Abstract
Development of a vaccine that targets blood-stage malaria parasites is imperative if we are to sustainably reduce the morbidity and mortality caused by this infection. Such a vaccine should elicit long-lasting immune responses against conserved determinants in the parasite population. Most blood-stage vaccines, however, induce protective antibodies against surface antigens, which tend to be polymorphic. Cell-mediated responses, on the other hand, offer the theoretical advantage of targeting internal antigens that are more likely to be conserved. Nonetheless, few of the current blood-stage vaccine candidates are able to harness vigorous T cell immunity. Here, we present what we believe to be a novel blood-stage whole-organism vaccine that, by combining low doses of killed parasite with CpG-oligodeoxynucleotide (CpG-ODN) adjuvant, was able to elicit strong and cross-reactive T cell responses in mice. Our data demonstrate that immunization of mice with 1,000 killed parasites in CpG-ODN engendered durable and cross-strain protection by inducing a vigorous response that was dependent on CD4+ T cells, IFN-γ, and nitric oxide. If applicable to humans, this approach should facilitate the generation of robust, cross-reactive T cell responses against malaria as well as antigen availability for vaccine manufacture.
Introduction
Vaccines are among the most cost-effective strategies for preventing infectious disease. However, a highly effective vaccine against malaria remains elusive (1). Historically, vaccines have been most successful against diseases in which natural infection leads to long-lasting immunity, and the best formulations have usually been the ones closely mimicking natural infection (2, 3). However, in the case of malaria, natural immunity is slow to develop and incomplete even after years of continuous exposure (4). While some protection from severe disease can be achieved, “immune” individuals can rapidly become reinfected following cure (5). These observations have indicated that a vaccine-mimicking natural immunity is unlikely to be effective, and alternative paradigms have been investigated.
RTS,S, the most advanced malaria vaccine, for instance, relies on administration of a complex construct containing a large part of the circumsporozoite protein (CSP) fused to the HBV surface antigen (HBV-S Ag) and free HBV-S Ag, formulated in a 3-component adjuvant. This preerythrocytic vaccine induces an approximately 30%–50% reduction in the risk of clinical malaria through antibody- and cell-mediated responses, thus positioning it close to licensure trials (6, 7). In the case of blood-stage malaria, however, success has been limited. The main targets have been proteins on the surface of the merozoite or the infected red cell; and thus, antibodies have been recognized as the main mediators of protection (8). Therefore, several vaccine candidates have been selected using sera from immune individuals (9, 10), and many of the current candidates aim to induce antibody responses of the same type as those induced through natural infection (11). Antigenic variation and polymorphism will probably limit their protective efficacy (12, 13).
In contrast to antibodies, T cells mainly recognize antigen after it has been processed and presented in the context of MHC molecules. This process allows T cells to target internal and possibly more conserved epitopes and/or antigens. In rodent malaria, for example, CD4 T cells are able to recognize cryptic epitopes of polymorphic antigens such as apical membrane antigen 1 (AMA-1) or CSP (14, 15). Similarly, rodent CD4 T cells can recognize invariant regions on surface antigens such as CSP (16) or internal and highly conserved proteins such as hypoxanthine-xanthine-guanine phosphoribosyl transferase (HGXPRT) (17). More importantly, rodent and human CD4 T cells are able to target invariant epitopes of polymorphic antigens such as CSP in the context of different MHC-II molecules (16, 18, 19), suggesting that malaria-specific CD4 T cells can be broadly reactive. This phenomenon has already been documented in B cell–deficient mice in which cross-protection against heterologous parasites can be readily induced in the absence of humoral responses (20).
Therefore, it would be expected that a malaria vaccine able to induce vigorous T cell responses against multiple antigens would engender some degree of cross-protection. However, most blood-stage candidates induce suboptimal T cell responses and only against 1 or 2 antigens. To maximize the spectrum of antigens, whole-organism immunization has been proposed and tested against liver or blood stages (1, 21). The available data suggest that (a) immunization of humans with irradiated sporozoites or (b) mice with genetically attenuated sporozoites (22, 23) or (c) humans with blood-stage parasites curtailed by antimalarials (24, 25) can achieve sterile immunity or delayed parasitemia through the induction of vigorous T cell responses.
Here, we set out to develop a whole-organism vaccine able to stimulate vigorous and broadly reactive T cell responses against malaria. Four considerations were essential prior to development. First was the need to keep a broad spectrum of target antigens. To circumvent the limitations of using live parasites yet retain the maximum spectrum of antigens, a formulation based on killed parasites was ideal. Second was the selection of a potent T cell adjuvant. Here, the selection of an adjuvant such as CpG-oligodeoxynucleotides (CpG-ODN), used extensively in human trials, easy to admix with parasites and conducive of vigorous Th1 differentiation, was suitable (26). Third and more importantly was the determination of the right antigenic dose. Here, administration of low doses of parasite was desired in order to elicit better T cell responses. This was based on extensive evidence demonstrating that low doses of live parasite induce vigorous T cell responses (24, 25), while higher parasite burdens result in deletion of effector cells (27). Finally, a high-dose whole-parasite vaccine would not be possible due to logistical constraints.
By addressing these issues, we developed a 3-prong vaccine approach combining (a) low doses of (b) whole-killed parasite (c) adjuvanted in CpG-ODN. By applying this strategy, we show that rodent immunization with only 1,000 killed parasites in CpG-ODN induces durable protection against homologous and heterologous challenge. Strong, durable, and cross-reactive CD4 T cells were easily induced by this type of vaccine, thus providing useful insight for the development of better T cell vaccines against malaria.
Results
Definition of a low-dose whole-parasite vaccine.
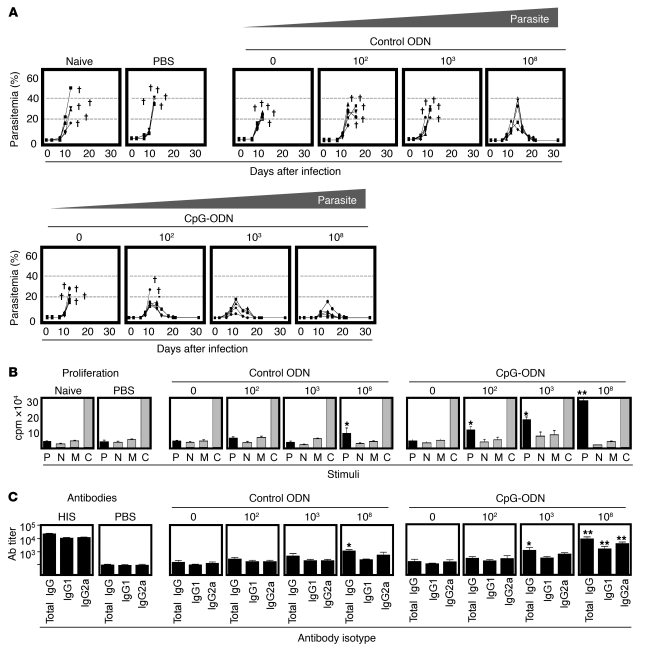
To define an effective vaccine containing low doses of Plasmodium antigen, A/J mice were immunized with decreasing doses of P. c. chabaudi AS antigen equivalent to 108, 103, or 102 parasitized rbc (prbc) in CpG-ODN or control ODN. Two weeks after immunization, mice were given a lethal parasite challenge. Parasitemia (Figure 1A) and disease severity (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI39222DS1) were monitored. Similar to naive mice, animals immunized with adjuvant alone (0 prbc), ultra-low (102 prbc), or low (103 prbc) doses of parasite in control ODN experienced high mortality and severe disease. Mice immunized with high doses of parasite (108 prbc) in control ODN survived challenge but exhibited high parasitemia and severe disease. In contrast, high doses of parasite (108 prbc) in CpG-ODN achieved 100% protection, markedly reducing parasitemia and disease severity. Of greater interest, however, low doses of parasite (103 prbc) in CpG-ODN induced complete protection with limited disease severity and low parasitemia. Ultra-low doses (102 prbc) in CpG-ODN still resulted in 60% protection.
Figure 1. Definition of a low-dose whole-parasite vaccine.
(A) To define the low-dose vaccine, A/J mice were immunized with decreasing doses of P. c. chabaudi AS (AS) antigen equivalent to 108, 103, 102, or 0 (adjuvant alone) prbc in CpG-ODN or control ODN. 2 weeks after immunization, mice were challenged i.v. with 105 homologous (AS) prbc and the outcome of infection monitored as parasitemia. Disease severity is also described in Supplemental Figure 1. Data for individual mice are shown. †Animals that succumbed to infection. (B) To assess cellular immune responses induced by vaccination, antigen-specific proliferation of splenic lymphocytes was assessed at the time of challenge infection. Spleen cells were cultured for 96 hours in the presence of fresh (AS) parasitized rbc (P), normal rbc (N), medium (M), or ConA (C) and proliferation assessed by thymidine uptake. Results show mean ± SEM. (C) To assess vaccine-induced antibody responses, parasite-specific IgG titers against homologous (AS) antigen were assessed by ELISA at the time of challenge. Reciprocal median total IgG titers and interquartile ranges are shown. All results are representative of 5 mice per group of 3 independent experiments performed. Significant differences compared with adjuvant alone (control ODN or CpG-ODN) are shown. *P < 0.05; **P < 0.01. HIS, hyperimmune sera.
To investigate the induction of cellular and humoral immunity following vaccination, parasite-specific proliferation (Figure 1B) and antibody titers (Figure 1C) were assessed in all groups. PBS-control mice showed no parasite-specific proliferation (<5 × 103 cpm) or antibody responses (IgG titers: <1:500). In accordance with their susceptibility to challenge, animals immunized with adjuvant alone (CpG-ODN or control ODN), ultra-low (102 prbc), or low (103 prbc) doses of parasite in control ODN also failed to exhibit proliferative or antibody responses (<5 × 103 cpm and IgG titers: <1:500). Animals immunized with high doses of parasite in control ODN showed modest T cellular (10 ± 3 × 103 cpm) and humoral responses (IgG titer: 1:1,000–2,000) while the same doses of parasite (108 prbc) in CpG-ODN induced robust proliferation (30.0 ± 3 × 103 cpm) and higher antibody levels (IgG titer: 1:9,000-12,000). Interestingly, animals immunized with low doses (103 prbc) of antigen in CpG-ODN exhibited strong cellular responses (20 ± 4 × 103 cpm) yet only modest antibody levels (IgG titer: 1:3,000–5,000). Ultra-low doses (102 prbc) of parasite in CpG-ODN induced limited cellular (12.0 ± 2 × 103 cpm) and no measurable antibody responses (IgG titer: <1:500). Thus, lower doses of antigen (102–103 prbc) induced protection by the elicitation of strong cellular and modest antibody responses.
Mechanisms of protection.
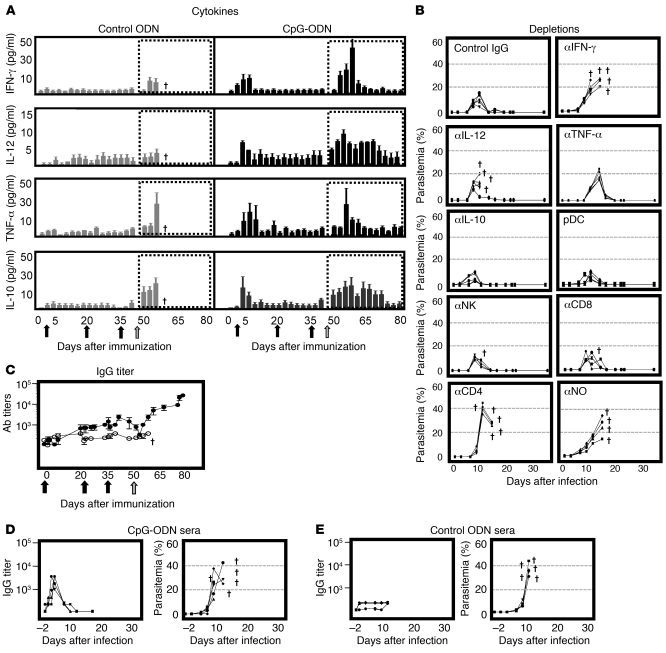
Because our strategy was to investigate the most effective low-dose vaccine, we focused on the formulation containing the lowest dose of parasite able to elicit a stable and protective response (i.e., 103 prbc in CpG-ODN). To define the protective mechanisms, first, we assessed the pattern of serum cytokine secretion during immunization (priming and boost injections) and challenge. During priming, administration of parasite in CpG-ODN, but not parasite in control ODN, induced rapid secretion of IFN-γ, IL-12, TNF-α, and IL-10 (Figure 2A). Following boost injections (parasite alone), minimal cytokine release was observed. Upon lethal challenge, however, mice immunized with parasite in CpG rapidly produced IFN-γ, IL-12, TNF-α, and IL-10 (Figure 2A). Mice immunized with parasite in control ODN also produced significant amounts of TNF-α and IL-10 but succumbed to infection. This finding suggested that neither TNF-α nor IL-10 were required for protection. To ascertain this hypothesis, mice immunized with parasite in CpG were depleted of each cytokine, IFN-γ, IL-12, TNF-α, or IL-10, and then challenged (Figure 2B). In accordance with serum cytokine data, depletion of IFN-γ and IL-12 abrogated protection, while blockade of TNF-α or IL-10 had no effect on survival. Because plasmacytoid DC (pDC), conventional DC (cDC), NK, and T cells were shown to be responsible for the rapid production of IL-12 and IFN-γ following CpG-ODN administration (Supplemental Figure 2), cell-depletion studies were also performed (Figure 2B). Depletion of pDC had no effect on survival, suggesting additional sources of IL-12 (i.e., cDC) as shown in supplemental data (Supplemental Figure 2). Similarly, depletion of NK or CD8 T cells had no effect on survival, while depletion of CD4 T cells completely abrogated protection. Finally, given the requirement for IFN-γ and IL-12 to drive nitric oxide (NO) production for parasite elimination (28), NO depletion was also performed. In keeping with the requirement for CD4 T cells, IFN-γ and IL-12, depletion of NO abrogated protection.
Figure 2. Mechanisms of protection.
(A) To determine the profile of cytokine secretion, levels of IFN-γ (first panel), IL-12 (second panel), TNF-α (third panel), and IL-10 (fourth panel) were assessed in sera collected from A/J mice during immunization with 103 (AS) prbc in control ODN (gray bars) or CpG-ODN (black bars) as well as following challenge (dotted boxes). Immunizations (1 priming and 2 boost injections) are indicated as black arrows and challenge as a gray arrow on the x axis (days after immunization). Results represent mean ± SEM. †Animals that succumbed to infection. (B) To identify cytokines and cell types essential for protection, A/J mice were immunized with 103 prbc in CpG-ODN and subsequently depleted of IFN-γ, IL-12, TNF-α, IL-10, pDC, NK, CD8, CD4, or NO as described in Methods. Mice were then challenged i.v. with 105 homologous (AS) prbc and the outcome of infection monitored. Data for individual mice are shown. (C) To determine the kinetics of antibody responses during immunization and challenge, A/J mice were immunized with 103 (AS) prbc in CpG-ODN (black circles) or control ODN (white circles) and titers of parasite-specific IgG against homologous antigen assessed. Reciprocal median total IgG titers and interquartile ranges are shown. (D, E) To ascertain a role for antibodies in protection, naive A/J mice were adoptively transferred on days –1, 0, and 1 with 200 ml i.p. of serum collected from immune (103 [AS] prbc in CpG-ODN) or control (103 [AS] prbc in control ODN) donors. Recipients were challenged with homologous 105 (AS) prbc on day 0. Parasitemia and parasite-specific IgG titers against homologous (AS) were monitored. Reciprocal median total IgG titers and interquartile ranges are shown. All data sets are representative of 5 mice per group of 2 independent experiments performed.
Despite the moderate level of parasite-specific antibodies induced by the low-dose vaccine, we set out to study their role in protection. First, we determined the kinetics of the antibody response during immunization and challenge. Total parasite-specific IgG antibody levels increased slowly through immunization to reach a titer of 1:2,000–5,000 prior to challenge (day 49). After challenge and coincident with increasing parasitemia, IgG titers decreased to less than 1:500, the lowest at the time of peak parasitemia (days 57–60). During parasite clearance (days 61–80), however, antibody levels progressively increased, reaching a final titer of 1:20,000–30,000. The initial reduction and subsequent increase suggested that vaccine-induced antibodies were being adsorbed during acute infection and produced during recovery. To better define a role for antibodies, adoptive transfer studies were also performed. For this purpose, sera from mice immunized with parasite in CpG-ODN were collected after completion of the immunization regime (day 49 after immunization) and adoptively transferred into naive recipients (Figure 2D). Here, we found that recipients of sera from CpG-immunized mice reached antibody levels comparable to donors (3 to 5 × 103) after 3 injections (Figure 2D). Upon challenge, however, titers decreased, suggesting adsorption of parasite-specific antibodies as observed following direct immunization (Figure 2C). Despite this, all recipients of sera from CpG-immunized mice succumbed to infection (Figure 2E) as did recipients of sera from ODN-immunized mice. Therefore, the modest antibody response induced by vaccination appeared not to play a role in protection.
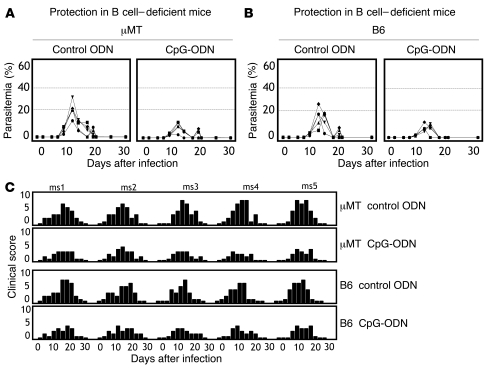
To further examine the requirement for B cells in the establishment of protective responses after immunization, B cell knockout mice (μMT) were used. These mice are derived from a C57BL/6 (B6) background [H2b] and are known to display functional T cell activation and memory formation (29). However, unlike A/J mice, B6 mice do not succumb to P. c. chabaudi. Therefore, protection is indicated by reduced parasitemia and disease severity. As shown in Figure 3, A–C, protection was equivalent in CpG-immunized B cell–deficient (μMT; Figure 3A) or B cell–sufficient (B6; Figure 3B) mice. Altogether, the serum transfer and B cell KO experiments confirmed that neither antibodies nor B cells were necessary for protection following low-dose immunization.
Figure 3. Protection in B cell–deficient mice.
To define the role of B cells in protection following immunization, B cell–deficient (μMT) or B cell–sufficient (B6) mice were immunized with 103 (AS) prbc in CpG-ODN or control ODN. 2 weeks later, mice were challenged i.v. with 105 homologous (AS) prbc and (A) parasitemia or (B) disease severity monitored. Disease severity was assessed by determining clinical scores (C). Data for individual mice are shown and are representative of 3 independent experiments. Note that mice of B6 background (B6 or μMT) are naturally resistant to P. c. chabaudi AS and thus, protection was determined by reduced parasitemia and disease severity. ms1, mouse 1.
Cross-reactivity and longevity of cellular responses.
The known promiscuity of CD4 T cells in human and rodent malarias (16, 18, 19) prompted us to also investigate whether broadly reactive CD4 T cell responses were elicited by the low-dose vaccine. Different lethal strains and species of parasite were selected to stringently assess heterologous immunity in vitro and in vivo. First, we assessed the type of CD4 T cell responses against different strains of parasite in vitro. Mice were immunized with P. c. chabaudi AS (AS), and the profile of CD4 T cell cytokine secretion (Th1 vs. Th2) against homologous (AS) and heterologous (P. c. chabaudi AJ [AJ] and P. Yoelii YM [YM]) parasites was assessed after in vitro restimulation (Figure 4A). In accordance with the results described in Figure 2, cytokine secretion indicated the predominance of a Th1 profile. As such, high levels of TNF-α, IFN-γ, and IL-2 were produced not only in response to AS but also to AJ and YM parasites (Figure 4A).
Figure 4. Cellular responses and cross-reactivity.
(A) To determine CD4 response, A/J mice were immunized with 103 (AS) prbc in CpG-ODN (black bars) or control ODN (gray bars). Spleen cells collected 2 weeks after immunization were cultured with homologous (AS) or heterologous (P. c. chabaudi AJ [AJ]; P. yoelii YM [YM]) parasites, normal rbc (nrbc), or ConA. After 96 hours, supernatants were assayed for TNF-α, IFN-γ, IL-2 (Th1 cytokines), and IL-4 or IL-5 (Th2 cytokines) assessed. Results are mean ± SEM. (B) For proliferation, spleen cells from A/J mice immunized as above were purified at 2, 8, or 12 weeks and incubated for 96 hours with AS, AJ or YM prbc, nrbc, medium, or ConA. Numbers represent percentage of CFSEdim CD4 T cells. (C) For cytokine secretion, spleen cells from A/J mice immunized as above and purified at 2, 8, or 12 weeks were incubated for 96 hours with AS, AJ, or YM prbc, nrbc, or medium and stained for intracellular IFN-γ. Numbers represent percentage of IFN-γ+ CD4 T cells. (D) For cross-reactive antibodies, sera from A/J mice immunized with 103 (AS) prbc in CpG-ODN (black bars), control ODN (gray bars), or hyperimmune sera (HIS, white bars) were tested for IgG titers against homologous (AS) or heterologous (AJ and YM) parasites. Results represent reciprocal median total IgG titers and interquartile ranges. All data sets are representative of 5 mice per group of 3 independent experiments performed. Significant differences compared with controls are shown. *P < 0.05.
To determine the longevity of this response, proliferation (Figure 4B) and IFN-γ secretion (Figure 4C) were assayed at 2, 8, and 12 weeks after immunization. We found that a significant proportion of CD4 T cells from mice immunized with parasite in CpG-ODN proliferated (Figure 4B) and secreted IFN-γ (Figure 4C) against all parasites at 2, 8, and even 12 weeks after immunization. We also tested any antibody cross-reactivity against all parasite strains (Figure 4D). Here, modest IgG titers against homologous parasite (AS) were detected at 2 weeks after immunization (3 to 5 × 103) but declined to less than 1 × 103 at 12 weeks. In contrast to cellular responses, antibody titers against heterologous parasites were negligible (less than 1 × 103) at all time points, suggesting that humoral responses were neither sustained nor cross-reactive.
Heterologous immunity.
Next, we set out to ascertain whether the broad recognition of multiple parasites observed in vitro would afford some cross-protection in vivo. For this purpose, strains of mice with differing genetic background (A/J [H-2a], B6 [H-2b], and BALB/c [H-2d]) were immunized with P. c. chabaudi AS and challenged with homologous (AS) or heterologous (AJ or YM) parasites at 2, 8, or 12 weeks (Figure 5). Consistent with the results described in Figure 4, B–C, A/J mice were protected against challenge with all parasites at 2, 8, and 12 weeks after immunization. Interestingly, B6 and BALB/c mice demonstrated long-term protection against AS and AJ but limited protection against YM parasites. Better protection against YM challenge, however, was achieved when animals were immunized with low doses of homologous parasite (103 YM prbc) in CpG-ODN (Figure 6). These results showed that cross-protection against highly virulent YM parasites was only achieved in A/J mice despite cross-protection against AS and AJ parasites being achieved in all mouse strains.
Figure 5. Heterologous immunity.
To assess for cross-protection, different strains of mice including A/J [H-2a], B6 [H-2b] and BALB/c [H-2d] mice were immunized with 103 (AS) prbc and challenged i.v. with homologous (AS) or heterologous (AJ or YM) parasites at 2, 8, or 12 weeks after vaccination. Rechallenge was performed at 12 weeks after parasite clearance. The outcome of infection was monitored by assessing parasitemia. Data for individual mice are shown. nd, not determined. †Animals that succumbed to infection.
Figure 6. Homologous immunity.
To assess for homologous protection against P. yoelii YM, different strains of mice including A/J, B6 and BALB/c mice were immunized with 103 (YM) prbc in CpG-ODN and challenged i.v. with homologous parasites at 2 weeks after vaccination. The outcome of infection was monitored by assessing parasitemia. In all data sets, results for individual mice are shown and are representative of 3 independent experiments performed. †Animals that succumbed to infection.
Induction of CD4 T cell memory.
Because a population of cross-reactive CD4 T cells was shown to persist up to 12 weeks after immunization (Figure 4B), we set out to determine whether these cells were required for long-term protection. Two approaches were undertaken. First, to ascertain an effector role for memory CD4 T cells, 12 weeks after immunization, CD4 T cells were depleted and mice given a lethal challenge. Similar to the results described when depletion was undertaken at 2 weeks (Figure 2B), removal of CD4 T cells at 12 weeks resulted in a severe course of infection and death (Figure 7A). Second, to exclude a helper role in recall responses, CD4 T cells were depleted as above, but mice were only challenged 21 days later. This allowed for naive but not memory T cells to replenish. Here, despite the presence of naive/helper CD4 T cells, all animals succumbed to severe infection (Figure 7A). Therefore, vaccine-primed memory CD4 T cells, but not unprimed CD4 T cells, were essential for long-term protection.
Figure 7. Induction of CD4 T cell memory.
(A) To assess the role of CD4 T cells in protracted protection, A/J mice immunized with 103 (AS) prbc in control ODN or CpG-ODN were depleted of CD4 T cells 12 weeks after immunization. Mice were challenged i.v. with 105 homologous (AS) prbc immediately after depletion (left panels, effector) or 21 days after depletion to allow for naive/helper CD4 T cells to replenish (right panels, helper). Parasitemias were monitored. Results are representative of 3 separate experiments and data for individual mice are shown. †Animals that succumbed to infection. (B) To assess for cytokine secretion in memory CD4 T cells, spleen cells from A/J mice immunized with 103 (AS) prbc in CpG-ODN were purified 12 weeks after immunization, incubated for 96 hours in the presence of homologous (AS) prbc, and cells stained for intracellular IFN-γ and IL-2. Numbers represent percentages of cytokine+ CD4 T cells. (C) To identify the specificity of CD4 T cell memory subsets, IFN-γ secretion and expression of lymph node homing receptor (CD62L) were assessed by harvesting spleen cells from A/J mice immunized with 103 (AS) prbc in CpG-ODN at 12 weeks after immunization. Cells were incubated for 96 hours in the presence of homologous (AS) prbc and stained. Numbers represent percentages of IFN-γ+ CD62LloCD4 T cells. (D) To determine the existence of memory populations, spleen cells from A/J mice immunized with 103 (AS) prbc in CpG-ODN were FACS-sorted into CM (CD44hiCD62Lhi) and EM (CD44hiCD62Llo) populations directly ex vivo. CM and EM cells were then incubated for 96 hours in the presence of homologous (AS) prbc and stained for intracellular cytokine (IFN-γ) and proliferation (BrDU). Numbers represent percentages of CD4 T cells in corresponding quadrants. Results are representative of 3 independent experiments performed.
Next, we assessed the profile of cytokine secretion of memory CD4 T cells, particularly IL-2 and IFN-γ, as these cytokines exert a profound influence on the maintenance of CD4 T cell memory (30). For this purpose, we estimated the frequency of CD4 T cells able to secrete IL-2, IFN-γ, or both cytokines in the memory phase (Figure 7B). Interestingly, we found that 5.9% ± 0.7% of CD4 T cells (approximately 30%–36% of the parasite-specific CD4 T cell response) were able to secrete both IL-2 and IFN-γ in response to parasite antigen. These data suggested that the low-dose vaccine elicited multifunctional CD4 T cells with potential for memory differentiation.
To formally assess this hypothesis, we analyzed the memory phenotype of CD4 T cells at 12 weeks after immunization. Here, we subdivided CD4 T cell memory cells into central (CM, CD62Lhi) or effector (EM, CD62Llo) subsets based on their expression of the lymph node homing receptor (CD62L) (31). To delineate parasite specificity, we assessed IFN-γ secretion upon in vitro restimulation (Figure 7C). By this method, we found that more than 95% of parasite-specific CD4 T cells were CD62Llo. The scarcity of the CD62Lhi population and the fact that CD62L downregulates upon antigenic stimulation prompted us to also purify individual subsets of CM (CD44hiCD62Lhi) and EM (CD44hiCD62Llo) CD4 T cells prior to antigenic stimulation (Figure 7D). By doing so, we confirmed the presence of both types of parasite-specific memory CD4 T cells in CpG-immunized mice. We found that parasite-specific CD62Lhi CD4 T cells (CM) decreased expression of CD62L (data not shown) and exhibited strong proliferative capacity (Figure 7D). In fact, a marked proportion of CD62Lhi CD4 T cells (CM) proliferated and acquired effector function as defined by BrDU incorporation and IFN-γ secretion (97% BrDU+ and 39% IFN-γ+; Figure 7D). In contrast, CD62Llo CD4 T cells (EM) exhibited less proliferative capacity yet produced considerable more amounts of IFN-γ regardless of their proliferative status (60% BrDU+ and 45% IFN-γ+; Figure 7D). In conclusion, the low-dose vaccine was able to induce both types of memory subsets where CD62Lhi CD4 T cells (CM) exhibited strong proliferative potential while CD62Llo CD4 T cells (EM) demonstrated rapid effector function on antigen encounter.
Discussion
The complexity of the malaria parasite coupled with the lack of evidence for robust infection-induced immunity has made malaria vaccine development especially difficult. Most successful vaccines work by mimicking natural immunity to elicit long-lived antibody responses (32). However, humoral immunity to malaria is incomplete and does not eradicate all parasites or provide protection against future challenge (4, 5). For blood-stage malaria, numerous approaches have been pursued, including the use of subunit antigens, prime-boost strategies, or combination of adjuvants to induce robust humoral responses. Here, we report on what we believe to be a novel T cell vaccine against blood-stage malaria that builds on previous observations that (a) IL-12 and ODN are effective adjuvants for immunization against blood-stage infection (33, 34) and (b) low doses of antigenic stimulus facilitate cellular immunity. We combined these strategies with a whole-parasite approach to demonstrate that administration of extremely low doses of killed blood-stage parasites in CpG-ODN is able to engender broad protection against lethal blood-stage challenge through the generation of cross-reactive T cells.
From a vaccine perspective, understanding the elicitation of such a vigorous Th1 response is essential to harness any T cell vaccine candidate into human trials. It is known, for instance, that vaccine-induced Th1 responses are controlled by the type of APC (35), the dose of antigen (36–38), or the cytokine environment induced during vaccination (39). To develop vigorous T cell responses against blood stage, a number of considerations were of paramount importance for us. First was the selection of a potent adjuvant such as CpG-ODN. While it is still under development, there have been extensive trials of ODN in humans in which it has been shown to activate immune cells to secrete cytokines conducive of Th1 differentiation (26). In the case of malaria, for instance, CpG-ODN has been successfully used as an adjuvant to immunize nonhuman primates and mice (40–42). Second was the identification of an optimal antigenic dose. This was based on evidence that low doses of live parasite facilitate cellular immunity (24, 25), while higher parasite burdens tend to result in apoptosis of Th1 effectors (27). Third was our desire to maintain a broad spectrum of target antigens while avoiding the limitations of live parasites. All of the above led us to combine CpG-ODN with low doses of whole killed parasite in a simple prime-boost regime aiming to afford strong cellular immunity against blood-stage infection.
Our results confirmed that CpG-ODN was crucial to providing vigorous polarization signals when the dose of antigen was limiting. In fact, the rapid activation of DCs, B cells, and macrophages to secrete IL-12, IL-10, and TNF-α and NK and T cells to secrete IFN-γ confirmed that the strong recognition of pathogen-associated molecular patterns was central to determining the type of immune response after vaccination. Clearly, the cytokine environment and the broad range of target antigens available facilitated the priming of broadly reactive CD4 T cells, which upon challenge, rapidly engaged to deliver macrophage activation signals (i.e., IFN-γ) to activate NO production for parasite clearance, reminiscent of the protective mechanisms of the bacillus Calmette-Guérin vaccine (43).
Because we aimed to achieve protective cellular responses, it was essential to ensure that the low-dose vaccine was efficacious at inducing memory CD4 T cells. In this case, the induction of sufficient but not excessive cytokine signals was desired for the generation of both effector and memory CD4 T cells. It is known, for example, that CD4 effector cells that secrete only IFN-γ have limited capacity to develop into memory cells, while effectors able to secrete IL-2 or IL-2 and IFN-γ are more likely to differentiate into memory cells (44). By combining a strong T cell adjuvant with low doses of killed parasite, we were able to induce a significant proportion of multifunctional parasite-specific CD4 T cells. More importantly, these cells persisted up to 12 weeks after immunization and correlated with protection. It remains to be shown, however, whether similar responses can be induced in humans.
A final consideration for our vaccine was the target population. Here, the development of a formulation able to induce rapid yet long-lived T cell responses is essential if we are to complement current vaccine efforts. This is principally so because in contrast to the induction of protective antibodies that can be inhibited by maternal antibodies, T cell responses can be rapidly elicited in young children (45). BCG immunization, for instance, can be given as single injection at birth to protect throughout childhood (46). While our data indicated that protective responses can be induced in young mice within 2 weeks of a single s.c. injection of low doses of parasite in CpG-ODN (Supplemental Figure 3), preliminary results suggest that subsequent parasite exposure (boost injections or low-grade infection) is required for protracted protection (data not shown). Strong protection is also achieved using other, more human-compatible routes, such as i.m. injections (Supplemental Figure 4). While experimentation is still underway, it is difficult to predict how such a vaccine would perform in a pediatric population. Studies in infants immunized against measles suggest that the rapid generation of cellular responses should provide solid protection against disease yet only limited protection against infection (47, 48).
In our model, the low-dose vaccine conferred partial protection, preventing severe disease and facilitating the generation of cross-reactive immunity. However, the magnitude of the cross-reactive response generated against heterologous parasites was not equivalent to that induced against the homologous challenge. While a mixed parasite vaccine would constitute a plausible alternative, our preliminary evidence suggests that a combination of multiple parasites may deliver an excessive antigenic load, thus altering the nature of the protection (A. Pinzon-Charry et al., unpublished observations). Definition of an effective low-dose mixed-parasite vaccine will require careful titration of parasite doses to maintain the fine balance between antigenic dose and spectrum of antigens.
On the other hand, our studies also suggest that vaccine-induced immunity can be boosted upon reexposure and cross-protection can improve after repeat challenge (Figures 5 and 6). Here, the vigorous and broadly reactive T cell responses induced through vaccination were able to reduce parasite burdens to protect from severe disease (Figure 1 and Supplemental Figure 1) and, in combination with antibody responses generated during low-grade infection (i.e., challenge), provided almost complete, long-term (~24 weeks) protection upon rechallenge (Figures 5 and 6). If similar responses can be induced in humans, a whole-parasite T cell vaccine would afford some rapid parasite clearance to prevent severe disease and facilitate subsequent immunity. However, as the infected-erythrocyte challenge does not directly translate to natural challenge, our results should be compared with other studies encompassing sporozoite challenge. In this regard, remarkably, a recent study has demonstrated that protection against a homologous malaria challenge in humans can be induced by the inoculation of small doses of intact sporozoites together with chloroquine chemoprophylaxis to limit blood-stage parasite growth. Protection against subsequent challenge was associated with the induction of blood-stage parasite-specific pluripotent effector memory T cells (49). Other studies have shown that low doses of whole irradiated or genetically modified sporozoites can induce vigorous T cell responses in humans and mice (21, 23, 50). Altogether, these findings will likely add momentum to the general issue of whole-parasite vaccines advancing toward clinical testing (21, 23).
In conclusion, this study demonstrates that a simple vaccine based on low doses of whole killed parasite in CpG-ODN can elicit the robust T cellular response usually associated with live (attenuated) vaccines while, at the same time, retaining some of the safety features of a killed (subunit) vaccine. While the hazards involved in the administration of a blood-based product to thousands of otherwise healthy individuals must be addressed, the risk of alloimmunization, blood-borne infections, and parasite purification as well as parasite nonviability are currently being investigated. We envisage that the data provided here will shed some light into the elicitation of cellular responses through whole-organism vaccination and aid in advancing the field of T cell vaccines against other organisms.
Methods
Mice.
Female A/J [H-2a], BALB/c [H-2d], B6 [H-2b], and μMT [H-2b] mice were obtained from the Animal Resources Center (Willetton, Western Australia). μMT mice were bred in-house. All animals were maintained in a specific pathogen–free environment and tested negative for pathogens in routine screening. All animal studies were approved by the Queensland Institute of Medical Research Animal Ethics Committee.
Parasites.
The rodent malaria parasites used included P. c. chabaudi AS, P. c. chabaudi AJ, and P. yoelii YM. Parasites were maintained by i.p. passaging of 105 prbc into naive recipient mice. Challenge infections were performed by i.v. injection of 105 prbc (AS or AJ) or 104 prbc (YM). Parasitemia was monitored by performing Giemsa-stained thin tail blood smears. Disease severity was assessed by performing clinical scores monitoring weight loss (from 0–2), posture (from 0–2), activity (from 0–2), fur texture (from 0–2), and percentage of parasitemia (from 0–2) for a maximal score of 10. Refer to Supplemental Table 1.
ODN.
Phosphorothioate-modified ODN sequence 1826 containing 2 CpG motifs (underlined: TCCATGACGTTCCTGACGTT) and control sequence 1982 (TCCAGGACTTCTCTCAGGTT) were purchased from Sigma-Aldrich.
Immunization.
Whole-parasite vaccines were prepared using blood from A/J mice infected with P. c. chabaudi AS. When the parasitemia of infected mice was 30%–40%, blood collected into heparinized tubes was washed (1,000 g for 10 minutes) and resuspended in sterile PBS. Total rbc counts and parasitemias were determined and parasites adjusted to desired concentrations. Subsequently, parasites were killed by repetitive cycles of freeze-thawing, and vaccine formulation was prepared as described (34). Briefly, an amount of malaria antigen equivalent to 102 to 108 prbc was admixed with 50 μg of CpG-ODN or control ODN and aluminium hydroxide (Pierce) and mixed thoroughly to a final volume of 100 μl. Mice were immunized s.c. on the abdomen. Three weeks later, mice were boosted (i.p.) with the same amount of antigen in normal saline and boosted (i.p.) again 2 weeks later with the same amount of antigen. Animals were challenged 2, 8, or 12 weeks after immunization.
Flow cytometry and serum cytokines.
FACS was performed on a FACSCalibur flow cytometer (BD Biosciences) and analyzed using Summit V4.3 (DakoCytomation) or CellQuest Pro (BD Biosciences) software. Serum cytokines were measured using the Cytometric Bead Array (CBA) for mouse inflammation or Th1/Th2 cytokine kits (BD Biosciences). Acquisition was performed on a FACScan cytometer equipped with CellQuest Pro and CBA Software (BD Biosciences) according to manufacturer’s instructions.
Serum antibody ELISA.
Crude parasite antigen from AS, AJ, or YM parasites was prepared by collecting blood, washing in PBS, and incubating in 0.01% (w/v) in saponin (Sigma-Aldrich) at 37°C for 20 minutes. The pellet was washed in PBS, resuspended in 1.5 ml of PBS, and sonicated. MaxiSorp Nunc immunoplates (Nalge Nunc) were coated with parasite antigen overnight at 4°C with 5–10 μg of parasite antigen/ml in bicarbonate coating buffer (pH 9.6) as described (25). Hyperimmune sera was generated by infecting naive mice 3 times as outlined above and drug curing with 0.2 mg of atovaquone and 0.08 mg of proguanil in 10 μl of water by oral gavage daily for 4 days starting on day 7 of infection.
Passive antibody transfers.
For passive transfer study, naive A/J mice were injected on days –1, 0, and 1 with 200 ml i.p. of serum collected from immune (103 P. c. chabaudi AS prbc in CpG-ODN) or control (103 P. c. chabaudi AS prbc in control ODN) donors. Recipients were challenged with 105 P. c. chabaudi AS prbc on day 0 and parasitemia or parasite-specific IgG against P. c. chabaudi AS antigen assessed by ELISA
Lymphocyte proliferation and intracellular cytokine staining.
Spleen cells were diluted to 5 × 106 cells/ml in culture medium (Eagle’s Minimum Essential Medium; Trace Scientific) with 5% heat-inactivated FCS, 50 μg/ml streptomycin (CSL), 100 μg penicillin (CSL), and 55 μM 2-mercaptoethanol (Gibco BRL; Invitrogen) and aliquotted into 96-well plates (5 × 105/well). Triplicate wells were cultured with concanavalin A (10 μg/ml), fresh prbc (5 × 105/well), or normal rbc adjusted to give equivalent concentrations, or medium alone. Cells were cultured for 96 hours. For proliferation, cells were pulsed with 0.25 μCi of 3[H]-thymidine (NEN)/well during the last 24 hours. The cells were harvested onto fiberglass mats and radioactivity measured. Alternatively, cells were stained with CFSE prior to culture using a Vybrant CFDA SE Cell Tracer Kit (Molecular Probes) following the manufacturer’s instructions. For intracellular staining, cells were incubated with PMA (0.025 μg/ml; Sigma-Aldrich), ionomycin (1 μg/ml; Sigma-Aldrich), and brefeldin A (10 μg/ml; Sigma-Aldrich) for the final 4–6 hours as described (51), stained with CD4-APC or -FITC (BD Pharmingen), fixed with 1% paraformaldehyde, and stained with PE- or APC-conjugated IFN-γ and/or IL-2 (BD Biosciences — Pharmingen). Proliferation and cytokine secretion of FACS-purified (>95% purity) CD44hiCD62Lhi and CD44hiCD62Llo CD4 T cells was performed using a BrdU Flow Kit (BD Biosciences — Pharmingen) following the manufacturer’s instructions.
Depletions.
To deplete IFN-γ, mice received 1 mg i.p. of anti–IFN-γ antibody (clone XMG-6 provided by H, Xu, Queensland Institute of Medical Research) on days –1, 0, and 1 relative to day of challenge. IL-12 depletion was performed by injecting 1 mg i.p. of the mAb (clone C17.8) on days –1 and 0. TNF-α blockade was performed by injecting 100 μg i.p. of Etanercept (Wyeth) on days –1, 0, 2, 4, 6, and 8. IL-10 depletion was performed by injecting 1 mg i.p. (clone 1B1.3a) on days –1, 0, and 1. Depletion of pDC was performed by administering 500 μg of anti-PDCA i.p. (Miltenyi Biotec) on days –1 and 1 relative to day of challenge. To deplete NK cells, mice received a single dose of 675 μg i.v. (anti-asialo GM1; Wako Chemicals) on days –2, 0, and 4. For CD8 or CD4 depletion, mice were injected with 1 mg i.p. (clone 58.5.8 or clone GK1.5, respectively) on days –4, –3, –2, –1, and 0. NO depletion was performed by injecting amino guanidine (AG; Sigma-Aldrich) at 50 mg/kg of body weight in 0.5 ml of saline by gastric lavage twice daily on days –1, 0, 1, 2, 4, 6, and 8. In all studies, control mice received parallel doses of rat Ig control (Sigma-Aldrich) from day –4 to day 10 in the same manner as the test antibodies.
Statistics.
All comparisons among experimental groups to establish significance were determined by 2-tailed Student’s t test, assuming unequal variances, or 1-way ANOVA followed by Bonferroni’s comparison test. All analyses were performed using the statistical program GraphPad Prism. Results were considered to be statistically significant at P < 0.05.
Supplementary Material
Acknowledgments
The authors would like to thank Huji Xu, Michelle Wykes, and Fiona Amante for kind provision of hybridoma lines and reviewing the manuscript. We are also very grateful to Alejandro Lopez for detailed review of the manuscript as well as to Denise Doolan, Tonia Woodberry, and Alex Loukas for critical comments. This project was funded by the National Health and Medical Research Council (NHMRC) and the Australian Rotary Health Research Fund (ARHRF). A. Pinzon-Charry is the recipient of a NHMRC Peter Doherty Postdoctoral Fellowship (ID:443041) and a GlaxoSmithKline Australia Postgraduate Award.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2010;120(8):2967–2978. doi:10.1172/JCI39222.
References
- 1.Good MF. Vaccine-induced immunity to malaria parasites and the need for novel strategies. Trends Parasitol. 2005;21(1):29–34. doi: 10.1016/j.pt.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Fox JP. Modes of action of poliovirus vaccines and relation to resulting immunity. Rev Infect Dis. 1984;6 suppl 2:S352–S355. doi: 10.1093/clinids/6.supplement_2.s352. [DOI] [PubMed] [Google Scholar]
- 3.Mielcarek N, et al. Live attenuated B. pertussis as a single-dose nasal vaccine against whooping cough. PLoS Pathog. 2006;2(7):e65. doi: 10.1371/journal.ppat.0020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGregor IA. Mechanisms of acquired immunity and epidemiological patterns of antibody responses in malaria in man. Bull World Health Organ. 1974;50(3–4):259–266. [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffman SL, et al. Naturally acquired antibodies to sporozoites do not prevent malaria: vaccine development implications. Science. 1987;237(4815):639–642. doi: 10.1126/science.3299709. [DOI] [PubMed] [Google Scholar]
- 6.Alonso PL, et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366(9502):2012–2018. doi: 10.1016/S0140-6736(05)67669-6. [DOI] [PubMed] [Google Scholar]
- 7.Alonso PL, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet. 2004;364(9443):1411–1420. doi: 10.1016/S0140-6736(04)17223-1. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S, McGregor IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 9.Coppel RL, et al. Immune Sera Recognize on Erythrocytes a Plasmodium-Falciparum Antigen Composed of Repeated Amino-Acid-Sequences. Nature. 1984;310(5980):789–792. doi: 10.1038/310789a0. [DOI] [PubMed] [Google Scholar]
- 10.Holder AA, Freeman RR. Biosynthesis and processing of a plasmodium-falciparum schizont antigen recognized by immune serum and a monoclonal-antibody. J Exp Med. 1982;156(5):1528–1538. doi: 10.1084/jem.156.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genton B, Reed ZH. Asexual blood-stage malaria vaccine development: facing the challenges. Curr Opin Infect Dis. 2007;20(5):467–475. doi: 10.1097/QCO.0b013e3282dd7a29. [DOI] [PubMed] [Google Scholar]
- 12.Cortes A, Mellombo M, Mueller I, Benet A, Reeder JC, Anders RF. Geographical structure of diversity and differences between symptomatic and asymptomatic infections for Plasmodium falciparum vaccine candidate AMA1. Infect Immun. 2003;71(3):1416–1426. doi: 10.1128/IAI.71.3.1416-1426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy MC, et al. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infect Immun. 2002;70(12):6948–6960. doi: 10.1128/IAI.70.12.6948-6960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Good MF, Branigan J, Smith G, Houghten RA. Peptide immunization can elicit malaria protein-specific memory helper but not proliferative T cells. Pept Res. 1990;3(3):110–115. [PubMed] [Google Scholar]
- 15.Amante FH, Crewther PE, Anders RF, Good MF. A cryptic T cell epitope on the apical membrane antigen 1 of Plasmodium chabaudi adami can prime for an anamnestic antibody response: implications for malaria vaccine design. J Immunol. 1997;159(11):5535–5544. [PubMed] [Google Scholar]
- 16.Sinigaglia F, et al. A malaria T-cell epitope recognized in association with most mouse and human MHC class II molecules. Nature. 1988;336(6201):778–780. doi: 10.1038/336778a0. [DOI] [PubMed] [Google Scholar]
- 17.Makobongo MO, et al. The purine salvage enzyme hypoxanthine guanine xanthine phosphoribosyl transferase is a major target antigen for cell-mediated immunity to malaria. Proc Natl Acad Sci U S A. 2003;100(5):2628–2633. doi: 10.1073/pnas.0337629100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinigaglia F, et al. Epitopes recognized by human T lymphocytes on malaria circumsporozoite protein. Eur J Immunol. 1988;18(4):633–636. doi: 10.1002/eji.1830180422. [DOI] [PubMed] [Google Scholar]
- 19.Fern J, Good MF. Promiscuous malaria peptide epitope stimulates CD45Ra T cells from peripheral blood of nonexposed donors. J Immunol. 1992;148(3):907–913. [PubMed] [Google Scholar]
- 20.Grun JL, Weidanz WP. Antibody-independent immunity to reinfection malaria in B-cell-deficient mice. Infect Immun. 1983;41(3):1197–1204. doi: 10.1128/iai.41.3.1197-1204.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luke TC, Hoffman SL. Rationale and plans for developing a non-replicating, metabolically active, radiation-attenuated Plasmodium falciparum sporozoite vaccine. J Exp Biol. 2003;206(pt 21):3803–3808. doi: 10.1242/jeb.00644. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman SL, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185(8):1155–1164. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 23.Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433(7022):164–167. doi: 10.1038/nature03188. [DOI] [PubMed] [Google Scholar]
- 24.Pombo DJ, et al. Immunity to malaria after administration of ultra-low doses of red cells infected with Plasmodium falciparum. Lancet. 2002;360(9333):610–617. doi: 10.1016/S0140-6736(02)09784-2. [DOI] [PubMed] [Google Scholar]
- 25.Elliott SR, Kuns RD, Good MF. Heterologous immunity in the absence of variant-specific antibodies after exposure to subpatent infection with blood-stage malaria. Infect Immun. 2005;73(4):2478–2485. doi: 10.1128/IAI.73.4.2478-2485.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–760. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 27.Xu H, et al. The mechanism and significance of deletion of parasite-specific CD4(+) T cells in malaria infection. J Exp Med. 2002;195(7):881–892. doi: 10.1084/jem.20011174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevenson MM, Tam MF, Wolf SF, Sher A. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-gamma and TNF-alpha and occurs via a nitric oxide-dependent mechanism. J Immunol. 1995;155(5):2545–2556. [PubMed] [Google Scholar]
- 29.Phillips JA, Romball CG, Hobbs MV, Ernst DN, Shultz L, Weigle WO. CD4+ T cell activation and tolerance induction in B cell knockout mice. J Exp Med. 1996;183(4):1339–1344. doi: 10.1084/jem.183.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Younes SA, et al. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J Exp Med. 2003;198(12):1909–1922. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 32.Robinson HL, Amara RR. T cell vaccines for microbial infections. Nat Med. 2005;11(4 suppl):S25–S32. doi: 10.1038/nm1212. [DOI] [PubMed] [Google Scholar]
- 33.Hirunpetcharat C, et al. CpG oligodeoxynucleotide enhances immunity against blood-stage malaria infection in mice parenterally immunized with a yeast-expressed 19 kDa carboxyl-terminal fragment of Plasmodium yoelii merozoite surface protein-1 (MSP1(19)) formulated in oil-based Montanides. Vaccine. 2003;21(21–22):2923–2932. doi: 10.1016/S0264-410X(03)00132-4. [DOI] [PubMed] [Google Scholar]
- 34.Su Z, Tam MF, Jankovic D, Stevenson MM. Vaccination with novel immunostimulatory adjuvants against blood-stage malaria in mice. Infect Immun. 2003;71(9):5178–5187. doi: 10.1128/IAI.71.9.5178-5187.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 36.Power CA, Wei G, Bretscher PA. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by the intravenous, subcutaneous, or intradermal route. Infect Immun. 1998;66(12):5743–5750. doi: 10.1128/iai.66.12.5743-5750.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8(1):89–95. doi: 10.1016/S1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 38.Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202(5):697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8(3):275–283. doi: 10.1016/S1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 40.Jones TR, et al. Protection of Aotus monkeys by Plasmodium falciparum EBA-175 region II DNA prime-protein boost immunization regimen. . J Infect Dis. 2001;183(2):303–312. doi: 10.1086/317933. [DOI] [PubMed] [Google Scholar]
- 41.Jones TR, et al. Synthetic oligodeoxynucleotides containing CpG motifs enhance immunogenicity of a peptide malaria vaccine in Aotus monkeys. Vaccine. 1999;17(23–24):3065–3071. doi: 10.1016/S0264-410X(99)00145-0. [DOI] [PubMed] [Google Scholar]
- 42.Kumar S, et al. CpG oligodeoxynucleotide and Montanide ISA 51 adjuvant combination enhanced the protective efficacy of a subunit malaria vaccine. Infect Immun. 2004;72(2):949–957. doi: 10.1128/IAI.72.2.949-957.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baumann S, Nasser Eddine A, Kaufmann SH. Progress in tuberculosis vaccine development. Curr Opin Immunol. 2006;18(4):438–448. doi: 10.1016/j.coi.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 44.Wu CY, et al. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat Immunol. 2002;3(9):852–858. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- 45.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19(25–26):3331–3346. doi: 10.1016/S0264-410X(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 46.Soares AP, et al. Bacillus calmette-guerin vaccination of human newborns induces T cells with complex cytokine and phenotypic profiles. J Immunol. 2008;180(5):3569–3577. doi: 10.4049/jimmunol.180.5.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gans HA, et al. IL-12, IFN-gamma, and T cell proliferation to measles in immunized infants. J Immunol. 1999;162(9):5569–5575. [PubMed] [Google Scholar]
- 48.Giuliano M, Mastrantonio P, Giammanco A, Piscitelli A, Salmaso S, Wassilak SG. Antibody responses and persistence in the two years after immunization with two acellular vaccines and one whole-cell vaccine against pertussis. J Pediatr. 1998;132(6):983–988. doi: 10.1016/s0022-3476(98)70395-6. [DOI] [PubMed] [Google Scholar]
- 49.Roestenberg M, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361(5):468–477. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 50.Chakravarty S, Cockburn IA, Kuk S, Overstreet MG, Sacci JB, Zavala F. CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat Med. 2007;13(9):1035–1041. doi: 10.1038/nm1628. [DOI] [PubMed] [Google Scholar]
- 51.Zaph C, Scott P. Th1 cell-mediated resistance to cutaneous infection with Leishmania major is independent of P- and E-selectins. J Immunol. 2003;171(9):4726–4732. doi: 10.4049/jimmunol.171.9.4726. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.