Abstract
Objective
To compare levels of expression of mucin gene 2 (MUC2), a major secretory mucin, in the middle ear of patients with otitis media (OM) and control patients.
Design
Case-control study.
Setting
Children’s Hospital of Wisconsin, Milwaukee.
Patients
Nineteen patients aged 6 months to 15 years undergoing routine ventilation tube insertion for recurrent OM or chronic OM with effusion and 8 controls with no history of OM undergoing cochlear implantation.
Interventions
Biopsy of middle ear epithelium for RNA extraction.
Main Outcome Measure
Expression of MUC2 by real-time reverse transcription–polymerase chain reaction.
Results
Twenty-seven OM samples (17 recurrent and 10 with effusion) from 19 patients were analyzed and compared with 9 control samples from 8 patients. The mean MUC2 expression was 6.12 (95% confidence interval, 3.32–8.89) times that of the controls in the OM samples overall, 5.00 (95% confidence interval, 2.79–7.21) times that of controls in the recurrent OM samples, and 7.98 (95% confidence interval, 1.58–14.38) times that of controls in the OM with effusion samples.
Conclusions
Levels of MUC2 expression in human middle ear epithelium are significantly increased in patients with OM overall, patients with recurrent OM, and patients with OM with effusion compared with controls. Mucins are fundamentally important in the middle ear, controlling viscoelastic properties of secretions and providing mucosal protection and bacterial clearance. Demonstration of these differences between patient groups highlights the need for greater understanding of molecular responses in OM, which may provide novel interventions for this common problem.
Otitis media (OM) is both common, representing the most frequent diagnosis of illness in US pediatrician visits, and expensive, accounting for approximately $5 billion in spending annually on the treatment of OM in the United States.1,2 Despite the prevalence of OM, its potential for morbidity, the enormous health care expenditures resulting from its treatment, the frequent need for surgical intervention, and the increasing therapeutic challenges imposed by antimicrobial resistance, much is still unknown about the cellular, molecular, immunologic, and inflammatory events in this disease process.
Mucins are glycoproteins that are secreted in response to various stimuli, including inflammatory cytokine exposure, from the pseudostratified columnar epithelium of the middle ear space.3 A variety of mucins are secreted from this epithelium, and variation in the quantity and character of these mucins is known to be important in the pathophysiologic mechanisms of OM.3,4 Mucins are the only component of middle ear effusions responsible for their viscoelastic properties and are responsible for creating a viscous fluid that can prevent normal mucociliary clearance.5 This increased viscosity can lead to pathological conditions such as chronic otitis media with effusion (OME) and hearing loss. However, mucins also play an important protective function within the middle ear space, assisting with mechanical clearance of pathogens and debris as well as providing a protective barrier to the epithelium and assisting with the host’s innate immune function.6 Given this important role of mucin in the physiology of the middle ear space, investigations that provide insight into middle ear mucin function and regulation may allow meaningful new intervention strategies for OM by incorporating a concept of modulating mucin production by middle ear epithelium. Despite this understanding of the importance of mucin in OM, little has been done to investigate specific cellular and molecular mechanisms that may be related to differences in middle ear mucin production between patients with OME or recurrent OM (RecOM) and patients without an underlying history of OM. The aim of this study was to investigate the regulation in the middle ear of a specific mucin gene, MUC2, which has been shown to respond to inflammatory mediators,3 in patients with and without a diagnosis of OM.
METHODS
PATIENTS
Institutional review board approval was obtained from the Children’s Hospital of Wisconsin Human Research Review Committee. Patients were invited to participate at the time of consultation for either ventilation tube insertion (VTI) (study group) or cochlear implantation (control group). Written informed consent was obtained from the parents or guardians of the children in the study and control groups. Each patient fulfilling the study criteria between February 1, 2004, and April 30, 2005, from a single tertiary referral pediatric otolaryngology practice (J.E.K.) was invited to participate. The participation rate was approximately 50% in the VTI group. Patients meeting control criteria between February 1, 2004, and April 30, 2005, from a single tertiary referral otology practice (P.A.W.) were invited to participate as well. Basic demographic information including age, sex, and race was collected.
Inclusion criteria for the study group included age between 6 months and 15 years and meeting the clinical criteria for VTI for either OME or RecOM. Otitis media with effusion was defined as the persistence of a middle ear effusion for longer than 3 months. Recurrent OM was defined as 3 or more acute OM presentations within a 6-month period in which clinical evidence of OM and middle ear effusion resolved between episodes. Inclusion criteria for the control group included patients in the same age range who met the clinical criteria for cochlear implantation and who had fewer than 1 episode of diagnosed OM per 12 months of life. Because of the small quantity of RNA extracted from each control sample and the difficulty enrolling age-matched cochlear implant control patients who met the inclusion criterion of no significant history of OM, adults undergoing cochlear implantation who had no history of OM were enrolled as well. Exclusion criteria included immunologic abnormality, either intrinsic or pharmacologic; anatomic or physiologic defect of the ear (except in the control group, who all had an anatomic or physiologic defect of the ear with resulting profound sensorineural hearing loss requiring cochlear implantation); syndrome associated with OM (eg, Down syndrome or cleft palate); and chronic mastoiditis, cholesteatoma, or other OM complications except for conductive hearing loss. In addition, control patients were excluded if there was any evidence of middle ear disease at the time of cochlear implantation.
SPECIMEN ACQUISITION AND TISSUE PREPARATION
In the patient group, general mask anesthesia was used during the surgical procedure. With the use of an operating microscope (Carl Zeiss Inc, Thornwood, New York), a myringotomy incision was made in the anteroinferior tympanic membrane. Middle ear effusion was collected, if present, with a sterile tympanostomy trap for culture. A cup forceps was inserted through the myringotomy incision under high-power magnification, and middle ear mucosa specimens (<1 mm2) were obtained from the middle ear promontory in proximity to the eustachian tube orifice.
In the control group, a mastoidectomy was performed per routine for the approach for cochlear implantation. When the middle ear was exposed, a small (approximately 1 mm2) middle ear mucosa biopsy specimen was taken from a location similar to that in the patient group with a cup forceps.
All samples were placed in preservative-free normal saline, placed on ice, and transferred directly to the laboratory. Total RNA was immediately extracted from the biopsy specimens by means of a purification kit (RNeasy Mini Kit; Qiagen, Valencia, California) according to the manufacturer’s instructions. DNase digestion was performed on-column by means of a commercial kit (RNase-Free DNase Set; Qiagen). The yield and purity were determined by spectrophotometric determination. Purified RNA was stored at −80°C until reverse transcription–polymerase chain reaction (RT-PCR) analysis was performed.
Total RNA was reverse transcribed by means of a kit (Superscript III; Invitrogen, Carlsbad, California). Conventional RT-PCR was performed with both a human sample and cultured human middle ear epithelial cells to verify that RT-PCR could be accomplished on the human samples, with the following primer set: sense 5′-ccgtcctcctaccacatcat-3′, antisense 5′-ctctccaggccgttgaagt-3′. The products were separated on aga-rose gel to verify correct size of 149 base pairs (bp). The RT-PCR products were also cut by means of PvuII, which appropriately divided the amplicon into 52-bp and 97-bp fragments.
Real-time PCR was carried out (BioRad iCycler iQ Real-Time system; BioRad, Hercules, California) by means of assays (TaqMan Gene Expression Assays; Applied Biosystems, Foster City, California) specific for human MUC2 and the housekeeping gene HPRT. (Primers are commercially available from Applied Biosystems and are verified by the company to amplify the gene of interest.) HPRT was chosen because it reaches the linear phase during the RT-PCR within 3 to 4 cycles of MUC2, allowing a consistent comparison of threshold cycle values. Relative expression levels were then calculated by comparing the threshold cycle values of HPRT and MUC2 as described in the literature.7
All OM samples were run simultaneously with control samples, so that levels of MUC2 expression could be expressed as a fold increase over the mean of control samples for that run. The mean control expression level was set, by definition, at 1. Means for OM overall, OME, and RecOM were analyzed with a 95% confidence interval (CI). Mean increases in expression for patients with RecOM with and without effusions at the time of specimen collection were also compared.
RESULTS
Conventional RT-PCR resulted in an amplicon appearing at the correct length of 149 bp, with an appearance similar to the result obtained from RNA from cultured human middle ear epithelial cells (Figure 1). Incubation with restriction endonuclease PvuII led to appropriate cutting of the amplicon into fragments of 52 bp and 97 bp. These results indicated that RT-PCR was feasible with the RNA extracted from human middle ears.
Figure 1.
Conventional reverse transcription–polymerase chain reaction for MUC2. Lane 1,50– to 1000–base pair ladder (M); lane 2, human otitis media (OM) sample RNA; lane 3, cultured human middle ear epithelial cell (HMEEC) RNA. bp indicates base pairs.
A total of 27 OM samples were obtained from 19 patients undergoing VTI, and 9 control samples were obtained from 8 patients undergoing cochlear implantation. Ten samples from 8 patients were included in the OME group, and 17 samples from 11 patients were included in the RecOM group. Table 1 gives the characteristics of the patients with OM. In the OME group, 7 of 8 patients (88%) had effusions collected at the time of VTI, while only 5 of 11 patients in the RecOM group (45%) had effusions at the time of VTI.
Table 1.
Characteristics of Patients With Otitis Media
| Characteristic | No. (%) |
||
|---|---|---|---|
| Overall (N=19) | OME (n=8) | RecOM (n=11) | |
| Sex, F | 6 (32) | 4 (50) | 2 (18) |
| Age, mean, mo | 29 | 32 | 27 |
| Race | |||
| White | 14 (74) | 6 (75) | 8 (73) |
| Asian | 1 (5) | 0 (0) | 1 (9) |
| Hispanic | 1 (5) | 1 (12) | 0 (0) |
| Other | 3 (16) | 1 (12) | 2 (18) |
Abbreviations: OME, otitis media with effusion; RecOM, recurrent otitis media.
The cochlear implant control group included 6 children and 2 adults. One child under went bilateral cochlear implantation, and a sample was collected at each surgical procedure. Five (83%) of the children were male, 5 (83%) were white, and 1(17%) was Asian, with a mean age of 23 months. Two adults were included because of the scarcity of cochlear implant controls. One was a 32-year-old white man, and the other, a 55-year-old white woman. The mean MUC2 expression for the adults fell within 1 SD of the mean for all pediatric samples, so these samples were included in the analysis. Table 2 details the comparison between pediatric and adult control patients. While additional age-matched controls could have been obtained from patients undergoing other ear procedures such as tympanoplasty, these patients almost always have a history of OM (leading to the operative indication). In addition, patients with dry perforations of the tympanic membrane would likely have changes in their middle ear mucosa because it was exposed to the air of the external auditory canal. Neither of these situations represents a true control for baseline levels of MUC2 expression.
Table 2.
Control MUC2 Expression Valuesa
| Cochlear Implant Controls | MUC2 Expression |
|---|---|
| Pediatric | |
| CI-01 | 3.59 |
| CI-02 | 0.35 |
| CI-03 | 0.80 |
| CI-04 | 0.20 |
| CI-05 | 1.03 |
| CI-06 | 4.85 |
| CI-05 | 3.59 |
| CI-06 | 0.35 |
| CI-05 | 0.48 |
| CI-06 | 6.79 |
| CI-19 | 1.17 |
| Pediatric mean | 2.11 |
| SD | 2.24 |
| Adult | |
| CI-14 | 0.27 |
| CI-15 | 2.24 |
Abbreviation: CI, cochlear implant control patients.
Comparison of MUC2 expression in pediatric and adult cochlear implant controls. Values represent the comparison of threshold cycle of MUC2 with the housekeeping gene HPRT for each sample.7
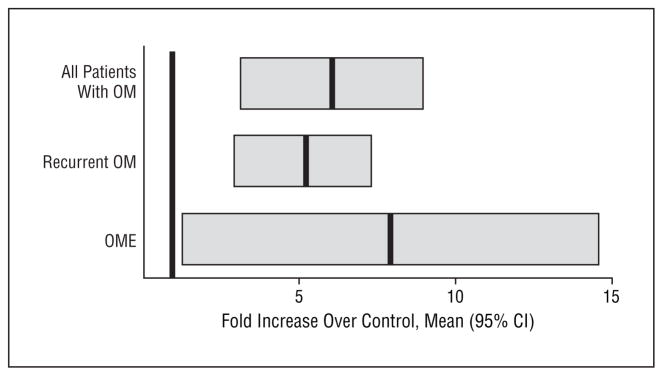
Data from each run of real-time RT-PCR are given in Table 3. Taking all patients with OM as a group, the mean level of MUC2 expression was 6.12 times that of the controls, with a 95% CI of 3.32 to 8.89. The MUC2 expression in patients with OME averaged 7.89 times the control value (95% CI, 1.58–14.38). The MUC2 expression in patients with RecOM was 5.00 times the control value (95% CI, 2.79–7.21). Because none of these CIs overlaps 1 (which is, by definition, the mean for controls), all of these levels are statistically significant. When the OME and RecOM groups were compared, there was a trend toward higher expression in patients with OME, but this difference was not statistically significant (Figure 2).
Table 3.
MUC2 Expression Levels by Run of RT-PCRa
| Run | Sample | MUC2 Expression | Fold Increase |
|---|---|---|---|
| Run 1 | CI-01 | 3.59 | |
| CI-02 | 0.35 | ||
| CI-03 | 0.80 | ||
| RecOM-1 | 11.40 | 7.22 | |
| RecOM-2 | 15.05 | 9.52 | |
| RecOM-3 | 1.16 | 0.73 | |
| OME-1 | 51.20 | 32.41 | |
| OME-2 | 36.62 | 23.18 | |
| OME-3 | 13.72 | 8.68 | |
| OME-4 | 7.35 | 4.65 | |
| Run 2 | CI-04 | 0.20 | |
| CI-05 | 1.03 | ||
| CI-06 | 4.85 | ||
| RecOM-4 | 1.36 | 0.67 | |
| OME-5 | 3.76 | 1.85 | |
| OME-6 | 2.72 | 1.34 | |
| OME-7 | 3.51 | 1.73 | |
| Run 3 | CI-05 | 3.59 | |
| CI-06 | 0.35 | ||
| RecOM-5 | 11.58 | 5.88 | |
| RecOM-6 | 2.96 | 1.50 | |
| RecOM-7 | 2.83 | 1.44 | |
| RecOM-8 | 30.57 | 15.52 | |
| RecOM-9 | 13.94 | 7.08 | |
| RecOM-10 | 2.64 | 1.34 | |
| Run 4 | CI-14 | 0.27 | |
| CI-15 | 2.24 | ||
| RecOM-11 | 17.54 | 13.98 | |
| RecOM-12 | 1.89 | 1.50 | |
| OME-8 | 1.50 | 1.19 | |
| OME-9 | 2.86 | 2.27 | |
| Run 5 | CI-05 | 0.48 | |
| CI-06 | 6.79 | ||
| CI-14 | 2.87 | ||
| CI-15 | 1.08 | ||
| CI-19 | 1.17 | ||
| RecOM-13 | 12.97 | 5.23 | |
| RecOM-14 | 10.29 | 4.15 | |
| RecOM-15 | 2.00 | 0.81 | |
| RecOM-16 | 18.34 | 7.40 | |
| RecOM-17 | 2.96 | 1.19 | |
| OME-10 | 6.26 | 2.53 |
Abbreviations: CI, cochlear implant controls; OME, otitis media with effusion; RecOM, recurrent otitis media; RT-PCR, reverse transcription–polymerase chain reaction.
MUC2 expression levels by run of real-time RT-PCR. MUC2 values represent the comparison of threshold cycle of MUC2 to the housekeeping gene HPRT for each sample.7 “Fold increase” indicates the fold increase of each sample over the mean of CI samples for that run of real-time RT-PCR.
Figure 2.
Mean relative expression of MUC2 in patients with otitis media (OM) compared with control patients. The mean for controls is, by definition, equal to 1, because all OM samples are expressed as a fold increase over controls (shown as the dark bar at a value of 1). The dark bars for each OM group are at the mean, with thick gray bars representing the 95% confidence interval (CI). None of the CIs overlaps 1, indicating statistical significance. OME indicates OM with effusion.
The patients with RecOM with or without effusions at the time of VTI were also compared. Nine of 17 RecOM samples (53%) came from ears without effusions at the time of VTI. These samples had a mean MUC2 expression 5.67 times that of controls (95% CI, 2.61–8.73). Of the samples with effusions, the mean MUC2 expression was 4.26 times the control value (95% CI, 0.93–7.59).
COMMENT
In OME, which frequently results in significant hearing loss and requirement for surgical intervention, middle ear epithelium often produces mucin for a longer period and with greater viscosity than in patients with RecOM. The increased viscosity of the mucin inhibits mucociliary clearance. Its persistence in the middle ear space leads to hearing loss and may contribute to future acute infections. Recent advances in molecular techniques have allowed significant advances in the understanding of mucin structure and the roles of various mucin genes. Of the 20 currently identified human mucin genes, the genes MUC1, MUC2, MUC4, MUC5AC, and MUC5B all appear to play some role in OME.5,8 Animal studies have demonstrated mucin gene up-regulation in response to inflammatory mediators, and cultured human middle ear epithelial cells are affected similarly.3 However, there have been no large studies to date comparing the mucin gene expression from middle ear epithelium of human patients with OME and RecOM to delineate possible differential expression contributing to the underlying pathologic mechanism of these disease processes.
Of particular interest in respiratory epithelial pathology, including the middle ear, are gel-forming mucins. These mucins are the major contributors to the viscoelastic properties of mucous secretion. As such, these mucins are associated with airway epithelial abnormalities owing to their ability to compromise normal mucociliary clearance. This is especially important in chronic disease states such as chronic bronchitis, asthma, and chronic OM.9 The protein products of MUC2, MUC5AC, and MUC5B have been previously identified as gel-forming mucins.9 Inflammatory cytokines are important in the pathogenesis of infection and inflammation, including in OM, and cytokines have been shown to upregulate mucin secretion from middle ear epithelium.10,11 MUC2 and MUC5AC have demonstrated up-regulation in response to the inflammatory cytokines interleukin 1α (IL-1α) and tumor necrosis factor β (TNF-β), and MUC2 has shown the greatest response.3 Given that only very small amounts of tissue were sampled, limiting the number of RT-PCR reactions that could be performed, this study focused on the expression of MUC2 in biopsy specimens of human middle ear epithelium.
The findings from this investigation provide further evidence that mucin gene regulation can be linked to middle ear epithelial inflammatory events, and specifically OM, in an in vivo human tissue model. In addition, although mucin gene up-regulation might be expected during an acute otitis event, these results demonstrate that changes in mucin gene expression persist in patients who have chronic OM in the form of either OME or recurring acute infections. This ongoing change in mucin gene expression may lead to persistent production of more highly viscous fluid, given that MUC2 represents a gel-forming mucin, and contribute to the production of a middle ear effusion that is difficult to clear through the eustachian tube. This, in turn, might suggest that dysfunction of the eustachian tube is not the only causative factor in the development of OME. Aside from focusing on mechanical aspects in this disease process, molecular changes of the middle ear epithelium must be considered as well. In addition, the findings comparing patients with RecOM with and without effusions is intriguing, especially the fact that MUC2 expression remains significantly elevated in patients without effusion. The finding that there is a persistent increase in MUC2 levels almost as great as in patients with OME was somewhat unexpected. In these patients, mucin expression levels may remain high after an initial infectious event despite the ability to clear the middle ear effusion, or perhaps these patients may express mucin at a higher baseline level than controls. These possibilities raise intriguing questions about the pathophysiologic characteristics of OM. This includes the potential for genetic variability at a molecular level linked to gene expression profiles influencing underlying patients’ risk of and susceptibility to OM.
These results highlight the concept that OM is a complex interaction between host and pathogen; it is a conflict between host and pathogen genomes. In addition to the well-known sociologic risk factors that have been associated with OM, there are likely genomic host factors that predispose individuals to OM outside of anatomic or syndromic associations. One possibility is that there are differences among individuals in the mucin genes themselves. Although the presence of polymorphisms in mucin genes and the genes that regulate their expression has been well described, the importance of these polymorphisms is less well understood.12–15 It has been demonstrated that polymorphisms in the variable numbers of tandem repeats (VNTR) region of the MUC1 mucin gene affect an individual’s susceptibility to Helicobacter pylori gastritis, and longer MUC2 mucin genes protect atopic individuals from developing asthma.12–14 These results may be explained by changes in protein functionality associated with different-length mucin glycoproteins, which in turn produce clinically relevant differences in response to pathogens, infection, inflammation, and overall epithelial physiologic characteristics. Mucin gene polymorphisms may have similar implications in middle ear epithelium. Polymorphism may lead to different glycoprotein folding properties or variable levels of glycosylation. Clinically, these changes may alter mucin function by altering middle ear fluid viscosity, mucociliary flow, bacterial or viral adherence, and bacterial- or viral-host interactions. The possibility of mucin gene VNTR polymorphism contributing to the severity or incidence of OM has not been reported but is currently being investigated in our laboratories.
Another host factor that may predispose to OM is an alteration in cell signaling leading to up-regulation of mucin expression. This may begin with higher levels of inflammatory cytokines in response to an inflammatory stimulus. Otitis media susceptibility has been associated with high-cytokine–producing genotypes of TNF-α and IL-6.16 Cultured middle ear epithelial cells have shown increased expression in response to TNF-α, IL-1β, and IL-6.3,10,11 However, the middle ear epithelial cells of some individuals may respond to inflammation with a different mucin secretion profile. This may result from polymorphisms in the promoters of mucin genes. For instance, MUC5B shows little variation in the number of VNTRs, but its promoter region has demonstrated a large number of polymorphisms. These different polymorphisms display variation in transcriptional activity, and polymorphisms with decreased transcriptional activity show a negative association with diffuse panbronchiolitis, while polymorphisms with increased transcriptional activity show a positive association with the disease.17 Similar variations in promoter sequences may have a role in OM as well.
Pathogen factors contributing to continued expression of mucin genes should be considered as well. Analysis of chinchilla middle ear mucosa from animals inoculated with viable Haemophilus influenzae, then treated with amoxicillin, demonstrated the presence of biofilms.18 In addition, middle ear mucosa from children with RecOM or OME undergoing VTI demonstrated biofilms in 92% of cases and complete absence in control patients.19 These results suggest that biofilms play an ongoing role in the pathophysiologic mechanisms of RecOM and OME. It is unclear how these biofilms stimulate the inflammatory pathway, but it seems likely that continued presence of viable bacteria would be associated with continued middle ear epithelial inflammation. This has been linked to up-regulation of mucin secretion and, in particular, MUC2 expression. Again, these findings lead to more questions than answers, but clearly the relationship between biofilm formation in the middle ear and mucin, which is recognized as a primary mucosal defense against pathogen invasion, deserves additional study.
CONCLUSIONS
This study demonstrates the increased expression of MUC2 in patients requiring VTI for the RecOM and OME groups over controls. Variation in the quantity and character of middle ear secretions, and specifically mucin secretion, during periods of inflammation is known to be important in the pathophysiologic mechanisms of OM. The data from this study further demonstrate the importance of understanding the function and regulation of mucins on a cellular and molecular level. Efforts at developing meaningful new intervention strategies for OM should, therefore, consider possibilities of modulating mucin production as one avenue to achieve improved success in treating this common childhood illness.
Acknowledgments
Funding/Support: This study was supported by National Institutes of Health grants NIDCD DC00192 (Dr Kerschner) and DC007903 (Dr Kerschner), an American Society of Pediatric Otolaryngology Resident Research Award (Dr Ubell), and the Toohill Research Fund of the Department of Otolaryngology and Communication Sciences, Medical College of Wisconsin (Dr Ubell).
Footnotes
Author Contributions: Drs Ubell and Kerschner had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Ubell, Kerschner, Wackym, and Burrows. Acquisition of data: Ubell, Kerschner, Wackym, and Burrows. Analysis and interpretation of data: Ubell and Kerschner. Drafting of the manuscript: Ubell and Kerschner. Critical revision of the manuscript for important intellectual content: Ubell, Kerschner, Wackym, and Burrows. Statistical analysis: Ubell, Kerschner, and Burrows. Obtained funding: Kerschner. Administrative, technical, and material support: Ubell, Kerschner, Wackym, and Burrows. Study supervision: Kerschner and Wackym.
Financial Disclosure: None reported.
Previous Presentation: This article was presented at The American Society of Pediatric Otolaryngology 2007 Annual Meeting; April 29, 2007; San Diego, California.
Additional Information: This article was awarded the William P. Potsic Honorable Mention Award for Basic Science Research.
References
- 1.Bluestone CD, Klein JO. Otitis media, atelectasis and eustachian tube dysfunction. In: Bluestone CD, Stool SE, Kenna MA, editors. Pediatric Otolaryngology. Vol. 1. Philadelphia, PA: WB Saunders; 1996. pp. 388–582. [Google Scholar]
- 2.Rosenfeld RM, Casselbrant ML, Hannley MT. Implications of the AHRQ evidence report on acute otitis media. Otolaryngol Head Neck Surg. 2001;125 (5):440–448. doi: 10.1067/mhn.2001.119326. [DOI] [PubMed] [Google Scholar]
- 3.Kerschner JE, Meyer TK, Burrows A. Chinchilla middle ear epithelial mucin gene expression in response to inflammatory cytokines. Arch Otolaryngol Head Neck Surg. 2004;130(10):1163–1167. doi: 10.1001/archotol.130.10.1163. [DOI] [PubMed] [Google Scholar]
- 4.Brown DT, Litt M, Potsic WP. A study of mucus glycoproteins in secretory otitis media. Arch Otolaryngol. 1985;111(10):688–695. doi: 10.1001/archotol.1985.00800120082011. [DOI] [PubMed] [Google Scholar]
- 5.Pearson JP, Rankin BJ, Hutton DA, Birchall JP, Wilson J. Selective upregulation of MUC 5B expression in otitis media with effusion (O.M.E.). Abstracts of the Seventh International Symposium on Recent Advances in Otitis Media; June 1–5, 1999; Fort Lauderdale, FL. Abstract 52. [Google Scholar]
- 6.Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev. 2000;173:27–38. doi: 10.1034/j.1600-065x.2000.917304.x. [DOI] [PubMed] [Google Scholar]
- 7.Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques. 2002;32(6):1372–1374. 1376, 1378–1379. [PubMed] [Google Scholar]
- 8.Kerschner JE, Yang C, Burrows A. Signaling pathways and blockade in interleukin-1β–mediated middle ear epithelial mucin secretion. Laryngoscope. 2006;116 (2):207–211. doi: 10.1097/01.mlg.0000191467.63650.9e. [DOI] [PubMed] [Google Scholar]
- 9.Kubba H, Pearson JP, Birchall JP. The aetiology of otitis media with effusion: a review. Clin Otolaryngol Allied Sci. 2000;25(3):181–194. doi: 10.1046/j.1365-2273.2000.00350.x. [DOI] [PubMed] [Google Scholar]
- 10.Kerschner JE, Meyer TK, Wohlfeill E. Middle ear epithelial mucin production in response to interleukin 1βexposure in vitro. Otolaryngol Head Neck Surg. 2003;129(1):128–135. doi: 10.1016/S0194-59980300532-1. [DOI] [PubMed] [Google Scholar]
- 11.Kerschner JE, Meyer TK, Yang C, Burrows A. Middle ear epithelial mucin production in response to interleukin-6 exposure in vitro. Cytokine. 2004;26(1):30–36. doi: 10.1016/j.cyto.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Vinall LE, Hill AS, Pigny P, et al. Variable number tandem repeat polymorphism of the mucin genes located in the complex on 11p15.5. Hum Genet. 1998;102 (3):357–366. doi: 10.1007/s004390050705. [DOI] [PubMed] [Google Scholar]
- 13.Vinall LE, Fowler JC, Jones AL, et al. Polymorphism in human mucin genes in chest disease: possible significance of MUC2. Am J Respir Cell Mol Biol. 2000;23(5):678–686. doi: 10.1165/ajrcmb.23.5.4176. [DOI] [PubMed] [Google Scholar]
- 14.Vinall LE, King M, Novelli M, et al. Altered expression and allelic association of the hypervariable membrane mucin MUC1 in Helicobacter pylori gastritis. Gastroenterology. 2002;123(1):41–49. doi: 10.1053/gast.2002.34157. [DOI] [PubMed] [Google Scholar]
- 15.Allen RD. Polymorphism of the human TNF-alpha promoter—random variation or functional diversity? Mol Immunol. 1999;36(15–16):1017–1027. doi: 10.1016/s0161-5890(99)00127-3. [DOI] [PubMed] [Google Scholar]
- 16.Patel JA, Nair S, Revai K, et al. Association of proinflammatory cytokine gene polymorphisms with susceptibility to otitis media [published correction appears in Pediatrics. 2007;119(6):1270] Pediatrics. 2006;118(6):2273–2279. doi: 10.1542/peds.2006-0764. [DOI] [PubMed] [Google Scholar]
- 17.Kamio K, Matsushita I, Hijikata M, et al. Promoter analysis and aberrant expression of the MUC5B gene in diffuse panbronchiolitis. Am J Respir Crit Care Med. 2005;171(9):949–957. doi: 10.1164/rccm.200409-1168OC. [DOI] [PubMed] [Google Scholar]
- 18.Ehrlich GD, Veeh R, Wang X, et al. Mucosal biofilm formation on middle-ear mucosa in the chinchilla model of otitis media. JAMA. 2002;287(13):1710–1715. doi: 10.1001/jama.287.13.1710. [DOI] [PubMed] [Google Scholar]
- 19.Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296 (2):202–211. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]