Abstract
Class 3 semaphorins (Sema3s) regulate axon guidance, angiogenesis, tumor growth, and tumor metastasis. Neuropilins (NRPs; NRP1 and NRP2) are the cell surface receptors for the Sema3s. However, to signal, interaction of Sema3s and NRPs with plexins is obligatory. In this issue of the JCI, Casazza and colleagues report data that challenge the conventional wisdom about the role of Sema3s in tumor metastasis. As a rule, Sema3B and Sema3F, for example, are inhibitors of tumor angiogenesis, progression, and metastasis. However, Casazza et al. found that Sema3E inhibited tumor growth but atypically promoted invasiveness and metastasis. This metastatic potential was dependent on Plexin D1 expression but was independent of NRP expression. Of clinical importance, Sema3E and Plexin D1 were found to be upregulated in human colon cancer, liver metastasis, and melanoma progression.
Semaphorins and their receptors
There are eight classes of semaphorins characterized by structural heterogeneity (1, 2). Class 3 semaphorins (Sema3s; Sema3A–Sema3G) are secreted proteins of approximately 100 kDa; they bind neuropilins (NRPs; NRP1 and NRP2) as their cell surface receptors. However, Sema3s also require interactions with plexins to signal. Plexins are large 200-kDa transmembrane proteins that act as substrates for kinases, such as feline sarcoma oncogene (Fes) and Src (3). Plexins form complexes with NRPs to transduce the Sema3 signal. Nine plexins have been identified so far (A1–A4, B1–B3, C1, and D1). Sema3s were originally demonstrated to be axon guidance proteins that repelled axons and collapsed growth cones via NRPs (4). They have since been implicated as regulators of angiogenesis and tumor progression (1, 2). Sema3A was the first Sema3 to be studied in a vascular context. It was shown to inhibit EC migration and capillary sprouting (5). Subsequent studies showed that Sema3s inhibit adhesion and migration of tumor cells (2). NRPs also bind VEGF family members (6). VEGF-NRP interactions regulate angiogenesis by acting as coreceptors for a receptor tyrosine kinase, VEGFR-2. The puzzle of how two such structurally disparate groups of ligands (Sema3s and VEGF family members) could bind to the same receptor was resolved when it was shown that VEGF binds to the NRP–B domain and that Sema3s bind to the NRP–A domain (7). A critical role for NRPs in angiogenesis, likely as a result of their ability to bind VEGF family members, was shown in mice lacking NRPs (8) and in zebrafish in which NRP levels had been knocked down (9). The convention has been that Sema3s are inhibitors of tumor angiogenesis, progression, and metastasis and that their function requires NRPs. However, in this issue of the JCI, Casazza and colleagues put a new twist on the semaphorin cancer story, particularly in the area of semaphorin effects on metastasis, by demonstrating that Sema3E inhibits tumor growth but promotes metastasis and that it does this in an NRP-independent manner (10).
p61 is the biologically active species of Sema3E
Sema3E is synthesized as an 85- to 90-kDa protein. However, it has furin-sensitive sites that are cleaved to generate p61, which is the main species of endogenous Sema3E. p61 induced lung metastasis in mice (11). In tumor cells, it promoted in vitro cell motility, invasiveness, transendothelial migration, and extravasation. Furin-induced cleavage is a feature of many of the Sema3s (12). For example, Sema3B found in the conditioned medium of cancer cells is almost completely cleaved by furin-like proprotein convertases, generating inactive fragments. So, furin-induced proteolytic processing of Sema3s does not necessarily result in a bioactive form, as it does for Sema3E.
Sema3E binds Plexin D1 but is NRP independent
Sema3E binds Plexin D1 directly and with high affinity, the only Sema3 to do so (13). A role in angiogenesis for this ligand/receptor pair has been clearly defined. For example, Plexin D1 expression is restricted to ECs in mouse early development and is upregulated in tumor vasculature (14). Sema3E and Plexin D1 control EC positioning and patterning of the developing vasculature (13). The pattern of Sema3E expression is markedly similar to that of Plexin D1, with both localizing to the vasculature. In addition, Plexin D1 expression is necessary for cardiovascular development (15). Plexin D1–knockout mice survive for up to 2 days after birth and then succumb to structural cardiovascular defects. In zebrafish, the Plexin D1 gene is expressed throughout the vasculature, and knockdown of Plexin D1 results in highly abnormal intersegmental vessels (16). A very important discovery was the demonstration in mouse embryogenesis that, unlike the other Sema3s, Sema3E is NRP independent and instead signals directly via Plexin D1 (13). NRPs had been previously presumed to be obligate Sema3 coreceptors.
Sema3E inhibits tumor growth but promotes metastasis
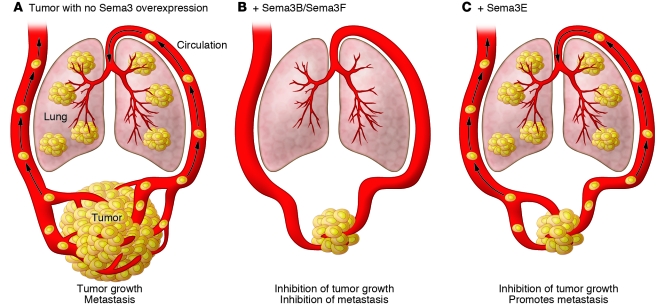
A central point of the article by Casazza and colleagues (10) is the involvement of Sema3E and Plexin D1 in promoting metastasis, as shown in vitro and in preclinical and clinical studies. Earlier, Christensen and colleagues, using differential display, had demonstrated in a limited study that mouse SemaH (this name changed later to Sema3E) was expressed in 12 out of 12 metastatic cell lines and in only 2 out of 6 nonmetastatic cell lines (17). In general, Sema3s (e.g., Sema3B and Sema3F) have been considered to be inhibitors of tumor growth and metastasis (Figure 1). However, Casazza et al. have now demonstrated that Sema3E and Plexin D1 expression correlates positively with the metastatic progression of three human tumors: colon, liver, and melanoma (10). Whereas Plexin D1 was expressed in all human colon carcinoma samples, Sema3E was detected in just a few. However, Sema3E was expressed more highly in metastatic disease compared with nonmetastatic disease. Dramatically, Sema3E was expressed in 100% of liver metastases derived from colon carcinoma. Furthermore, Sema3E was expressed in only a fraction of benign human skin lesions but almost 100% of metastatic melanomas of Clark levels III and IV. Taken together, analysis of colon, liver, and melanoma sections showed that Sema3E expression correlated strongly with metastasis. However, these correlative data did not show that Sema3E actually promoted metastasis. Casazza and colleagues were able to show this in a more direct manner: knockdown of either Sema3E or Plexin D1 hampered the metastatic potential of human cancer cells upon xenotransplantation, whereas overexpression of Sema3E promoted metastatic spreading (10). The surprise here is that even though Sema3E inhibited tumor growth, as other Sema3s do, it promoted metastasis (Figure 1). It should be noted, however, that Roodink et al. have reported that overexpression of Sema3E in a model of metastatic melanoma decreases, rather than increases, metastasis (18). On the other hand, Plexin D1 expression did correlate with metastatic potential.
Figure 1. The effect of Sema3s on tumor growth and metastasis.
(A) Tumors grow and metastasize. Tumor growth is defined as increased tumor volume. (B) Sema3B and Sema3F block tumor growth and metastasis. Inhibition of tumor growth is ascribed to inhibition of angiogenesis and lowered microvascular density. (C) Sema3E inhibits tumor growth but promotes metastasis.
The findings of Casazza et al. (10) are paradoxical, since inhibiting tumor angiogenesis, as Sema3E does, is usually thought to inhibit metastasis by inhibiting the formation of blood vessel conduits for delivering primary tumor cells to distant sites. This raises the question of why Sema3E and Sema3B/Sema3F, which have such similar structures, act so differently in a tumor metastasis context? At this point, we speculate that Plexin D1 is responsible, because of its close and unique association with blood vessels. In this scenario, blood vessel Plexin D1 would uniquely bind Sema3E, which was shown by Casazza et al. (10) to stimulate tumor cell motility, invasiveness, transendothelial migration, and extravasation. These events could result in increased tumor cell escape from blood vessels. On the other hand, the inability of Sema3B/Sema3F to promote metastasis suggests that binding to Plexin D1 is obligatory for this process.
ErbB2 transactivates Plexin D1
A previous study has shown that Sema4D, a class 4 semaphorin that does not bind NRPs, stimulates cell migration and invasive growth (19). Interestingly, the mechanism underlying this was that binding of soluble Sema4D (approximately 61 kDa) to Plexin B1 triggered the activation of Met tyrosine kinase, thereby promoting cell dissociation and invasive growth. By analogy, Casazza et al. hypothesized that Sema3E and Plexin D1 would also interact with a receptor tyrosine kinase to promote invasive behavior (10). A microarray identified ErbB2 but not Met as a candidate. ErbB2 would fit the bill nicely, since it is an oncogene implicated in cancer invasion and metastasis. Indeed, Casazza et al. showed that p61 induced ErbB2 phosphorylation in tumor cells but not in ECs (10). Plexin D1 and ErbB2 formed receptor complexes in which Plexin D1 became tyrosine phosphorylated in response to p61. Furthermore, an ErbB2 kinase inhibitor blocked p61-induced migration and inhibited formation of lung metastatic colonies. ErbB2 function in this context required MAPK and PLC-γ activation.
Perspectives
In summary, Sema3E is clearly an outlier within the Sema3 family (Table 1). Sema3s, such as Sema3B and Sema3F, have been long considered inhibitors of tumor growth and metastasis; however, in contrast, Casazza et al. show that Sema3E inhibits tumor growth but promotes metastasis (10). The Sema3E story is reminiscent of recent controversial studies, indicating that angiogenesis inhibitors that block tumor growth actually promote metastasis (20, 21). Thus, the conventional wisdom that tumor growth is a necessary prelude to induce metastasis needs reexamination. These studies by Casazza and colleagues are important, because they provide new information that could be clinically useful in inhibiting metastasis, for example, by targeting Sema3E, Plexin D1, and ErbB2/Plexin D1 heterodimerization. Hopefully, further clinical data from this laboratory will confirm the importance of Sema3E as a key contributor to metastasis.
Table 1 .
Activity differences between Sema3B and Sema3F compared with Sema3E
Acknowledgments
We thank Andrew Dudley and Diane Bielenberg for useful discussions about the manuscript. We thank Melissa Herman for help in writing the manuscript and Kristin Johnson for preparing the figure. This commentary was supported by NIH grants CA37392 and CA45548.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2010;120(8):2658–2660. doi:10.1172/JCI44110.
See the related article beginning on page 2684.
References
- 1.Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008;8(8):632–645. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- 2.Bielenberg DR, Klagsbrun M. Targeting endothelial and tumor cells with semaphorins. Cancer Metastasis Rev. 2007;26(3–4):421–431. doi: 10.1007/s10555-007-9097-4. [DOI] [PubMed] [Google Scholar]
- 3.Franco M, Tamagnone L. Tyrosine phosphorylation in semaphorin signalling: shifting into overdrive. EMBO Rep. 2008;9(9):865–871. doi: 10.1038/embor.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90(4):739–751. doi: 10.1016/S0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 5.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146(1):233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92(6):735–745. doi: 10.1016/S0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 7.Geretti E, Shimizu A, Kurschat P, Klagsbrun M. Site-directed mutagenesis in the B-neuropilin-2 domain selectively enhances its affinity to VEGF165, but not to semaphorin 3F. J Biol Chem. 2007;282(35):25698–25707. doi: 10.1074/jbc.M702942200. [DOI] [PubMed] [Google Scholar]
- 8.Takashima S, et al. Targeting of both mouse neuropilin-1 and neuropilin-2 genes severely impairs developmental yolk sac and embryonic angiogenesis. Proc Natl Acad Sci U S A. 2002;99(6):3657–3662. doi: 10.1073/pnas.022017899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee P, Goishi K, Davidson AJ, Mannix R, Zon L, Klagsbrun M. Neuropilin-1 is required for vascular development and is a mediator of VEGF-dependent angiogenesis in zebrafish. Proc Natl Acad Sci U S A. 2002;99(16):10470–10475. doi: 10.1073/pnas.162366299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casazza A, et al. Sema3E–Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. J Clin Invest. 2010;120(8):2684–2698. doi: 10.1172/JCI42118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen C, et al. Proteolytic processing converts the repelling signal Sema3E into an inducer of invasive growth and lung metastasis. Cancer Res. 2005;65(14):6167–6177. doi: 10.1158/0008-5472.CAN-04-4309. [DOI] [PubMed] [Google Scholar]
- 12.Varshavsky A, et al. Semaphorin-3B is an angiogenesis inhibitor that is inactivated by furin-like pro-protein convertases. Cancer Res. 2008;68(17):6922–6931. doi: 10.1158/0008-5472.CAN-07-5408. [DOI] [PubMed] [Google Scholar]
- 13.Gu C, et al. Semaphorin 3E and plexin-D1 control vascular pattern independently of neuropilins. Science. 2005;307(5707):265–268. doi: 10.1126/science.1105416. [DOI] [PubMed] [Google Scholar]
- 14.Roodink I, et al. Plexin D1 expression is induced on tumor vasculature and tumor cells: a novel target for diagnosis and therapy? Cancer Res. 2005;65(18):8317–8323. doi: 10.1158/0008-5472.CAN-04-4366. [DOI] [PubMed] [Google Scholar]
- 15.Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell. 2004;7(1):107–116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Torres-Vazquez J, et al. Semaphorin-plexin signaling guides patterning of the developing vasculature. . Dev Cell. 2004;7(1):117–123. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Christensen CR, Klingelhofer J, Tarabykina S, Hulgaard EF, Kramerov D, Lukanidin E. Transcription of a novel mouse semaphorin gene, M-semaH, correlates with the metastatic ability of mouse tumor cell lines. Cancer Res. 1998;58(6):1238–1244. [PubMed] [Google Scholar]
- 18.Roodink I, et al. Semaphorin 3E expression correlates inversely with Plexin D1 during tumor progression. Am J Pathol. 2008;173(6):1873–1881. doi: 10.2353/ajpath.2008.080136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conrotto P, et al. Sema4D induces angiogenesis through Met recruitment by Plexin B1. Blood. 2005;105(11):4321–4329. doi: 10.1182/blood-2004-07-2885. [DOI] [PubMed] [Google Scholar]
- 20.Paez-Ribes M, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15(3):220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15(3):232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]