Abstract
Targeted cancer therapeutics can be effective when patients are preselected to maximize the chance of response. Increasingly, molecular markers such as oncogenic DNA mutations are being exploited to help guide patient preselection. These DNA lesions can predict for either a positive or negative response to a given drug. Finding such predictive biomarkers is an ongoing challenge. New work by Di Nicolantonio and colleagues in this issue of the JCI demonstrates that PI3K catalytic α subunit (PIK3CA) mutations can sensitize cancer cells to the mammalian target of rapamycin (mTOR) inhibitor everolimus. In addition, they show that the concurrent presence of PIK3CA mutations and mutations in either KRAS or BRAF predict for resistance to this drug. These data suggest that mTOR inhibitors currently in use will be ineffective against cancers that have a mutation in either KRAS or BRAF despite having PI3K/AKT/mTOR pathway activation.
In the past few decades, developers of new anticancer therapies have moved away from cytotoxic drugs that simply target the proliferative hallmark of all cancer cells. Currently, targeted therapies dominate cancer drug development with the aim of blocking the growth and spread of cancer by interfering with specific molecules involved in the progression of a given tumor. One of the most successful targeted anticancer therapies developed is the kinase inhibitor imatinib, which targets the product of the BCR-ABL oncogene that drives disease in all patients with chronic myeloid leukemia (CML) (1). However, for most targeted therapies, only a subset of the patients predicted to respond do so. For example, EGFR-directed therapies were thought to inhibit the growth of non–small-cell lung cancers with EGFR overexpression, but only those cancers with certain activating EGFR mutations respond to these small molecule inhibitors (2, 3). It has therefore become critically important to develop predictive biomarkers for patients who are likely to respond to a given therapy and, equally important, for those who will not. As an example, testing for KRAS mutations has become mandatory for colorectal cancer patients receiving EGFR-directed therapies because the presence of KRAS mutations predicts for resistance to this class of drugs (4). In this issue of the JCI, Di Nicolantonio and colleagues have now uncovered mutations that seem to predict for response to the anticancer drug everolimus, which targets mammalian target of rapamycin (mTOR) (5).
The PI3K/AKT/mTOR pathway and mTOR inhibitors
The PI3K/AKT/mTOR signaling pathway mediates key cellular processes, including cell growth, proliferation, and survival (6) (Figure 1). Activation of the downstream component mTOR can lead to features of transformation through its known role in regulating factors involved with the initiation of protein synthesis of critical growth-promoting genes (7). Furthermore, activating mutations that contribute to carcinogenesis are commonly found in genes encoding proteins within this pathway (8). In particular, oncogenic mutations of PI3K catalytic α subunit (PIK3CA) are among the most frequently reported genetic aberrations in human cancers (9). These mutations activate the PI3K/AKT/mTOR pathway and contribute to carcinogenesis, providing the rationale for inhibiting this pathway for cancer therapy. The development of agents to target components of this pathway has resulted in a class of drugs that specifically target mTOR. However, despite the fact that early-phase clinical trials indicate that mTOR inhibitors may have activity in a number of cancers, only a fraction of patients receiving these drugs derived substantial clinical benefit (10). Developing biomarkers able to stratify patients into those likely to respond and those unlikely to respond is now critical if mTOR inhibitors are to become widely used for the treatment of cancer.
Figure 1. The PI3K/AKT/mTOR pathway.
Receptor tyrosine kinases bind with ligand and initiate the signaling pathway via intermediate molecules (IRS). PI3K becomes activated, which results in phosphorylation of phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 3,4,5-trisphosphate (PIP3), a process that is reversed by PTEN. At the cell membrane, proteins with pleckstrin homology domains then become phosphorylated via PIP3 (phosphoinositide-dependent protein kinase–1 [PDK1] and AKT). PDK1 can also phosphorylate critical residues on AKT. The tumor suppressor complex of TSC1/TSC2 normally inhibits mTOR activation via Ras homolog enriched in brain (Rheb). Activated AKT prevents this inhibition, leading to activation of the mTOR/Raptor complex known as mTOR complex 1 (mTORC1). This complex can be inhibited by rapamycin and its analogs, including everolimus. Ultimately, mTORC1 leads to the activation of downstream proteins involved in the initiation of protein synthesis, resulting in cellular growth. Receptor tyrosine kinase activation also initiates MAPK pathway signaling, which leads to cell cycle progression and proliferation. MAPK pathway activation can also augment PI3K signaling. MEK, MAPK/ERK kinase.
Cancer mutations and the response to the mTOR inhibitor everolimus
In this issue of the JCI, Di Nicolantonio and colleagues used a panel of isogenic human cell lines to characterize the response to the mTOR inhibitor everolimus, which is a rapamycin analog originally known as RAD001 (5). This group and others have previously demonstrated that somatic cell gene targeting in non-tumorigenic human cell lines can accurately recapitulate oncogenic mutations and their response to drug therapies (11, 12). By using paired cell lines that are isogenic, or as close to isogenic as possible, drug sensitivity versus resistance can accurately be assessed, and any phenotypic changes are the direct result of the introduced mutations (Figure 2). In the current study by Di Nicolantonio and colleagues (5), isogenic human cell lines were generated containing hotspot mutations in PIK3CA (H1047R or E545K) and were found to be selectively sensitive to rapamycin and its analog everolimus. This was true in both spontaneously immortalized non-tumorigenic human breast epithelial cells (MCF10A) and human breast epithelial cells immortalized via telomerase overexpression (hTERT-HME1).
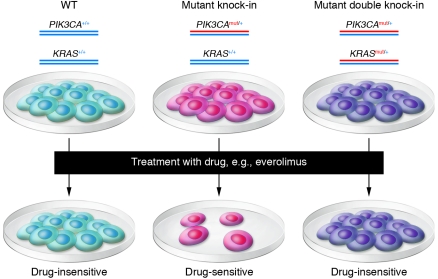
Figure 2. Predicting responses using genetically engineered isogenic human cell lines.
Human mammary epithelial cells (WT) are subjected to gene targeting to create isogenic derivatives that contain a single PIK3CA oncogenic mutation (Mutant knock-in) or the same PIK3CA mutation along with a KRAS oncogenic mutation (Mutant double knock-in). Cells are then subjected to drugs in parallel, and resistance versus sensitivity can be assessed. Because the cell lines are isogenic, this allows for a clean interpretation of whether drug sensitivity is mediated by the presence or absence of a given mutation or set of mutations.
Di Nicolantonio et al. then sought to assess whether the increased sensitivity to everolimus in the non-transformed PIK3CA isogenic human cells could be recapitulated in transformed cancer cells carrying PIK3CA mutations along with multiple other genetic alterations (5). This assessment included screening for everolimus sensitivity using a panel of cancer cell lines with known genetic alterations in PIK3CA or phosphatase and tensin homolog (PTEN), a tumor suppressor gene that encodes for an inhibitor of the PI3K/AKT/mTOR signaling pathway. It was in this key experiment that Di Nicolantonio and colleagues discovered that the response to everolimus could be divided into two groups: everolimus-sensitive cancer cells that contained mutations in the PI3K pathway and everolimus-resistant cancer cells that contained mutations in both the PI3K and the MAPK pathways, the latter being characterized as cells with either a KRAS or BRAF mutation.
To test the hypothesis that the presence of a KRAS mutation results in resistance to everolimus, the authors performed additional cell proliferation experiments using cell lines derived from the HCT116 colorectal cancer cell line, which naturally contains a heterozygous PIK3CA mutation as well as a heterozygous KRAS mutation. The team employed HCT116 derivatives that had been previously modified via gene targeting such that the mutant or wild-type KRAS allele had been deleted (13). The cells that contained only a single wild-type copy of KRAS were sensitive to everolimus, while derivatives of HCT116 containing mutant KRAS were resistant (5). To obtain further evidence that the KRAS mutant was responsible for everolimus resistance, the authors performed rescue experiments on the KRAS wild-type–only HCT116 derivative cell line. By exogenously introducing a mutant copy of KRAS and then treating the cells with everolimus, the authors found that they were able to restore the resistance phenotype.
Di Nicolantonio et al. provide further evidence of the contribution of mutant KRAS in mediating everolimus resistance by assessing whether this finding was relevant in an in vivo setting (5). The authors evaluated this by recapitulating their in vitro data using the above HCT116 system grown as xenografts in immunocompromised mice, as well as a second cell line, ME-180, which is an endometrial cancer cell line that has a PIK3CA mutation but is wild type for KRAS and BRAF. As before, the group generated a derivative of this cell line carrying a transgene overexpressing a mutant KRAS allele. In both mouse xenograft models, the authors found that the presence of mutant PIK3CA as well as a mutant KRAS resulted in abrogation of everolimus’s antiproliferative effects.
Importantly, the authors provide data to indicate a potential mechanism by which these KRAS mutations might abrogate the antiproliferative effects of everolimus on cells expressing activating PIK3CA mutations. Using biochemical analyses, they provide evidence that mutant KRAS leads to mTOR-independent protein synthesis, possibly through the activation of p90 ribosomal S6 kinase (p90RSK).
Clinical implications
Ultimately, the goal in understanding the mechanism of cancer resistance is to be able to use the information gathered from these laboratory-based experiments and apply them accurately to a clinical setting. Di Nicolantonio and colleagues took their findings and hypothesized that patients with tumors containing both PIK3CA and KRAS mutations would be resistant to everolimus treatment. The authors were able to acquire a small number of tumor samples from patients who had received everolimus therapy and assessed their cancers for PIK3CA mutations and PTEN loss along with BRAF and KRAS mutations. Despite the small number of patients (i.e., 43), the data support the notion that activation of the PI3K pathway by a PIK3CA mutation or PTEN loss does lead to sensitivity to mTOR inhibitors, but that the concurrent presence of either a KRAS or BRAF mutation abrogates this effect. Although it is difficult to know the validity of such analyses given the small sample size, these data strongly support the idea that patients with KRAS or BRAF mutations in their cancers who are receiving everolimus will likely circumvent mTOR inhibition and receive little to no clinical benefit from rapamycin derivatives.
How are we to use the findings of this important study? Like most preclinical data and retrospective studies, the results presented can be viewed as hypothesis generating but not hypothesis proving. Further prospective trials are ultimately needed to validate these results. That said, the results of this elegant study using isogenic cell lines along with mouse models and retrospective patient samples suggest that the new paradigms invoked by the authors may play out to be true in future prospective studies. Moreover, the work of Di Nicolantonio and colleagues (5) presented here provides a framework whereby the use of precise genetic manipulations within human cell lines could be starting points for assessing positive or negative predictors of response to newer targeted therapies. Ultimately, this may lead to more effective identification of patient populations that would be the “right” candidates for a given inhibitor resulting in truly individualized treatments for cancer therapy. The work by Di Nicolantonio et al. is a significant step toward this goal.
Acknowledgments
M. Mohseni is supported by a Department of Defense Breast Cancer Research Program Predoctoral Award (W81XWH-09-1-0100). B.H. Park acknowledges support from the Breast Cancer Research Foundation, Susan G. Komen for the Cure, the NIH/National Cancer Institute (CA109274, CA88843), and the Avon Foundation.
Footnotes
Conflict of interest: Ben Ho Park is a consultant for, and has received research funding from, GlaxoSmithKline and is a consultant for Horizon Discovery Ltd.
Citation for this article: J Clin Invest. 2010;120(8):2655–2658. doi:10.1172/JCI44026.
See the related article beginning on page 2858.
References
- 1.Druker BJ. STI571 (Gleevec) as a paradigm for cancer therapy. Trends Mol Med. 2002;8(4 Suppl):S14–S18. doi: 10.1016/s1471-4914(02)02305-5. [DOI] [PubMed] [Google Scholar]
- 2.Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. . N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 3.Paez JG, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304(5676):1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 4.Siena S, Sartore-Bianchi A, Di Nicolantonio F, Balfour J, Bardelli A. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. J Natl Cancer Inst. 2009;101(19):1308–1324. doi: 10.1093/jnci/djp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Nicolantonio F, et al. Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. . J Clin Invest. 2010;120(8):2858–2866. doi: 10.1172/JCI37539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8(8):627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Wood LD, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318(5853):1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 9.Karakas B, Bachman KE, Park BH. Mutation of the PIK3CA oncogene in human cancers. Br J Cancer. 2006;94(4):455–459. doi: 10.1038/sj.bjc.6602970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houghton PJ. Everolimus. Clin Cancer Res. 2010;16(5):1368–1372. doi: 10.1158/1078-0432.CCR-09-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Nicolantonio F, et al. Replacement of normal with mutant alleles in the genome of normal human cells unveils mutation-specific drug responses. Proc Natl Acad Sci U S A. 2008;105(52):20864–20869. doi: 10.1073/pnas.0808757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gustin JP, et al. Knockin of mutant PIK3CA activates multiple oncogenic pathways. Proc Natl Acad Sci U S A. 2009;106(8):2835–2840. doi: 10.1073/pnas.0813351106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirasawa S, Furuse M, Yokoyama N, Sasazuki T. Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science. 1993;260(5104):85–88. doi: 10.1126/science.8465203. [DOI] [PubMed] [Google Scholar]