Abstract
Basophils are the least abundant granulocytes found in the circulation. Until recently, their functions were poorly understood. In the past few years, the list of basophil functions in the context of immunity has dramatically increased. Thus, the need for basophil-deficient animal models to confirm these findings is imperative. In this issue of the JCI, Wada and colleagues introduce the first mouse model in which basophils are conditionally ablated in vivo. Using this model, they then uncover a nonredundant role for basophils in acquired immunity against tick infection.
Basophils, the least abundant granulocytes found in the circulation, have long been outsiders in the immunology community. This is in part because of their scarcity and phenotypic similarity to mast cells. Although mast cells are not classified as granulocytes, they share several characteristics with basophils; these include surface expression of the high-affinity Fc receptor for IgE (FcεRI) and the ability to release chemical mediators such as histamine after stimulation. In mice, mature basophils have a relatively small cytoplasm and few cytoplasmic granules (1). However, surface markers that are specific for basophils have not been identified; thus, an experimental tool to examine the unique functions of basophils in vivo has long been wanted. Recent studies have increased the need for this, as they have accumulated convincing evidence that basophils play crucial roles in the development of immune responses, particularly those associated with type 2 immunity (2, 3).
Basophil-mediated regulation of immune responses is primarily mediated by the Th2-type cytokines produced by basophils, including IL-4 and thymic stromal lymphopoietin. Unlike Th2 CD4+ T cells, which arise following stimulation of naive CD4+ T cells in the appropriate cytokine milieu, basophils do not require a differentiation step to produce Th2-type cytokines; instead, it has been shown that they spontaneously acquire the capacity to produce these cytokines (4). Importantly, basophils have been shown to enter antigen-draining lymph nodes where they support Th2 differentiation of naive CD4+ T cells (5). Furthermore, basophils have also been demonstrated to function as antigen-presenting cells that directly prime antigen-specific naive CD4+ T cells to become Th2 effector T cells even in the absence of CD11c+ dendritic cells (6). Despite this increasing evidence that basophils regulate type 2 immunity, their contribution to protective effector immunity remains less well characterized. A substantial increase in the number of IL-4–producing basophils was found in mice infected with the intestinal nematode Nippostrongylus brasiliensis (7), although their role as inducers of N. brasiliensis–specific Th2 immunity was recently challenged (8). Basophils also have recently been found in the lamina propria of the small intestine and the lung parenchyma during infection of mice with N. brasiliensis (9), and depletion of basophils using Ab treatment impaired parasite expulsion during primary and secondary infection with N. brasiliensis (9, 10), suggesting that basophils may play a protective role against the parasite infection.
Basophil-deficient mice
As the number of functions ascribed to basophils has risen, so the demand for an animal that lacks basophils has continued to rise. In vivo depletion using monoclonal Abs specific for FcεRI (MAR-1) or CD200R3 (Ba103) has at least allowed researchers to begin to test the functions of basophils in vivo (5, 11). However, concerns related to the possibility that basophils are activated by the Ab treatment during depletion, which would result in cytokine secretion, and/or the possibility that the Abs cross-react with nonbasophils, particularly mast cells, have always been an issue of uncertainty. In this issue of the JCI, Wada et al. report for the first time a mouse model of inducible basophil ablation (12). The mouse model was generated by introducing the diphtheria toxin receptor–encoding (DTR–encoding) gene into the 3′ untranslated region of the mast cell serine protease 8 (Mcpt8) gene in a bacterial artificial chromosome. Consistent with a report that mouse MCP8 is a basophil-specific differentiation marker (13), expression of DTR was only found in basophils and not in other cells, especially mast cells. Treatment of the mice, which were termed Mcpt8DTR mice, with diphtheria toxin (DT) led to a transient depletion of basophils in the bone marrow and periphery, while the majority of mast cells remained unaffected during the treatment.
Tick infection model
Studies using guinea pigs and ruminants have shown that repeated tick infestation induces cutaneous basophilia and that host Fc receptors are required for Ab-mediated resistance (14, 15). Moreover, the recruited basophils appear to play an important role in host resistance, as injection of anti-basophil serum partially impairs resistance to infestation (16). However, this notion was challenged by the finding that mast cell–deficient mice fail to acquire resistance to tick infestation (17) and by the difficulty in identifying infiltrating basophils in tissues stained with H&E.
However, using their mouse model of inducible basophil ablation, Wada et al. have provided indisputable evidence that it is indeed basophils that have a nonredundant role in Ab-mediated acquired immunity against reinfestation with the tick Haemaphysalis longicornis, which is the vector that mediates babesiosis, Q fever, and Russian encephalitis in humans (12). The authors established a model system in which mice infested with H. longicornis ticks acquire protective immunity during primary infestation, as determined by substantially reduced numbers and weights of engorged ticks (a measure known as tick repletion) during the second infestation compared with the first. Consistent with an earlier report (17), tick repletion remained unaltered in mice deficient in mast cells (KitW-sh/W-sh) and diminished to the level seen in wild-type mice upon reconstituting KitW-sh/W-sh mice with mast cells derived from wild-type bone marrow, suggesting that mast cells play a key role in the acquisition of resistance to tick reinfestation. Moreover, both antibody-deficient and Fcer1g-deficient mice failed to display tick resistance, supporting the idea that anti-tick antibodies (probably IgE isotype) mediate resistance. However, KitW-sh/W-sh mice that received mast cells derived from Fcer1g-deficient bone marrow were fully capable of lowering tick repletion, as seen in recipients of mast cells derived from wild-type bone marrow, suggesting that Ig receptors on mast cells are not necessary for Ab-mediated resistance to tick reinfestation. This unexpected observation prompted the authors to test the role of basophils in this experimental setting.
Wada et al. then used the Mcpt8DTR mice to directly test the role of basophils in acquired resistance to tick infection (12). Treatment of Mcpt8DTR mice with DT during the secondary infestation completely abolished resistance. Importantly, the total mast cell numbers in the tick feeding sites remained unchanged relative to control mice, strongly indicating that mast cells are not necessary for resistance. The importance of basophils was further supported by the fact that transfer of basophil-enriched CD49b+ splenocytes isolated from sensitized mice was sufficient to confer resistance in naive mice. Moreover, transferring CD49b+ splenocytes isolated from DT-treated (but not from PBS-treated) Mcpt8DTR mice that had been infested once was unable to diminish tick repletion. These results strongly suggest that basophils play a critical nonredundant role in acquired resistance to tick reinfestation. The authors further showed that the transfer of CD49b+ splenocytes isolated from tick-infested Fcer1g-deficient mice failed to confer resistance; in other words, the expression of Ig receptors on basophils rather than mast cells is a key determinant for protection, confirming their earlier finding of resistance following reconstitution of KitW-sh/W-sh mice with mast cells derived from Fcer1g-deficient bone marrow.
Future perspectives
The work of Wada et al. (12) raises several important questions for future study. A cellular mechanism underlying basophil recruitment to sites of tick infestation needs to be examined (Figure 1). Basophils are found within the draining lymph nodes after primary infection with N. brasiliensis, and IL-3 is necessary for this recruitment (8). It is therefore possible that circulating basophils may enter lymph nodes that drain tick antigens during primary infestation and that those basophils then present tick antigens and induce type 2 differentiation. The significance of basophil recruitment to the infestation sites during secondary infection is more likely related to their effector function. While the source of IL-3 in this setting is likely activated CD4+ T cells, the target cells of IL-3 are less well characterized. Because IL-3 is believed to induce the production of chemokines and expression of adhesion molecules that contribute to basophil transendothelial migration (18), finding the IL-3–responding target cells is likely to illuminate the mechanism involved in basophil migration. However, basophil migration into lymphoid versus nonlymphoid parenchymal tissues including skin or lung might utilize different mechanisms.
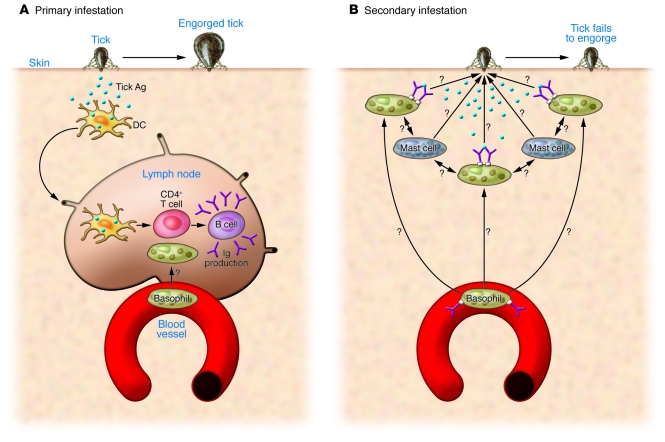
Figure 1. Basophil responses during primary and secondary tick infestation.
The model depicted here for the role of basophils during primary and secondary tick infestation as suggested by the data of Wada and colleagues (12). (A) Primary infestation. Upon infestation, tick antigens are likely to be taken up by tissue-resident dendritic cells, which subsequently migrate to the draining lymph nodes, where they present the antigen to CD4+ T cells. Activated CD4+ T cells are expected to provide “help” to B cells to produce tick antigen–specific Igs (including IgE and IgG). Whether basophils enter into the draining lymph nodes and influence the T cell responses as previously seen in the N. brasiliensis infection model remains unclear. Tick engorgement takes place. (B) Secondary infestation. Upon secondary infestation, basophils are rapidly recruited to the infestation sites, where they are expected to be activated by surface antibody crosslinking. How basophil recruitment is induced is unknown. Tissue mast cells play an important role in providing resistance, although antibody-mediated activation of mast cells is dispensable for the resistance. Antibody-activated basophils may cooperate with mast cells during this process. Tick engorgement fails to occur. The effector mechanisms that directly inhibit tick engorgement remain to be examined.
How recruited basophils mediate resistance also needs to be characterized. Given the failure of Fcer1g-deficient splenocytes to restore tick resistance in Mcpt8DTR mice treated with DT, it is likely that tick-specific antibodies crosslink Ig receptors on basophils that then secrete mediators that deter tick engorgement. However, whether basophils are directly involved in anti-tick immunity or recruit other effectors such as eosinophils into the sites (16) to mediate resistance remains to be explored. It is important to note that despite the fact that mast cells are necessary for acquired resistance to tick infestation, a mechanism mediating the resistance appears to be distinct from that mediated by basophils. What is particularly striking is that failure of resistance in mast cell–deficient mice is reversed by the transfer of mast cells derived from Fcer1g-deficient bone marrow. Given the fact that depletion of basophils alone is sufficient to abolish resistance, it is likely that mast cells are needed for basophils to execute anti-tick (Ig-dependent) effector immunity. Understanding the cellular cooperation between mast cells and basophils will be an important subject of investigation.
In sum, the results reported by Wada et al. (12) identify a previously unrecognized effector role for basophils during infestation with the tick H. longicornis, an important vector mediating critical human diseases. Moreover, the availability of an animal in which basophils can be conditionally deleted will allow us to better understand the mystery of these rare cells in the immune system.
Acknowledgments
The author is supported by NIH grant AI080908.
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2010;120(8):2648–2651. doi:10.1172/JCI44058
See the related article beginning on page 2867.
References
- 1.Dvorak AM, Nabel G, Pyne K, Cantor H, Dvorak HF, Galli SJ. Ultrastructural identification of the mouse basophil. Blood. 1982;59(6):1279–1285. [PubMed] [Google Scholar]
- 2.Min B. Basophils: what they ‘can do’ versus what they ‘actually do’. Nat Immunol. 2008;9(12):1333–1339. doi: 10.1038/ni.f.217. [DOI] [PubMed] [Google Scholar]
- 3.Karasuyama H, Mukai K, Tsujimura Y, Obata K. Newly discovered roles for basophils: a neglected minority gains new respect. Nat Rev Immunol. 2009;9(1):9–13. doi: 10.1038/nri2458. [DOI] [PubMed] [Google Scholar]
- 4.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. . J Immunol. 2005;174(2):1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 5.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9(3):310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siracusa MC, Perrigoue JG, Comeau MR, Artis D. New paradigms in basophil development, regulation and function. Immunol Cell Biol. 2010;88(3):275–284. doi: 10.1038/icb.2010.1. [DOI] [PubMed] [Google Scholar]
- 7.Min B, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J Exp Med. 2004;200(4):507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S, Prout M, Ramshaw H, Lopez AF, LeGros G, Min B. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol. 2010;184(3):1143–1147. doi: 10.4049/jimmunol.0902447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood. 2009;113(12):2816–2825. doi: 10.1182/blood-2008-05-154773. [DOI] [PubMed] [Google Scholar]
- 10.Ohnmacht C, Voehringer D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J Immunol. 2010;184(1):344–350. doi: 10.4049/jimmunol.0901841. [DOI] [PubMed] [Google Scholar]
- 11.Obata K, et al. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood. 2007;110(3):913–920. doi: 10.1182/blood-2007-01-068718. [DOI] [PubMed] [Google Scholar]
- 12.Wada T, et al. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest. 2010;120(8):2867–2875. doi: 10.1172/JCI42680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poorafshar M, Helmby H, Troye-Blomberg M, Hellman L. MMCP-8, the first lineage-specific differentiation marker for mouse basophils. Elevated numbers of potent IL-4-producing and MMCP-8-positive cells in spleen of malaria-infected mice. Eur J Immunol. 2000;30(9):2660–2668. doi: 10.1002/1521-4141(200009)30:9<2660::AID-IMMU2660>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 14.Brown SJ, Askenase PW. Amblyomma americanum: requirement for host Fc receptors in antibody-mediated acquired immune resistance to ticks. Exp Parasitol. 1985;59(2):248–256. doi: 10.1016/0014-4894(85)90079-7. [DOI] [PubMed] [Google Scholar]
- 15.Monteiro GE, Bechara GH. Cutaneous basophilia in the resistance of goats to Amblyomma cajennense nymphs after repeated infestations. Ann N Y Acad Sci. 2008;1149:221–225. doi: 10.1196/annals.1428.026. [DOI] [PubMed] [Google Scholar]
- 16.Brown SJ, Galli SJ, Gleich GJ, Askenase PW. Ablation of immunity to Amblyomma americanum by anti-basophil serum: cooperation between basophils and eosinophils in expression of immunity to ectoparasites (ticks) in guinea pigs. J Immunol. 1982;129(2):790–796. [PubMed] [Google Scholar]
- 17.Matsuda H, Fukui K, Kiso Y, Kitamura Y. Inability of genetically mast cell-deficient W/Wv mice to acquire resistance against larval Haemaphysalis longicornis ticks. J Parasitol. 1985;71(4):443–448. doi: 10.2307/3281535. [DOI] [PubMed] [Google Scholar]
- 18.Iikura M, et al. Transendothelial migration of human basophils. J Immunol. 2004;173(8):5189–5195. doi: 10.4049/jimmunol.173.8.5189. [DOI] [PubMed] [Google Scholar]