be expected to further reduce this activity, augmenting the stress-induced activation of CRF neurons and the HPA axis. In contrast, in rats self-administering nicotine, peri-PVN kynurenic acid had no affect on the corticosterone response to footshock, indicating that nicotine self-administration may further reduce the activity of glutamatergic inputs to peri-PVN GABAergic neurons during mild footshock stress.
Nicotine self-administration altered the HPA responsiveness to stress without affecting basal levels of PVN glutamate and GABA. This coheres with the fact that basal ACTH and corticosterone levels were unaffected by chronic nicotine self-administration in this study. Although both acutely injected and self-administered nicotine act as stressors, stimulating ACTH and corticosterone secretion, desensitization occurs during chronic nicotine self-administration (Chen et al. 2008, Yu et al. 2008). Similar to chronic stressors (Aguilera and Rabadan-Diehl 2000), chronic nicotine self-administration alters the phenotype of hypophysiotropic CRF neurons in pcPVN (Yu et al. 2008). Regarding potential plasticity within other functional units of the HPA, exposure to chronic stressors, including cocaine, has been shown to induce increased expression of proopiomelanocortin, CRF1 receptor and AVP V1b receptor in anterior pituitary corticotrophs (Aguilera and Rabadan-Diehl 2000, Zhou et al. 2003). However, the effects of nicotine on these structures have not been evaluated.
Homeostatic plasticity, one of multiple paradigms of brain plasticity, stabilizes the function of neurons and circuits despite alterations induced by other forms of plasticity (e.g., synaptic plasticity) (Nelson and Turrigiano 2008). Numerous molecular and cellular mechanisms promote homeostatic plasticity at the neuron level. For example, the firing rate of individual neurons is maintained within a preferred range by tuning synaptic strengths up or down (i.e., synaptic scaling), while holding constant the relative strength of each synapse (Turrigiano 2008). At the systems level, homeostatic plasticity, particularly synaptic scaling, is known to govern the ocular dominance plasticity that occurs in the visual cortex, and augments responses to the open eye after chronic closure of the opposite eye (Mrsic-Flogel et al. 2007). The present study suggests that circuits regulating the HPA stress response are dysregulated during chronic self-administration of nicotine. Although PVN glutamate release is enhanced, GABA release is diminished further rather than increased. This serves to facilitate the responses to increased glutamate neurotransmission. These findings, which are contrary to the precepts of homeostatic plasticity, may be characteristic of the dysregulation induced by drugs. Abused drugs exploit native brain circuitry through specific molecular interactions, altering the function of neurons and circuits, both short- and long-term (Kauer and Malenka 2007). This neuroplasticity can produce alterations in neuron, circuit and behavioral function that far outlast the presence of drug.
We have proposed that chronic nicotine self-administration functions as a chronic stressor by inducing the expression of CRF+/AVP+ neurons in pcPVN, which are activated by footshock, thereby contributing to the enhanced HPA responsiveness to footshock (Chen et al. 2008, Yu et al. 2008). Chronic nicotine self-administration also diminishes footshock-induced norepinephrine release in the PVN, yet enhances noradrenergic responsiveness concurrent with the application of a stressor (i.e., mild footshock stress) (Yu and Sharp 2010). The present study demonstrates that chronic nicotine self-administration differentially modulates glutamate and GABA transmission within the PVN. Both the augmented release of glutamate and the exaggerated reduction of GABA in the PVN are necessary for the enhanced HPA response to footshock. Disinhibition of the pcPVN, due to the reduction of GABA, is also the probable mechanism for the enhanced PVN responsiveness to norepinephrine during stress in rats self-administering nicotine (Yu and Sharp 2010). The net effect of these alterations in PVN neurotransmission is to increase the activation of pcPVN CRF+/AVP+ neurons, thereby sensitizing the HPA to a submaximal stressor such as mild footshock stress. These findings offer new mechanistic insights that run contrary to the concept of homeostatic plasticity and support the concept that smoking functions as a chronic stressor, sensitizing the HPA to stress.
Fig. 7.
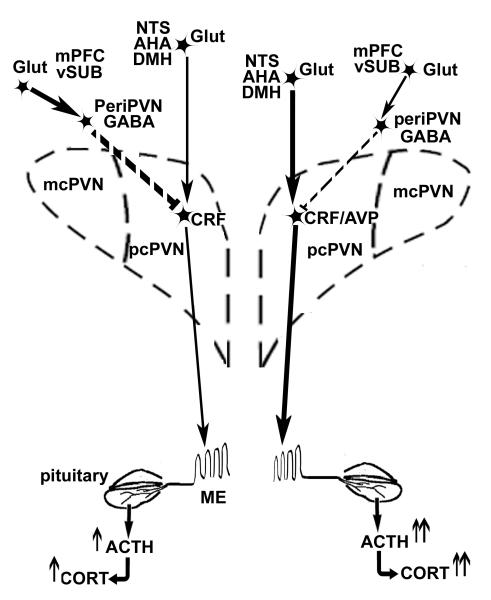
PVN neuropeptide and HPA axis responses to acute mild stress during saline vs. nicotine self-administration. The diagram illustrates (i) the changes in stress-induced neurotransmission observed during the self-administration of nicotine (right PVN) vs. saline (left PVN) and (ii) the multiple regions projecting excitatory glutamate (Glut) and inhibitory GABA afferents to PVN. Both glutamate and GABA inputs to PVN interact with corticotropin-releasing factor (CRF) neurons in the parvocellular division of PVN (pcPVN). Chronic nicotine self-administration induces the co-expression of arginine vasopressin (CRF/AVP) in most of the CRF neurons activated by mild footshock stress (Yu et al. 2008). Glutamatergic inputs to the pcPVN are primarily from the nucleus tractus solitarius (NTS), dorsomedial hypothalamus (DMH) and anterior hypothalamic area (AHA). GABAergic inputs to the pcPVN are partly from the peri-PVN region. Glutamatergic projections to the peri-PVN GABA neurons are primarily from medial prefrontal cortex (mPFC) and ventral subiculum (vSUB). During chronic nicotine self-administration (PVN on the right side), mild footshock stress stimulates more pcPVN glutamate release (thick solid arrow) and less GABA release (thin dashed stop); the latter may be due to the reduction in glutamatergic drive (thin solid arrow) from mPFC and vSUB to periPVN GABA neurons. Therefore, nicotine self-administration facilitates greater stress-induced release of CRF/AVP release (thick solid arrow) to median eminence (ME), enhancing the release of ACTH (↑↑) from the anterior pituitary gland and the secretion of corticosterone (CORT) (↑↑) from the adrenal cortex.
Acknowledgments
This research was supported by DA-03977 (B.M.S.) from NIDA. No financial or other conflict of interest was involved. We thank Ms Kathy McAllen for her technical contributions.
Abbreviations used
- ACTH
adrenocorticotropic hormone
- AVP
arginine vasopressin
- CRF
corticotropin-releasing factor
- HPA
hypothalamo-pituitary-adrenal
- OPA
O-phthalaldehyde
- PVN
hypothalamic paraventricular nucleus
- pcPVN
parvocellular division of the PVN
- mcPVN
magnocellular division of the PVN
References
- Aguilera G. Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol. 1994;15:321–350. doi: 10.1006/frne.1994.1013. [DOI] [PubMed] [Google Scholar]
- Aguilera G, Rabadan-Diehl C. Vasopressinergic regulation of the hypothalamic-pituitary-adrenal axis: implications for stress adaptation. Regul Pept. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- Chen H, Fu Y, Sharp BM. Chronic nicotine self-administration augments hypothalamic-pituitary-adrenal responses to mild acute stress. Neuropsychopharmacology. 2008;33:721–730. doi: 10.1038/sj.npp.1301466. [DOI] [PubMed] [Google Scholar]
- Cole RL, Sawchenko PE. Neurotransmitter regulation of cellular activation and neuropeptide gene expression in the paraventricular nucleus of the hypothalamus. J Neurosci. 2002;22:959–969. doi: 10.1523/JNEUROSCI.22-03-00959.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE. GABA(A) receptor subunit expression within hypophysiotropic CRH neurons: a dual hybridization histochemical study. J Comp Neurol. 2000;419:344–351. doi: 10.1002/(sici)1096-9861(20000410)419:3<344::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Ziegler DR, Herman JP. Functional role of local GABAergic influences on the HPA axis. Brain Struct Funct. 2008;213:63–72. doi: 10.1007/s00429-008-0192-2. [DOI] [PubMed] [Google Scholar]
- Decavel C, Van den Pol AN. GABA: a dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- Ebner K, Bosch OJ, Kromer SA, Singewald N, Neumann ID. Release of oxytocin in the rat central amygdala modulates stress-coping behavior and the release of excitatory amino acids. Neuropsychopharmacology. 2005;30:223–230. doi: 10.1038/sj.npp.1300607. [DOI] [PubMed] [Google Scholar]
- Feldman S, Weidenfeld J. Hypothalamic mechanisms mediating glutamate effects on the hypothalamo-pituitary-adrenocortical axis. J Neural Transm. 1997;104:633–642. doi: 10.1007/BF01291881. [DOI] [PubMed] [Google Scholar]
- Fu Y, Matta SG, Gao W, Brower VG, Sharp BM. Systemic nicotine stimulates dopamine release in nucleus accumbens: re-evaluation of the role of N-methyl-D-aspartate receptors in the ventral tegmental area. J Pharmacol Exp Ther. 2000;294:458–465. [PubMed] [Google Scholar]
- Fu Y, Matta SG, Valentine JD, Sharp BM. Adrenocorticotropin response and nicotine-induced norepinephrine secretion in the rat paraventricular nucleus are mediated through brainstem receptors. Endocrinology. 1997;138:1935–1943. doi: 10.1210/endo.138.5.5122. [DOI] [PubMed] [Google Scholar]
- Herman JP, Eyigor O, Ziegler DR, Jennes L. Expression of ionotropic glutamate receptor subunit mRNAs in the hypothalamic paraventricular nucleus of the rat. J Comp Neurol. 2000;422:352–362. [PubMed] [Google Scholar]
- Herman JP, Mueller NK, Figueiredo H. Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann N Y Acad Sci. 2004;1018:35–45. doi: 10.1196/annals.1296.004. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hilmas C, Pereira EF, Alkondon M, Rassoulpour A, Schwarcz R, Albuquerque EX. The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. J Neurosci. 2001;21:7463–7473. doi: 10.1523/JNEUROSCI.21-19-07463.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Mitrovic I, Riley RC, Jan LY, Basbaum AI. Immunohistochemical localization of GABA(B) receptors in the rat central nervous system. J Comp Neurol. 1999;405:299–321. doi: 10.1002/(sici)1096-9861(19990315)405:3<299::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- de Souza L. Marques, Franci CR. GABAergic mediation of stress-induced secretion of corticosterone and oxytocin, but not prolactin, by the hypothalamic paraventricular nucleus. Life Sci. 2008;83:686–692. doi: 10.1016/j.lfs.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Matta SG, Beyer HS, McAllen KM, Sharp BM. Nicotine elevates rat plasma ACTH by a central mechanism. J Pharmacol Exp Ther. 1987;243:217–226. [PubMed] [Google Scholar]
- Miklos IH, Kovacs KJ. GABAergic innervation of corticotropin-releasing hormone (CRH)-secreting parvocellular neurons and its plasticity as demonstrated by quantitative immunoelectron microscopy. Neuroscience. 2002;113:581–592. doi: 10.1016/s0306-4522(02)00147-1. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Nelson SB, Turrigiano GG. Strength through diversity. Neuron. 2008;60:477–482. doi: 10.1016/j.neuron.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani N, Sugano T, Ohta M. Alterations in monoamines and GABA in the ventromedial and paraventricular nuclei of the hypothalamus following cold exposure: a reduction in noradrenaline induces hyperphagia. Brain Res. 1999;842:6–14. doi: 10.1016/s0006-8993(99)01796-5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coorinates. Ed 2 Academic Press; New York: 1986. [Google Scholar]
- Pung T, Klein B, Blodgett D, Jortner B, Ehrich M. Examination of concurrent exposure to repeated stress and chlorpyrifos on cholinergic, glutamatergic, and monoamine neurotransmitter systems in rat forebrain regions. Int J Toxicol. 2006;25:65–80. doi: 10.1080/10915810500527119. [DOI] [PubMed] [Google Scholar]
- Rivier C, Vale W. Interaction of corticotropin-releasing factor and arginine vasopressin on adrenocorticotropin secretion in vivo. Endocrinology. 1983;113:939–942. doi: 10.1210/endo-113-3-939. [DOI] [PubMed] [Google Scholar]
- Soltis RP, Cook JC, Gregg AE, Stratton JM, Flickinger KA. EAA receptors in the dorsomedial hypothalamic area mediate the cardiovascular response to activation of the amygdala. Am J Physiol. 1998;275:R624–631. doi: 10.1152/ajpregu.1998.275.2.R624. [DOI] [PubMed] [Google Scholar]
- Soltis RP, DiMicco JA. Hypothalamic excitatory amino acid receptors mediate stress-induced tachycardia in rats. Am J Physiol. 1992;262:R689–697. doi: 10.1152/ajpregu.1992.262.4.R689. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine JD, Hokanson JS, Matta SG, Sharp BM. Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology (Berl) 1997;133:300–304. doi: 10.1007/s002130050405. [DOI] [PubMed] [Google Scholar]
- Valentine JD, Matta SG, Sharp BM. Nicotine-induced cFos expression in the hypothalamic paraventricular nucleus is dependent on brainstem effects: correlations with cFos in catecholaminergic and noncatecholaminergic neurons in the nucleus tractus solitarius. Endocrinology. 1996;137:622–630. doi: 10.1210/endo.137.2.8593811. [DOI] [PubMed] [Google Scholar]
- van den Pol AN, Wuarin JP, Dudek FE. Glutamate, the dominant excitatory transmitter in neuroendocrine regulation. Science. 1990;250:1276–1278. doi: 10.1126/science.1978759. [DOI] [PubMed] [Google Scholar]
- Verkuyl JM, Karst H, Joels M. GABAergic transmission in the rat paraventricular nucleus of the hypothalamus is suppressed by corticosterone and stress. Eur J Neurosci. 2005;21:113–121. doi: 10.1111/j.1460-9568.2004.03846.x. [DOI] [PubMed] [Google Scholar]
- Yu G, Chen H, Zhao W, Matta SG, Sharp BM. Nicotine self-administration differentially regulates hypothalamic corticotropin-releasing factor and arginine vasopressin mRNAs and facilitates stress-induced neuronal activation. J Neurosci. 2008;28:2773–2782. doi: 10.1523/JNEUROSCI.3837-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Sharp BM. Nicotine self-administration diminishes stress-induced norepinephrine secretion but augments adrenergic-responsiveness in the hypothalamic paraventricular nucleus and enhances adrenocorticotropic hormone and corticosterone release. J Neurochem. 2010;112:1327–1337. doi: 10.1111/j.1471-4159.2009.06551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Chen H, Sharp BM. Nicotine-induced norepinephrine release in hypothalamic paraventricular nucleus and amygdala is mediated by N-methyl-D-aspartate receptors and nitric oxide in the nucleus tractus solitarius. J Pharmacol Exp Ther. 2007;320:837–844. doi: 10.1124/jpet.106.112474. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, Schlussman SD, Ho A, Kreek MJ. Alterations in hypothalamic-pituitary-adrenal axis activity and in levels of proopiomelanocortin and corticotropin-releasing hormone-receptor 1 mRNAs in the pituitary and hypothalamus of the rat during chronic ‘binge’ cocaine and withdrawal. Brain Res. 2003;964:187–199. doi: 10.1016/s0006-8993(02)03929-x. [DOI] [PubMed] [Google Scholar]
- Zhu MY, Wang WP, Huang J, Feng YZ, Regunathan S, Bissette G. Repeated immobilization stress alters rat hippocampal and prefrontal cortical morphology in parallel with endogenous agmatine and arginine decarboxylase levels. Neurochem Int. 2008;53:346–354. doi: 10.1016/j.neuint.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DR, Herman JP. Local integration of glutamate signaling in the hypothalamic paraventricular region: regulation of glucocorticoid stress responses. Endocrinology. 2000;141:4801–4804. doi: 10.1210/endo.141.12.7949. [DOI] [PubMed] [Google Scholar]