Abstract
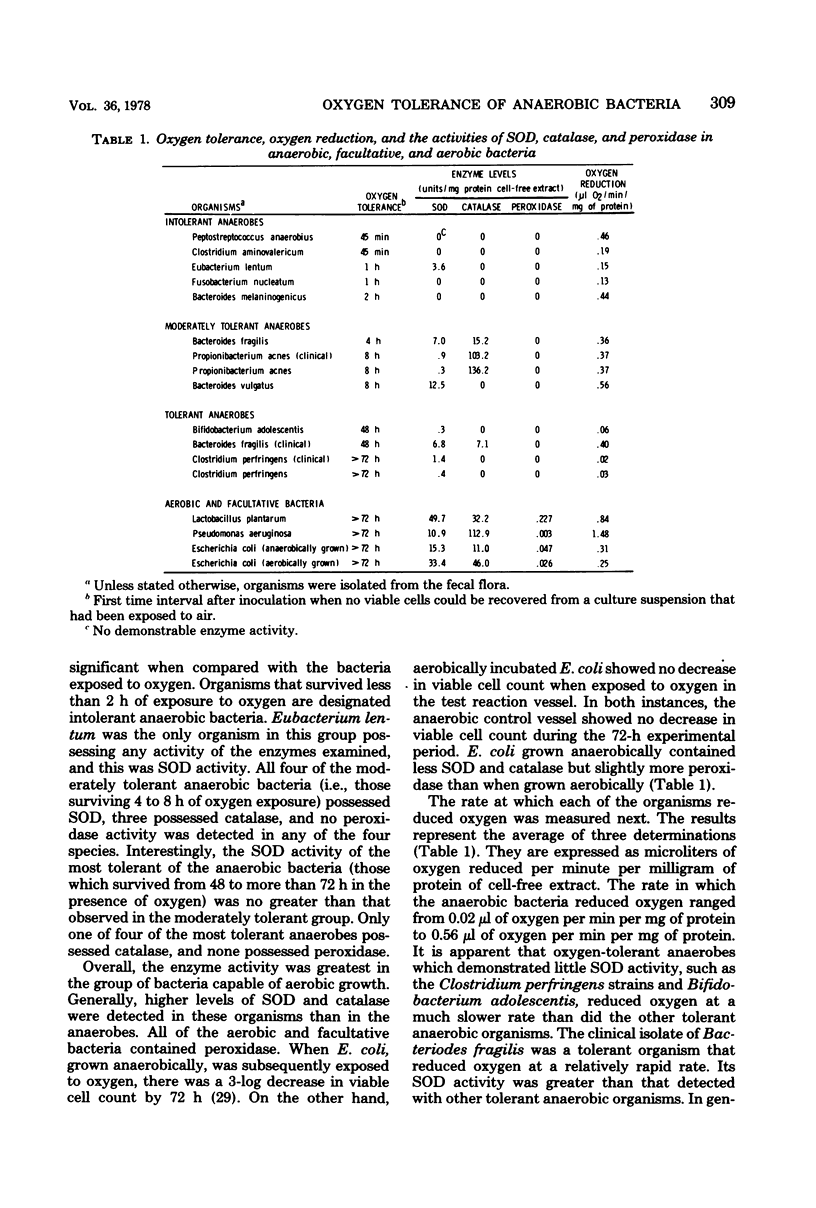
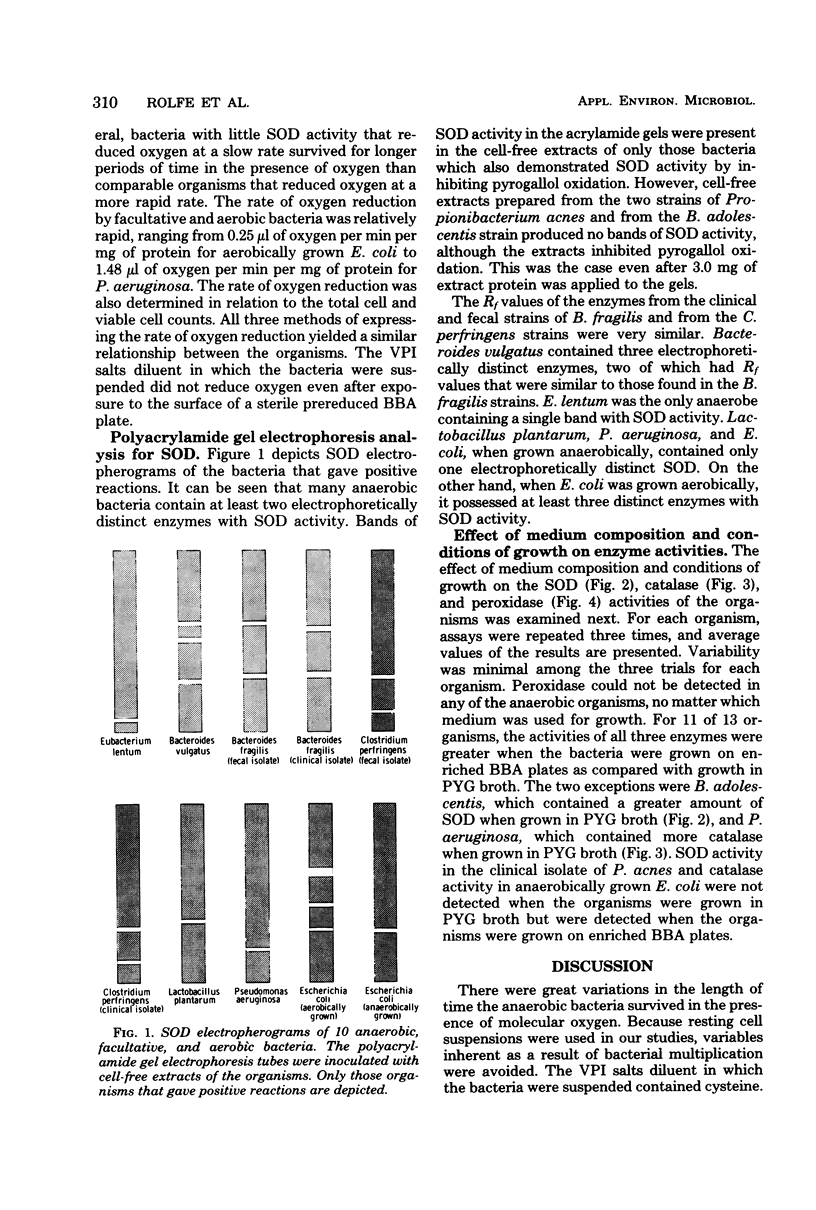
The effect of atmospheric oxygen on the viability of 13 strains of anaerobic bacteria, two strains of facultative bacteria, and one aerobic organism was examined. There were great variations in oxygen tolerance among the bacteria. All facultative bacteria survived more than 72 h of exposure to atmospheric oxygen. The survival time for anaerobes ranged from less than 45 min for Peptostreptococcus anaerobius to more than 72 h for two Clostridium perfringens strains. An effort was made to relate the degree of oxygen tolerance to the activities of superoxide dismutase, catalase, and peroxidases in cell-free extracts of the bacteria. All facultative bacteria and a number of anaerobic bacteria possessed superoxide dismutase. There was a correlation between superoxide dismutase activity and oxygen tolerance, but there were notable exceptions. Polyacrylamide gel electropherograms stained for superoxide dismutase indicated that many of the anaerobic bacteria contained at least two electrophoretically distinct enzymes with superoxide dismutase activity. All facultative bacteria contained peroxidase, whereas none of the anaerobic bacteria possessed measurable amounts of this enzyme. Catalase activity was variable among the bacteria and showed no relationship to oxygen tolerance. The ability of the bacteria to reduce oxygen was also examined and related to enzyme content and oxygen tolerance. In general, organisms that survived for relatively long periods of time in the presence of oxygen but demonstrated little superoxide dismutase activity reduced little oxygen. The effects of medium composition and conditions of growth were examined for their influence on the level of the three enzymes. Bacteria grown on the surface of an enriched blood agar medium generally had more enzyme activity than bacteria grown in a liquid medium. The data indicate that superoxide dismutase activity and oxygen reduction rates are important determinants related to the tolerance of anaerobic bacteria to oxygen.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arank A., Syed S. A., Kenney E. B., Freter R. Isolation of anaerobic bacteria from human gingiva and mouse cecum by means of a simplified glove box procedure. Appl Microbiol. 1969 Apr;17(4):568–576. doi: 10.1128/am.17.4.568-576.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Wrethén J., Beckman G. Superoxide dismutase in Bacteroides fragilis and related Bacteroides species. J Clin Microbiol. 1977 Sep;6(3):280–284. doi: 10.1128/jcm.6.3.280-284.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Douglas F., Hambleton R., Rigby G. J. An investigation of the oxidation-reduction potential and of the effect of oxygen on the germination and outgrowth of Clostridium butyricum spores, using platinum electrodes. J Appl Bacteriol. 1973 Dec;36(4):625–633. doi: 10.1111/j.1365-2672.1973.tb04148.x. [DOI] [PubMed] [Google Scholar]
- Fredette V., Planté C., Roy A. Numerical data concerning the sensitivity of anaerobic bacteria to oxygen. J Bacteriol. 1967 Dec;94(6):2012–2017. doi: 10.1128/jb.94.6.2012-2017.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide and evolution. Horiz Biochem Biophys. 1974;1:1–37. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35–97. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen metabolism in Lactobacillus plantarum. J Bacteriol. 1974 Jan;117(1):166–169. doi: 10.1128/jb.117.1.166-169.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Kowalski J. B., Holdeman L. V. Production and some properties of catalase and superoxide dismutase from the anaerobe Bacteroides distasonis. J Bacteriol. 1977 Mar;129(3):1298–1302. doi: 10.1128/jb.129.3.1298-1302.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Veltri B. J., Wagner D. L., Wilkins T. D. Carbohydrate repression of catalase synthesis in Bacteroides fragilis. J Bacteriol. 1977 Jan;129(1):534–535. doi: 10.1128/jb.129.1.534-535.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Yost F. J., Jr, Fridovich I. Superoxide dismutases of Escherichia coli: intracellular localization and functions. J Bacteriol. 1973 Sep;115(3):987–991. doi: 10.1128/jb.115.3.987-991.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Superoxide dismutase: a contaminant of bovine catalase. Biochem J. 1973 Oct;135(2):379–381. doi: 10.1042/bj1350379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J., Morris J. G. Superoxide dismutase in some obligately anaerobic bacteria. FEBS Lett. 1975 Feb 15;50(3):315–318. doi: 10.1016/0014-5793(75)80518-7. [DOI] [PubMed] [Google Scholar]
- Holdeman L. V., Moore W. E. Identification of anaerobes in the clinical laboratory. Am J Med Technol. 1975 Nov;41(11):411–416. [PubMed] [Google Scholar]
- Knaysi G., Dutky S. R. The Growth of a Butanol Clostridium in Relation to the Oxidation-Reduction Potential and Oxygen Content of the Medium. J Bacteriol. 1936 Feb;31(2):137–149. doi: 10.1128/jb.31.2.137-149.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loesche W. J. Oxygen sensitivity of various anaerobic bacteria. Appl Microbiol. 1969 Nov;18(5):723–727. doi: 10.1128/am.18.5.723-727.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden J., Hall D. O. Superoxide dismutase in photosynthetic organisms provides an evolutionary hypothesis. Nature. 1975 Oct 23;257(5528):670–672. doi: 10.1038/257670a0. [DOI] [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974 Sep 16;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa Hassan H., Fridovich I. Regulation of superoxide dismutase synthesis in Escherichia coli: glucose effect. J Bacteriol. 1977 Nov;132(2):505–510. doi: 10.1128/jb.132.2.505-510.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. W., Morris J. G. Oxygen and the growth and metabolism of Clostridium acetobutylicum. J Gen Microbiol. 1971 Nov;68(3):307–318. doi: 10.1099/00221287-68-3-307. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Johnston J., Mayhew J. W., Gorbach S. L. Effect of dissolved oxygen and Eh and Bacteroides fragilis during continuous culture. Appl Environ Microbiol. 1976 Feb;31(2):168–172. doi: 10.1128/aem.31.2.168-172.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe R. D., Hentges D. J., Barrett J. T., Campbell B. J. Oxygen tolerance of human intestinal anaerobes. Am J Clin Nutr. 1977 Nov;30(11):1762–1769. doi: 10.1093/ajcn/30.11.1762. [DOI] [PubMed] [Google Scholar]
- Tally F. P., Goldin B. R., Jacobus N. V., Gorbach S. L. Superoxide dismutase in anaerobic bacteria of clinical significance. Infect Immun. 1977 Apr;16(1):20–25. doi: 10.1128/iai.16.1.20-25.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tally F. P., Stewart P. R., Sutter V. L., Rosenblatt J. E. Oxygen tolerance of fresh clinical anaerobic bacteria. J Clin Microbiol. 1975 Feb;1(2):161–164. doi: 10.1128/jcm.1.2.161-164.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden W. C., Hentges D. J. Differential effects of oxygen and oxidation-reduction potential on the multiplication of three species of anaerobic intestinal bacteria. Appl Microbiol. 1975 Nov;30(5):781–785. doi: 10.1128/am.30.5.781-785.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshpe-Purer Y., Henis Y. Factors affecting catalase level and sensitivity to hydrogen peroxide in Escherichia coli. Appl Environ Microbiol. 1976 Oct;32(4):465–469. doi: 10.1128/aem.32.4.465-469.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousten A. A., Johnson J. L., Salin M. Oxygen metabolism of catalase-negative and catalase-positive strains of Lactobacillus plantarum. J Bacteriol. 1975 Jul;123(1):242–247. doi: 10.1128/jb.123.1.242-247.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



