Abstract
Metronomic cyclophosphamide treatment is associated with anti-angiogenic activity and is anticipated to generate exploitable hypoxia using hypoxia-activated prodrugs. Weekly administration of tirapazamine (TPZ; 5 mg/kg body weight i.p.) failed to inhibit the growth of 9L gliosarcoma tumors grown s.c. in scid mice. However, the anti-tumor effect of weekly cyclophosphamide (CPA) treatment (140 mg/kg BW i.p.) was substantially enhanced by weekly TPZ administration. An extended tumor free period and increased frequency of tumor eradication without overt toxicity were observed when TPZ was given 3, 4 or 5 days after each weekly CPA treatment. Following the 2nd CPA injection, Electron Paramagnetic Resonance (EPR) Oximetry indicated significant increases in tumor pO2, starting at 48 hr, which further increased after the 3rd CPA injection. pO2 levels were, however, stable in growing untreated tumors. A strong negative correlation (−0.81) between tumor pO2 and tumor volume during 21 days of weekly CPA chemotherapy was observed, indicating increasing tumor pO2 with decreasing tumor volume. Furthermore, CPA treatment resulted in increased tumor uptake of activated CPA. CPA induced increases in VEGF RNA, which reached a maximum on day 1, and in PLGF RNA which was sustained throughout the treatment, while anti-angiogenic host thrombospondin-1 increased dramatically through day 7 post-CPA treatment. Weekly cyclophosphamide treatment was anticipated to generate exploitable hypoxia. However, our findings suggest that weekly CPA treatment induces a functional improvement of tumor vasculature, which is characterized by increased tumor oxygenation and drug uptake in tumors, thus counter-intuitively, benefiting intratumoral activation of TPZ and perhaps other bioreductive drugs.
Keywords: Anti-angiogenic chemotherapy, Tirapazamine, cyclophosphamide, tumor, pO2, EPR oximetry
INTRODUCTION
Solid tumors present heterogeneous microenvironments characterized by distinct levels of oxygenation, growth, remodeling and death. However, hypoxia is thought to be the main force driving these changes including tumor responses to treatments [1], Continuous growth of tumor cells requires a greater supply of nutrients and oxygen, a demand that when unmet, leads to oxygen depletion or anoxia, followed by necrosis [2]. A similar outcome is observed when using angiogenesis inhibitors [3]. Classic angiogenesis inhibitors are cytostatic and as such their use suffers several limitations such as the need for long term administration with its associated high costs, as well as the potential to interfere with classic anti-cancer drugs [4]. Moreover, specific angiogenesis inhibitors could lead to tumor resistance by multiple factors such as angiogenic factor redundancy [5], angiogenesis independent vascular remodeling [6], and resistance to hypoxia due to p53 deficiency [7]. Multi-pronged angiogenesis inhibitors are therefore less susceptible to encounter tumor resistance.
Metronomic chemotherapy is a repetitive administration of small doses of chemotherapy drugs, which heavily target the tumor endothelium. The anti-angiogenic basis of a metronomic cyclophosphamide schedule and its direct cytotoxic effect constitute a combination approach that is absent with most angiogenesis inhibitors [8, 9]. The continuous administration of angiogenesis inhibitors, such as cyclophosphamide in the drinking water, can increase tumor hypoxia during the period of treatment [10]. However, under a weekly schedule of anti-angiogenic chemotherapy, with a rest period between treatments, the potential development of hypoxia episodes and their duration have yet to be investigated and could potentially follow a different pattern. The knowledge of the kinetic of tumor pO2 during metronomic chemotherapy regimens would likely help in the design of combination treatments with improved efficacy. Of particular interest is the use of hypoxia-activated drugs, which can be administered in conjunction with metronomic chemotherapy. TPZ is a leading bioreductive drug that exhibits striking specificity for hypoxic tumor cells [11]. TPZ is activated by various cellular reductases, including the flavoenzyme NADPH P450-reductase [12]. TPZ reduction by a one-electron step yields the TPZ nitroxide radical, which causes DNA single- and double-strand breaks under hypoxic conditions [13]. The significant level of NADPH P450-reductase activity in the NCI panel of 60 cell lines [14] is suggestive of the potential utility of combining bioreductive drugs with chemotherapy regimens. However, TPZ use could complicate the development of a rational treatment involving angiogenesis inhibitors especially in the context of metronomic chemotherapy, by virtue of its own potential anti-angiogenic effects [15] and vaso-active properties which can modulate blood flow in tumors [16]. It is therefore crucial to determine the best modalities to co-administer these complementary regimens.
In this study, we hypothesized that by virtue of its anti-angiogenic activity, metronomic CPA treatment could generate hypoxia in tumors, which could be exploited through a time-specific administration of TPZ. We therefore assessed the therapeutic efficacy of the combination of CPA and TPZ where TPZ is administered in a time-specific manner. The therapeutic effect of CPA/TPZ combination was correlated with the levels of molecular oxygen present in tumors using Electron Paramagnetic Resonance (EPR) oximetry. Similarly we measured drug uptake during this treatment. Tumor extracts were also analyzed for expression of major factors involved in endothelium growth and angiogenesis.
MATERIALS AND METHODS
Cyclophosphamide was purchased from Sigma-Aldrich Co., (St. Louis, MO). Tirapazamine (1,2,3 benzotriazine-3-amine 1,4 dioxide) was obtained from Dr. Michael P. Hay (Auckland Cancer Society, Auckland, New Zealand). Fetal bovine serum (FBS) and DMEM medium were purchased from Life Technologies (Grand Island, NY). TRIzol was purchased from Invitrogen Life Technologies Inc (Carlsbad, CA). Random hexamers, MuLV reverse transcriptase, RNase inhibitor, SYBR Green PCR Master Mix were purchased from Applied Biosystems (Foster City, CA). Rat 9L gliosarcoma tumor cells were obtained from the UCSF Brain Tumor Therapy Center and were grown as previously described [17].
Tumor Growth Delay Experiments
Six- to seven-week old (25–29 g) male ICR/Fox Chase scid immunodeficient mice (Taconic Farms, Germantown, NY) were housed in Boston University Laboratory of Animal Care Facilities in accordance with approved local protocols and federal guidelines. 9L cells used for tumor inoculation were grown in DMEM medium with 10% fetal bovine serum. Cells were resuspended in FBS-free DMEM and injected on both flanks as 4 × 106 cells in a volume of 0.5 ml of serum-free DMEM using a 0.5-inch 29-gauge needle and a 1 ml insulin syringe. Tumor areas (length × width) were measured twice a week using vernier calipers (Manostat Corp., Switzerland) and tumor vols were calculated based on: Vol = (π/6) (length × width)3/2. CPA treatment was initiated when the average tumor vol reached about ~350 mm3 or ~1200mm3. Freshly prepared CPA or TPZ solutions in PBS were filtered through a 0.2 μm Acrodisc syringe filter (Pall Corp. Ann Arbor, Ml) and administered to the tumor-bearing mice by i.p. injection at 140 mg CPA/kg and/or 5 mg TPZ/kg body weight (BW) every 7 d. Average tumor growth rates prior to CPA treatment were similar in all groups.
Electron Paramagnetic Resonance (EPR) Oximetry Method
All animal procedures for EPR oximetry were approved by the Institutional Animal Care and Use Committee (IACUC) of Dartmouth Medical School. EPR oximetry provides direct repeated measure of tissue pO2 and has been extensively used to measure and follow pO2 in various tissues including subcutaneous tumors [18–21].
After the insertion of the paramagnetic oxygen-sensitive probe into the tissue by minimally invasive procedures using 25–23ga needle/plungers, the measurements are completely non-invasive and can be performed for long periods of time (up to a few years) [21, 22]. The oximetry probes, lithium phthalocyanine (LiPc) crystals, used in this study were synthesized at Dartmouth. The EPR spectra of LiPc crystals exhibit a single sharp EPR line with a line width highly sensitive to pO2 and reflect the average pO2 on the surface of the crystals. The high density of the unpaired spins along with the narrow intrinsic line width of LiPc allows measurements of pO2 in the tumor tissue using few crystals with a total diameter of ~200 μm [21–23]. The mice were anesthetized (1.5% isoflurane with 30% O2) and two aggregates (40 μg each) of LiPc crystals were injected 4 mm apart and at a depth of 3 mm from the surface of the tumors with average tumor volume of 300 mm3, 24 h (day -1) prior to the experiments. These two LiPc implants reported tissue pO2 of the 9L tumors using low-resolution multi-site EPR oximetry [18, 20]. No significant difference in the pO2 observed from the two LiPc implants of each tumor was observed; therefore the pO2 values from the two implants were pooled to determine average tumor pO2 at each time point of the experiments. With tumor growth or shrinkage, the distance between the implants are expected to vary, however, the measurement of pO2 using two LiPc implants in each tumor allows an assessment of average tumor pO2 during the experiments.
The EPR oximetry measurements were performed on an L-band (1.2 GHz) EPR spectrometer equipped with a microwave bridge and an external loop resonator specially designed for in vivo experiments [21, 22]. For tumor pO2 measurements, the mice were anesthetized (1.5% isoflurane, 30% FiO2) and positioned in the EPR magnet. The two LiPc implants in each tumor were located along the lateral axis. For multi-site EPR oximetry measurements, the animals were positioned in the spectrometer such that this axis was parallel to the direction of the applied gradient in the main magnetic field. A gradient of up to 3.0 G/cm was used to separate the EPR signals of the two LiPc implants in each tumor. A warm air blower and a heated water pad were used to keep the animal warm. The rectal temperature of the animal was monitored and maintained at 37 ± 0.5°C during the measurements. Typical settings for the spectrometer were: incident microwave power, 2 mW; magnetic field center, 400 gauss; scan range, 1 gauss; modulation frequency, 24 kHz, and 180 scans with scan time of 10 seconds each. Modulation amplitude was set at less than one-third of the EPR line width and 6 EPR scans each were averaged to enhance the signal to noise ratio of the EPR signals. The EPR line widths were converted to pO2 using a calibration determined for the batch of LiPc crystals used in this study. A baseline tumor pO2 and tumor volume was measured on day 0 prior to any treatment. The treatment group received three doses of CPA (140mg/Kg) on day 0, day 7 and day 14 while the control group received an equal volume of phosphate buffered saline (PBS) at the same time-points. The experiment was terminated on day 21 for the treatment group. Untreated group was terminated on day 9 due to the very large tumor mass as per the IACUC guidelines.
Cytotoxicity Assays and P450 Reductase Activity
9L tumor cells were plated in triplicate at 4000 cells/well of a 48-well plate 18–24 hr prior to drug treatment. Cells were then treated (0 to 5 μM TPZ) and incubated for 4 days in a tissue culture incubator maintained either under hypoxia (1% O2, 5% CO2, 94% N2) or normoxia (19.6% O2, 5% CO2, 75.4% N2). Alternatively, cells were treated under normoxia with CPA (0 to 500 μM). Cell viability after this time was determined using a crystal violet/alcohol-extraction assay [17]. Data are presented as cell number relative to drug-free controls, mean ± SD values for triplicate samples. P450 reductase activity was assayed using 20 μg of tumor extract, lysed by sonication and then assayed for P450R-catalyzed, NADPH-dependent cytochrome C reduction (A550) as previously described [17].
Quantification of 4-OH-CPA
Rat 9L gliosarcoma tumors were grown in scid mice. When the tumor size reached 1 cm3, CPA was injected as a single dose at 140mg/Kg BW i.p. every 7 days (day 0 corresponds to 7 days after one CPA injection, and day 1, 2 or 3 correspond to 1, 2, or 3 days after the 2nd of two CPA injections). Drug flow into tumors was monitored 15 minutes after i.p. injection on each day by a small probing dose of CPA (50mg/kg). In 9L tumors, which do not metabolize CPA, peak concentrations of 4-OH-CPA are reached at the 15 min time point. Peak concentrations of 4-OH-CPA are detected in blood and liver as early as 6 min after i.p. CPA injection, while at 6 min the tumor content of 4-OH-CPA is only 40% of that of blood. This difference in Tmax indicates a delayed diffusion of 4-OH-CPA from systemic circulation into the tumor [24]. The liver-activated CPA circulating in blood and tumors was monitored by measuring 4OH-CPA in these two compartments. Analysis of 4-hydroxy-CPA production was carried out using 200–400 μl of blood collected from punctured hearts with a syringe containing 10 μl of heparin (100 U/ml). To stabilize 4-hydroxy-CPA in biological samples, 2–4 μl of 0.5 M semicarbazide (SCA) were added (final concentration 5 mM). Tumor and blood 4-hydroxy-CPA content was determined using ~0.4 g of tissue pre-washed with KPi buffer (0.1 M potassium phosphate buffer, pH 7.4 containing 0.1 M EDTA). Tissues were homogenized as described earlier [24]. 500 μl of tumor supernatant homogenates and 50 μl of blood samples were diluted with KPi buffer to a final volume of 500 μl, were deproteinized by the sequential addition of 250 μl containing 5.5% zinc sulfate and 250 μl of saturated barium hydroxide. The acrolein resulting from 4-hydroxy-CPA decomposition was derivatized to 7-hydroxyquinoline as previously described [24], and detected by fluorescence (excitation at 350 nm and emission at 515 nm) using an HPLC elution system equipped with a fluorescence scanner-detector. A standard curve was generated from 4-hydroperoxy-CPA (0–40 μM) dissolved in KPi buffer and processed in parallel with samples. The integrated peak areas determined by Millennium 32 software were then converted to tissue concentrations of metabolite (nmol/g tissue).
Real-time PCR Analysis
Rat 9L gliosarcoma tumors were grown in scid mice. When the tumor size reached 1 cm3, CPA was injected as a single dose at 140mg/Kg BW i.p. every 7 days (day 0 corresponds to 7 days after one CPA injection, and day 1 to 10 correspond to day 1 to 10 after the 2nd of two CPA injections). Tumor were harvested sequentially, snap frozen in liquid nitrogen and stored at −80°C until analysis. Total RNA from 9L tumors was isolated from frozen tumor samples (0.1–0.4 g) using TRIzol reagent according to the manufacturer’s protocol. Tissues were homogenized in a volume (~ 5 ml/g tissue) using a Polytron Homogenizer, Model PT-2000. The isolated RNA (1 μg) was treated with DNase I at 37°C for 60 min to remove any DNA contamination. After heating for 10 min at 75°C to inactivate the DNase, cDNA was synthesized following the manufacturer instructions. A 4 μl portion of the final reaction was diluted 1:50 in 50 ng/μl yeast tRNA and used as template DNA for real-time qPCR assays using the following primers: rat VEGF (sense 5′-TCCTGTGTGCCCCTAATGC-3′, reverse 5′-CCAGGGCTTCATCATTGCA-3′), mouse TSP-1 (sense 5′-TGTTCAAGAGGACCGGGCT-3′, reverse 5′-TGGATGGGTACATCCAGCTCC-3′), rat TSP1 (sense 5′-CCGGTTTGATCAGAGTGGT-3′, reverse 5′-GGTTCCGGAAGGTGCAAT-3′) rat PLGF (sense 5′-GATGTGCTCTGCGAATGCA-3′, reverse 5′-CCCCTTGGTTTTCCTCCTTT-3′), mouse VEGF receptor 2 (sense 5′-CCCTGCTGTGGTCTCACTACC, reverse 5′-AGTTTCACAGAGGTGCCGTTG-3′), mouse VEGFR receptor 1 (sense 5′-AACTAGGCAAATCGCTCGGA, reverse 5′ – AGGCTTGAACGACTTTCCCA-3′) rat HIF-a (sense 5′-AACAAACAGAATCTGTCCTCAAACC-3′, reverse 5′-CAGGTAATGGAGACATTGCCAG-3′) and rat Glut-1 (sense 5′-GGGCATGATTGGTTCCTTCTC-3′, reverse 5′-CCATAAGCACGGCAGACACA-3′) Relative levels were determined in triplicate wells of a 384-well plate and run through 40 cycles on ABS 7900HT sequence detection system (Applied Biosystems). Ct values determined for each mRNA were normalized to the 18S rRNA content of each RNA sample and the data is presented as a percentage of untreated tumor samples.
Statistical Analysis
Statistical significance of differences was determined by two-tailed Student’s test, or a one-way ANOVA paired and non-parametric Bonferroni’s Multiple Comparison test. All tests included comparisons to untreated samples or as indicated in the text. Statistical significance is indicated by *: p<0.05, **: p<0.01, ***: p<0.001. Analyses were performed using Prism software version 4 (GraphPad Software, San Diego, CA). Tumor volume in control group (Fig. (1E)) was modeled as a linear mixed model on the log scale assuming that the baseline tumor volume is mouse-specific [25, 26]. The dynamics of tumor volume in treatment groups (Fig. (1C)) were modeled as a quadratic function of time on the log scale, assuming that the baseline tumor volume is mouse specific. Quadratic function allows the determination of maximum increase in tumor volume and the day when this maximum occurs. Tumor pO2 is modeled as a cubic function of time on the log scale under the same assumption (Fig. (1B)). S-Plus 6.1 (Insightful, Inc. Seattle) was used to do computations involving estimation of mixed models.
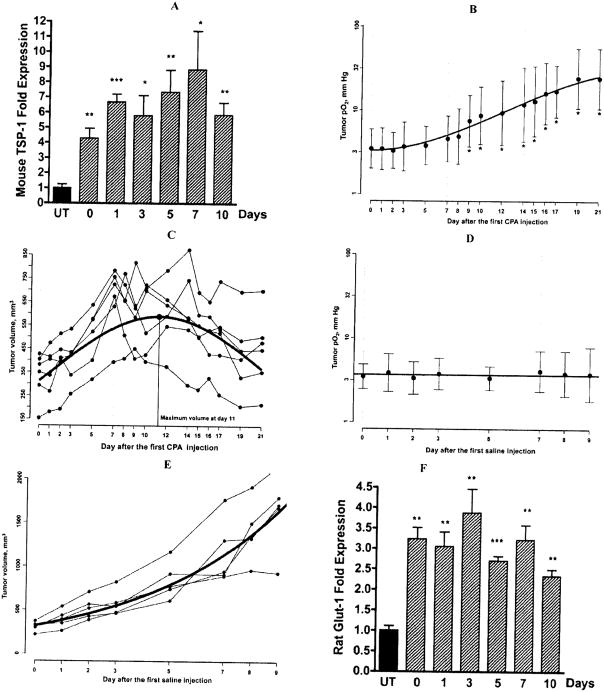
Fig. 1.
A, Expression of mouse TSP-1 mRNA was measured in the days following the second of two weekly CPA treatments (140mg/kg i.p.). TSP-1 expression is presented as fold increase over untreated tumor samples. Metronomic CPA induced more than 8-fold increase in endothelial TSP-1 above the untreated level, Mean ± SE n=4 individual tumors per group. B, Changes in tissue pO2 of subcutaneous 9L tumors in the experimental group. The mice were administered CPA (140mg/Kg, i.p.) on day 0, day 7 and day 14 and the changes in tumor pO2 were repeatedly measured by EPR oximetry, Mean ± SD, n = 6. C, Change in tumor volume of the experimental group, n = 6. D, Lack of change in tissue pO2 of 9L tumors in the control group injected with phosphate buffered saline. Mean ± SD, n = 5 and E, Control group volume measurement, n = 5. F, The expression of rat Glut-1 mRNA was measured in the days following the second of two weekly CPA treatments (140mg/kg i.p.). Metronomic CPA induced a 4-fold increase in rat Glut-1 expression above the untreated level, Mean ± SE n=4 individual tumors per group. Statistical comparisons using a two-tailed Student’s test were performed using Prism software version 4 (Graph-Pad Software, San Diego, CA), *: p< 0.05 and **: p < 0.01.
RESULTS
Metronomic Cyclophosphamide Treatment Significantly Increases Oxygenation in Subcutaneous 9L-GIiosarcoma Tumors
Both the 6-day CPA schedule [27] and continuous CPA in the drinking water regimen are associated with anti-angiogenic activity [3, 9] and are thought to exert their antitumor effect through severe hypoxia or anoxia [3]. However, unlike the weekly schedule of CPA, hypoxia under continuous CPA treatment such as through the drinking water is probably maintained as long as CPA intake is continued [10]. The repetitive and long-term nature of weekly metronomic chemotherapy treatment cycles offers a complex context in which it is difficult to assess the impact of each cycle of treatment. We therefore analyzed tumor responses following two to three cycles of a weekly dose of CPA (140mg/kg BW), when the anti-angiogenic impact of the treatment is clear. Consistent with this choice was our observation that the levels of mouse endothelial TSP-1 (Fig. (1A)), which mediates the anti-angiogenic effect of many angiogenesis inhibitors, including CPA [28, 29], were found to increase dramatically 7 days after the first CPA injection (day 0) and further in the days following the second CPA injection, thus confirming the anti-angiogenic effect of CPA during this time frame. 48 hr following the second intra-peritoneal CPA treatment, rat 9L tumors had a significant increase in pO2 levels (Fig. (1B)). Tumor oxygenation further increased after the 3rd CPA injection and was associated with tumor regression (Fig. (1C)). However, no increase in pO2 level was observed in untreated 9L tumors (Fig. (1D)), while tumors continued to grow (Fig. (1E)). Concomitant with the increased tumor oxygenation glucose transporter-1 (Glut-1) RNA, a limiting factor for glucose consumption and cellular metabolism, [30] was found to increase significantly, suggesting increased uptake of glucose (Fig. (1F)).
Metronomic Cyclophosphamide Treatment Improves Drug Uptake in 9L-Tumors
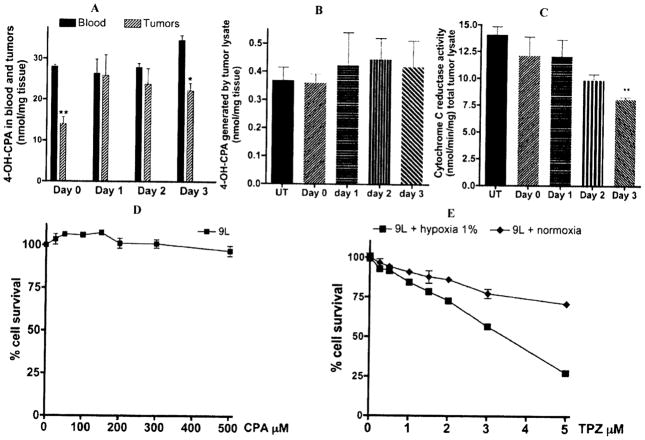
Since metronomic CPA treatment led to a period of increased oxygenation, we examined whether this period could also be associated with increased drug uptake in tumors. We therefore investigated the impact of two cycles of metronomic CPA treatment on liver-activated cyclophosphamide, 4-OH-CPA, flow into 9L tumors. 4OH-CPA was monitored in cardiac blood and in tumor extracts in animals 7 days after the first CPA injection (day 0) and in the days following the second CPA injection (day 1, 2 and 3). 4OH-CPA drug uptake in day 0 tumors reflects the status of drug flow in tumors that were allowed to recover until the 2nd CPA injection. Drug flow was monitored 15 minutes after i.p. injection on each day by a small probing dose of CPA (50mg/kg) as described in “material and methods”. (Fig. (2A)) shows that 7 days after a single CPA injection, tumors allowed a limited flow of 4OH-CPA, which corresponded to about 50 % of the amount found in a similar volume of circulating cardiac blood. We also previously reported similar reduced drug uptake in untreated 9L tumors [24]. However, following the second CPA injection an increased drug uptake by tumors was observed 24 hours later and was maintained up until day 3 post-CPA injection. Blood levels of 4OH-CPA did not differ significantly in animals receiving one or two CPA injections. Moreover, the increase of 4OH-CPA flux in tumors receiving two consecutive CPA injections was not attributable to increased intra-tumoral activation of CPA, since no increase in CPA activation (Fig. (2B)) or NADPH-P450 reductase activity (Fig. (2C)) was observed in assays using tumor extracts from animals undergoing metronomic CPA treatment. Increased drug access and the increased oxygenation observed during metronomic CPA are suggestive of tumor vasculature normalization [31].
Fig. 2.
Rat 9L gliosarcoma tumors were grown in scid mice. When the tumor size reached 1 cm3, CPA was injected as a single dose at 140mg/Kg BW i.p. every 7 days (day 0 corresponds to 7 days after one CPA injection, and day 1, 2 or 3 correspond to 1, 2, or 3 days after the 2nd of two CPA injections). A, To assess drug entry in tumors after CPA treatment, the amount of 4-OH-CPA in the blood and tumors were measured in animals killed 15 min after injection of a small test dose of 50 mg/kg CPA i.p. at day 0, 1,2, and 3 after the second CPA injection. Cardiac blood and tumor homogenates (n=4 tumors) were processed to determine their content of activated CPA. *: p< 0.05 and **: p< 0.01. B, Activated CPA metabolite, CPA activation by tumor extracts was assayed as previously described [24]. C, P450 reductase activity was assayed using 20 μg of tumor extracts, lysed by sonication and then assayed for P450R-catalyzed, NADPH-dependent cytochrome C reduction (DA550) at 30°C. Cytotoxicity assays – Cells were plated in triplicate at 4000 cells/well of a 48-well plate 18–24 hr prior to drug treatment. D, Cells treated with CPA (0 to 500 μM) were incubated for 4 days under normoxia. E, Cells treated with TPZ (0 to 5 μM), were incubated for 4 days in a tissue culture incubator maintained under hypoxic conditions 1% O2, or under normoxic conditions 19.6% O2. Cell viability after this time was determined using a crystal violet/alcohol-extraction assay. Data are presented as cell number relative to drug-free controls, mean ± SD.
Impact of Timed Weekly TPZ Administration on Tumors Undergoing Anti-Angiogenic CPA Treatment
9L cells do not express detectable cytochrome P450 enzymes known to activate CPA and consequently are totally insensitive to CPA in cell culture (Fig. (2D)). However, 9L cells express reasonable cytochrome P450-reductase activity (~24 nmol/min/mg cell lysate) and were able to activate TPZ more effectively under hypoxic conditions (Fig. (2E)). The enhanced oxygenation of tumors following metronomic CPA treatment is anticipated to reduce the efficacy of TPZ, and consequently a better anti-tumor effect is expected only when pO2 is lowest. Using 9L tumors (tumor size ~350mm3 at 1st CPA injection), we examined the impact of administering TPZ at different days during anti-angiogenic CPA treatment (1,2 and 3 days following each weekly CPA injection). Due to the long-term nature of this combination treatment and to avoid cumulative toxicity, we used a relatively small weekly dose of TPZ (5mg/kg BW). Table 1 shows that in the absence of any treatment 9L tumors showed a very aggressive growth leading to animal termination 15 days after the start of treatment. Similarly, animals that received a regimen consisting of only weekly TPZ (without CPA) experienced aggressive tumor growth, indicating the inability of this low TPZ dose to impact tumor growth. In accordance with previous experiments, weekly CPA administration affected tumor growth after the 3rd CPA injection and lead to dramatic tumor regression and the eradication of one tumor (n=8 tumors, Table 1). Co-administration of TPZ on day 1 and day 2 did not improve the efficacy of weekly CPA and did not result in reduced tumor growth at the initial phase of treatment or tumor eradication at the end of treatment, suggesting that TPZ administration on day 1 or day 2 did not substantially enhance its activation and anti-tumor activity. However, a statistically significant improvement was observed when TPZ was given on day 3, resulting in a faster tumor response (immediately after the 2nd injection), reduced tumor growth, longer tumor free periods and eradication of 50% of tumors. Overall, CPA/TPZ combinations were not associated with any observable signs of toxicity or severe animal weight losses (Table 1 and supplementary (Fig. (1)).
Table 1. Impact Timed Weekly TPZ Administration During Metronomic CPA Treatment on 9L Tumors (~350mm3).
Rat 9L gliosarcoma tumors were grown in male ICR scid mice. When tumor size reached ~350 mm3 (Table 1) or ~1200 mm3 (Table 2), drugs CPA was injected at a single dose of 140mg/Kg BW i.p. every 7 days, while TPZ was injected at 5mg/Kg every 7 days alone or in combination with CPA (140mg/Kg. i.p.) at days 1, 2, and 3 (Table 1) or at days 3, 4, 5 and 6 (Table 2) after each CPA injection. Tumor sizes were monitored twice a week along with BWs. A total of 9 CPA and 9 TPZ injections were used to treat all CPA and TPZ groups thus receiving the same cumulative doses of the drugs. Untreated and animals treated with CPA/TPZ at day 6 were killed due to large tumor sizes. Statistical comparison of treatment impacts at the initial phase of treatment (period between 2nd and 4th CPA/TPZ injections) between day 1 and 2 compared to day 3 (*: p<0.05), and at the end of treatment between days 1 and 2 compared to day 3 (*: p<0.05 and **: p<0.01 respectively) using One way anova Bonferroni’s Multiple Comparison test were performed using Prism software version 4 (GraphPad Software, San Diego, CA)
| Initial Response to CPA | |||||||
|---|---|---|---|---|---|---|---|
| Initial Tumor Vola | Continued Tumor Growth Periodb | Tumor Size Increasec | Maximal Tumor Regressiond | Tumor-free Periode | Tumors Eradicatedf | Body Weight Lossg | |
| mm3 | days | % | % | days | number | % | |
| 9L | 352 ± 55 | 15 (killed) | 2071 ± 437 | 0 | 0 | 0/8 | N/A |
| 9L + CPA/7 d | 346 ± 102 | 11–19 (16.7 ± 3.2) | 671 ± 213 | 100(3/8) | 27 | 1/8 | 7.4% |
| 9L + TPZ/7 d | 375 ± 80 | 15 (killed) | 2349 ± 514 | 0 | 0 | 0/8 | N/A |
| 9L + CPA7/TPZd1 | 342 ± 72 | 8–15 (12.7 ± 3.2) | 678 ± 142 | 100(5/8) | 25 ± 7.15 | 1/8 | 2.4% |
| 9L + CPA7/TPZ d2 | 379 ± 55 | 11–19 (16 ± 3.5) | 612 ± 96 | 100 (4/8) | 33 ± 11.5 | 1/8 | 4.6% |
| 9L + CPA7/TPZ d3 | 336 ± 66 | 8–19 (14.1 ± 4.4) | 463 ± 72 | 100(8/8) | 53 ± 2**/* | 4/8 | 1% |
Time, measured in days after first CPA injection, until tumor regression was first detected in tumor size measurements taken twice/week. Data are expressed as a range exhibited by the individual tumors in each group. Untreated 9L tumor controls, 9L TPZ treated tumors (Table 1) and 9L + CPA7/TPZ d6 (Table 2) did not regress; mice were killed 15 d or 21 d after initiation of CPA treatment in the other groups.
Percentage increase in tumor size from time of first CPA injection until mice were killed (untreated 9L controls) or tumor regression was first observed (treated tumors), mean ± SE.
Maximal decrease in tumor size, which generally was achieved after 6–9 CPA/TPZ injections. Percentage regression values were calculated based on the vol of each tumor at the time of first CPA injection (c.f., column 1) (mean ± SE).
Length of tumor-free periods before growth resumed after cessation of CPA/TPZ treatment. Data are expressed as mean ± SE days.
Number of tumors that were not palpable during the experiment and did not regrow.
Body weight loss was calculated as the change from untreated body weight of animals at the start of treatment.
Tumor vol at time of first CPA injection, mean ± SE for (n=8, 4 animals/group) individual tumors, calculated from measured tumor areas.
Impact of Tirapazamine Schedules on Anti-Angiogenic CPA Efficacy
We next examined the impact of scheduling TPZ administration at later days (3, 4, 5 and 6, which is 24 hours before the following weekly CPA injection), compared to day 3, which showed the best response in earlier experiments. However, we used a larger tumor size ~1200mm3 at the start of treatment vs. 350mm in Table 1. Table 2 shows that administration of TPZ at days 3, 4, and 5 produced similar initial anti-tumor effects and resulted in dramatic tumor regression in all groups. TPZ administration at day 5 resulted in the eradication of 50% of tumors, an effect not seen with the other groups. Surprisingly, TPZ administration at day 6 resulted in a far less effective anti-tumor regimen as tumor growth did not differ significantly from that of untreated tumors leading to animal termination due to enormous tumor sizes (Table 2 and supplementary (Fig. (2)).
Table 2. Impact Timed Weekly TPZ Administration During Metronomic CPA Treatment on 9L Tumors (~1200mm3).
Rat 9L gliosarcoma tumors were grown in male ICR scid mice. When tumor size reached ~350 mm3 (Table 1) or ~1200 mm3 (Table 2), drugs CPA was injected at a single dose of 140mg/Kg BW i.p. every 7 days, while TPZ was injected at 5mg/Kg every 7 days alone or in combination with CPA (140mg/Kg. i.p.) at days 1, 2, and 3 (Table 1) or at days 3, 4, 5 and 6 (Table 2) after each CPA injection. Tumor sizes were monitored twice a week along with BWs. A total of 9 CPA and 9 TPZ injections were used to treat all CPA and TPZ groups thus receiving the same cumulative doses of the drugs. Untreated and animals treated with CPA/TPZ at day 6 were killed due to large tumor sizes.Statistical comparison of treatment impacts at the initial phase of treatment (period between 2nd and 4th CPA/TPZ injections) between day 1 and 2 compared to day 3 (*: p<0.05), and at the end of treatment between days 1 and 2 compared to day 3 (*: p<0.05 and **: p<0.01 respectively) using One way anova Bonferroni’s Multiple Comparison test were performed using Prism software version 4 (GraphPad Software, San Diego, CA).
| Initial Response to CPA | |||||||
|---|---|---|---|---|---|---|---|
| Initial Tumor Vola | Continued Tumor Growth Periodb | Tumor Size Increasec | Maximal Tumor Regressiond | Tumor-free Periode | Tumors Eradicatedf | Body Weight Lossg | |
| mm3 | days | % | % | days | number | ||
| 9L | 1250 ± 444 | 21 (killed) | 548 ± 107 | 0 | 0 | 0/8 | N/A |
| 9L + CPA7/TPZ d3 | 1170 ± 270 | 8–19 (12.5 ± 4.2) | 345 ± 66.8 | 100(5/8) | 29.2 ± 8.8 | 1/8 | 6.7% |
| 9L + CPA7/TPZ d4 | 1184 ± 411 | 8–15 (13.2 ± 3.2) | 364 ± 119 | 100(8/8) | 38 ± 3.9 | 1/8 | 6.4% |
| 9L + CPA7/TPZ d5 | 1296 ± 522 | 8–15 (12.4 ± 3.6) | 316 ± 113 | 100(8/8) | 42.5 ± 7.4 | 4/8 | 0.6% |
| 9L + CPA7/TPZ d6 | 1263 ± 375 | 15 (killed) | 473 ± 118 | 0 | 0 | 0/8 | N/A |
Time, measured in days after first CPA injection, until tumor regression was first detected in tumor size measurements taken twice/week. Data are expressed as a range exhibited by the individual tumors in each group. Untreated 9L tumor controls, 9L TPZ treated tumors (Table 1) and 9L + CPA7/TPZ d6 (Table 2) did not regress; mice were killed 15 d or 21 d after initiation of CPA treatment in the other groups.
Percentage increase in tumor size from time of first CPA injection until mice were killed (untreated 9L controls) or tumor regression was first observed (treated tumors), mean ± SE.
Maximal decrease in tumor size, which generally was achieved after 6–9 CPA/TPZ injections. Percentage regression values were calculated based on the vol of each tumor at the time of first CPA injection (c.f., column 1) (mean ± SE).
Length of tumor-free periods before growth resumed after cessation of CPA/TPZ treatment. Data are expressed as mean ± SE days.
Number of tumors that were not palpable during the experiment and did not regrow.
Body weight loss was calculated as the change from untreated body weight of animals at the start of treatment.
Tumor vol at time of first CPA injection, mean ± SE for (n=8, 4 animals/group) individual tumors, calculated from measured tumor areas.
Impact of Initial Tumor Size on the Efficacy of CPA/TPZ Scheduling
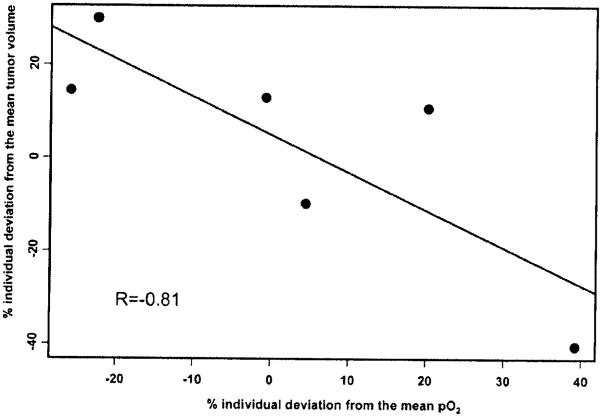
The efficacy of administering TPZ at day 3 in the context of metronomic CPA showed an apparent dependency on tumor sizes at the start of treatment. TPZ administration at day 3 using large tumors (~1200mm3) was not as effective as for smaller tumors (~350 mm3) (Tables 1 and 2). Under the same CPA/TPZ schedule, small tumors showed complete regression, extended tumor-free periods and tumor eradication of 50% of tumors compared to only 12% in the large tumor group. In tumors undergoing metronomic CPA we found an inverse correlation between tumor pO2 and tumor volume (Fig. (3)). In order to correlate the individual tumor volume with pO2, we estimated the respective random effects from the mixed models, which allowed the computation of individual deviations of the tumor volume and pO2 from the group mean. Results indicate a strong negative correlation (−0.81) between the changes in tumor pO2 and tumor volume during 21 days of metronomic chemotherapy. These results indicate that the decrease in tumor volume during treatment will result in an increase in tumor pO2 and could possibly be associated with increased TPZ uptake. More importantly, these changes in tumor pO2 could be used to predict approximately 60% of the changes in tumor volume (the coefficient of determination is 0.812 = 0.6) during metronomic chemotherapy. However, no correlation of tumor pO2 with tumor volume was found in the control group (the correlation coefficient is 0.06, data not shown). These results indicate that tumor pO2 could be potentially used as a prognostic indicator of the effectiveness of the chemotherapy. Additionally, this information could also be used to optimize therapies by timing subsequent doses at times of enhanced tumor oxygenation.
Fig. 3.
Plot of % individual deviation of pO2 versus tumor volume estimated from the mixed model described in Materials and Methods. Each point of the graph represents one mouse. There is a strong negative correlation (−0.81) between these two variables on an individual basis.
Expression of Angiogenesis Factors During Metronomic CPA
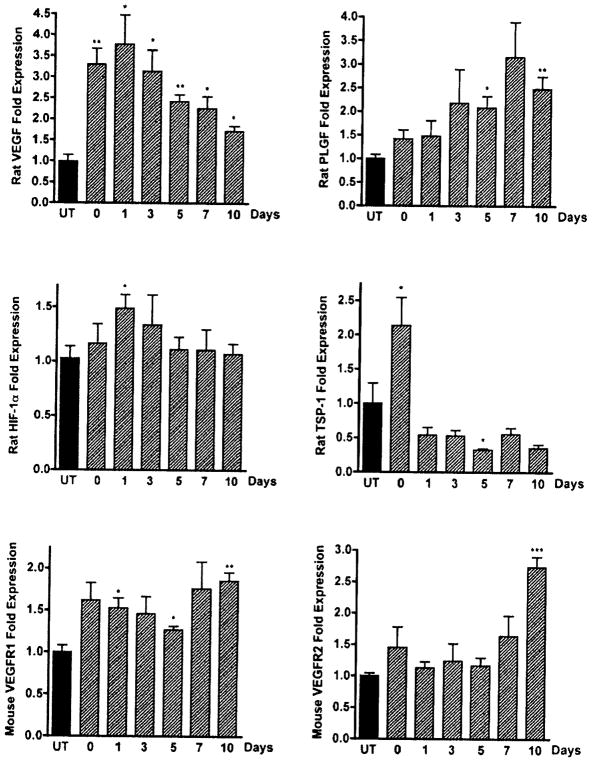
Metronomic CPA chemotherapy against 9L gliosarcoma tumors induced dramatic increases in mouse endothelial TSP-1 (Fig. (1A)). However, 9L rat TSP-1 showed a very different expression pattern with an ~2-fold increase after the 1st CPA injection, followed by a 2-fold decrease after the 2nd CPA injection (Fig. (4)). The transcriptional decline of 9L rat tumor TSP-1 while host TSP-1 increased might indicate a pro-angiogenic effort of the tumor. The expression of other pro-angiogenic factors increased throughout the treatment (Fig. (4)). Rat VEGF in particular reached a maximal level of expression following the 2nd CPA injection and then declined following pO2 increases. The maximal increase of VEGF occurred prior to the onset of increased pO2 during this treatment and coincides with a marginal increase of master hypoxia-effector HIF-1α transcripts in 9L-treated tumors. Interestingly, maximal rat PLGF expression levels were reached 7 to 10 days after the 2nd CPA injection, and showed a sustained gradual increase that extended after VEGF began to decline. The temporal delay between the maximal expression of VEGF and PLGF in this model suggests distinct temporal and perhaps spatial functions within the tumor. In the absence of CPA treatment this phase is thought to coincide with tumor vasculature recovery, suggesting that PLGF secreted by rat tumor cells might be involved in this reconstructive phase between two CPA injections. PLGF like VEGF, is known to signal through VEGFR1 and to cross talk with VEGFR2 and is involved in the mobilization of endothelial progenitor cells. However, the expression of VEGFR2 did not change significantly until 10 days after treatment cessation (Fig. (4)).
Fig. 4.
Expression profiles of factors involved in tumor angiogenesis during metronomic chemotherapy. Relative levels of rat VEGF, TSP-I, PLGF, HIF-1α, and mouse VEGF receptors 1 and 2 in 9L tumors were quantified by real-time PCR (qPCR) using SYBR Green 1 chemistry following the second of two weekly CPA treatments (140mg/kg i.p.). Total RNA was isolated from frozen tumor samples (0.1–0.4 g) using TRIzol reagent according to the manufacturer’s protocol. Ct values determined for each mRNA were normalized to the 18S rRNA content of each RNA sample and are plotted as fold increase over untreated tumor samples. Data are represented as mean ± SE relative mRNA levels of at least four individual tumors per group. *: p< 0.05 and **: p< 0.01.
We have found that the combination of CPA and TPZ is efficacious when TPZ is administered in a time-specific manner. However, this enhanced therapeutic effect did not correlate with the occurrence of hypoxia in tumors, but rather with increased tumor oxygenation, suggesting that moderate hypoxia and increased drug penetration are beneficial to intratumoral TPZ activation. The significantly increased tumor oxygenation at 48 hr post-2nd CPA injection coincided with maximal expression of VEGF, which peaked 24 hours before pO2 increase, while PLGF showed a steady increase during the treatment. As an indicator of the strong anti-angiogenic component of this treatment host mouse endothelial thrombospondin TSP-1 increased dramatically.
DISCUSSION
CPA and its isomer Ifosfamide (IFA) are activated by a 4-hydroxylation reaction mediated by several CYP enzymes with electron input from the flavoenzyme NADPH P450 reductase. CPA in combination with other drugs is used in various chemotherapy protocols. The pharmacokinetic of CPA is not easily predictable since CYP enzymes are inducible and vary in content between individuals [32]. Drug interactions in high dose chemotherapy and inter-individual P450 polymorphism could lead to severe toxicities in some patients. This suggests the need to tailor complex individualized protocols for high-dose chemotherapy, a prospect that is currently not practical.
Metronomic chemotherapy provides substantial advantages in comparison to high dose chemotherapy. Using substantially lower doses, fewer if any side effects are observed. The absence of toxicity allows for repeated treatment and in the case of certain alkylating agents an effective anti-angiogenic component was reported. In the case of CPA, this anti-angiogenic component is evident when the drug is administered in small doses either repeatedly or continuously [27, 33]. Under these treatments the rest period between administrations is either short or absent leaving less time for endothelial cells to recover leading to capillary “drop out” and tumor collapse. Several chemotherapeutic drugs were identified to induce anti-angiogenic effects similar to those of CPA. Drugs such as paclitaxel and docetaxel administered on a weekly basis at 30–40% of their MTDs were found to be effective treatments in women who had not benefited from MTD treatments every 3 weeks [34, 35]. Low, frequent doses of methotrexate were shown to be anti-angiogenic in in vitro and in vivo studies [36–38]. The underlying mechanism behind the anti-angiogenic activity of metronomic CPA treatment is not fully understood. However, the high sensitivity of proliferating endothelial cells to several anti-cancer drugs makes them a prime target of metronomic chemotherapy [39].
Most anti-angiogenic drugs administered as single agents can elicit only modest responses [40, 41] and are not able to enhance patient survival in clinical trials [42]. However, angiogenesis inhibitors in combination with chemotherapy produced remarkable increases in survival of colorectal cancer patients [43]. Administration of chemotherapeutic drugs in combination with anti-angiogenic compounds is, however, a conundrum since the destruction of tumor vasculature would severely compromise their delivery to solid tumors [44] or render them less susceptible to radiation as a result of a reduced oxygenation [45, 46].
A previous report using daily metronomic CPA (in drinking water ~20mg/kg/day) showed an increase of hypoxia in tumors. Combination of this treatment with weekly TPZ led to substantial growth delays of prostate and breast tumor xenografts but no tumor eradication. We found that in the course of our study and especially following the second CPA injection, the levels of pO2 increased significantly. Moreover, expression of glucose transporter glut-1, a reliable marker of glucose uptake, increased suggesting higher metabolic activity in 9L tumor cells.
Consistently, the increased oxygenation was associated with improved uptake of activated CPA into tumors to reach comparable levels found in circulating blood. These temporal increases in tumor oxygenation and drug uptake are suggestive of tumor blood vessel normalization and clearly confirm the association between increased oxygenation and drug penetration described earlier [31]. Administration of TPZ at day 1 or day 2 (Table 1) in the context of metronomic CPA did not yield a better anti-tumor effect and was less effective than when TPZ was given on day 3 (Table 1) or day 5 (Table 2). While we found that activated CPA entry into tumors is increased at day 1, it is not clear however if similar increases could be observed for TPZ or other drugs. Unlike 4-OH-CPA entry, TPZ entry into tumors is impacted by its metabolism [47, 48], which translates into a gradual depletion of diffusing TPZ through tumor cell layers, thus preventing its penetration deep into tumors. This phenomenon could be exacerbated in severely hypoxic tumors or large tumors and could potentially worsen in tumors overexpressing NADPH-P450 reductase (Jounaidi et al unpublished data). Several reports describe the ineffectiveness of TPZ when used as a monotherapy and the present report confirms those findings. This inefficacy might be due to two paradoxical physiological conditions, which are widespread in tumors; TPZ may either be unable to access severely hypoxic regions of tumors due to being prematurely metabolized or transit without activation through well-oxygenated tumor regions. Therefore, TPZ will be activated in a productive and effective fashion only when relatively efficient drug uptake and mild hypoxia are widespread in tumors. The counter-intuitive proposal of enhancing tumor oxygenation in order to enhance hypoxia-activated drug activation could be explained by the possibility that tumor oxygenation can improve bioreductive drug entry into tumors. Thus, the kinetic of the conversion from prodrug to toxic, active drug metabolite is slowed thereby allowing more time for the drug to penetrate the tumor and increase not absolute drug activation but intra-tumoral drug activation. In this regard, the mild levels of hypoxia observed on days 3 to 5 (Fig. (IF)), could reflect these conditions. However, the efficacy of administering TPZ at day 3 was also dependent on initial tumor size at the start of treatment. Large tumors offer a greater physical resistance for extravascular transit and could reduce TPZ efficacy and penetration deep into tumors.
By virtue of its own potential anti-angiogenic effects [15] and vasoactive properties TPZ can modulate blood flow in tumors [16]. This impact could possibly explain why TPZ administration on day 6 (one day prior to the next CPA injection, Table 2) translated into poor antitumor activity of metronomic CPA, leading to massive tumor growth and termination of animals. However, this result should be confirmed on smaller tumor size, as bigger sizes do not allow a long term tumor monitoring due to excessive tumor burden.
The anti-angiogenic effect of metronomic CPA was reflected by increased endogenous angiogenesis inhibitor thrombospondin-1 [28]. A high basal serum TSP-1 level correlates with prolonged response to metronomic treatment [49]. In the course of this metronomic CPA treatment, the global changes that occur in 9L tumors could be described in two phases: an initial response of the tumor (days 1–2), as manifested by changes in drug flow, a moderate increase in oxygenation and increased metabolism with maximal VEGF induction, followed by massive endothelial and tumor cell death [24, 27], and the following phase (days 3–7), which corresponds to recovery and rebuilding of tumor vasculature until the next round of treatment is applied, as characterized by the continued increases in 9L rat PLGF and a significant increase in pO2 levels. Our finding of a temporal delay between the maximal induction of VEGF and PLGF expression in our model suggests different functions within the tumor. The gradual steady increase in PLGF suggests a role in the following phase of tumor recovery. PLGF, like VEGF, was shown to signal through VEGFR1 and to cross talk with VEGFR2, and is involved in the mobilization of hematopoietic stem cells and EPCs [50].
The induction of VEGF is not in conflict with the anti-angiogenic nature of weekly CPA administration since this induction occurred prior to pO2 increase and then declined. Moreover, 9L tumors, which produce rat VEGF are probably less damaged after the second CPA injection comparatively to mouse endothelial cells which to a large extent bear the brunt of this treatment but recover between two cycles of treatment [27].
The overexpression of glucose transporter glut-1, a reliable marker of glucose uptake, suggests higher metabolic activity in 9L tumor cells. This increased uptake is supported by improved uptake of activated CPA into tumors to reach comparable levels found in circulating blood. It is possible this increased cellular metabolism could create an oxygen gradient whereby cells are experiencing some levels of moderate hypoxia, thus explaining the increase in VEGF. However, since TSP-1 increases due to the anti-angiogenic effect of weekly CPA, we expect endothelial cell death, which automatically will lead to some sort of hypoxic microenvironment. This sort of feedback loop between increased tumor metabolic activity and endothelial cell death could possibly explain our observations. Another potential explanation of VEGF induction during increased oxygenation is a hypoxia-independent VEGF induction by yet an unknown mechanism.
The reduced effectiveness of TPZ administration before pO2 increase, compared to days 3–5 when pO2 levels are significantly higher, is suggestive of complex tumor conditions that will potentiate TPZ activation in tumors. These conditions could be dependent on TPZ tumor penetration, pO2 levels, as well as tumor size and potentially vascular normalization [31]. Our results indicate that the hypoxia-activated prodrug TPZ can exert a superior anticancer activity in increasingly oxygenated tumors experiencing higher drug uptake.
In conclusion, our model of weekly CPA and timed weekly TPZ led to substantial anti-tumor effect with tumor eradication. The efficacy of this regimen was however not directly associated with hypoxia, but was rather enhanced when tumor oxygenation and drug uptake increased and coincided with a time period potentially bearing the characteristics of normalization. The efficacy of this regimen was also dependent on tumor size. Our results indicate that decreases in tumor volume correlate with increases in tumor pO2. This is likely to be associated with an increased drug penetration due to smaller tumor radius and increased perfusion. More importantly, these results indicate that changes in tumor pO2 may be used to predict approximately 60% of tumor volume changes (the coefficient of determination is 0.812 = 0.6) during metronomic chemotherapy. Repeated non-invasive measurements of tumor pO2 could potentially be used to optimize chemotherapy by scheduling multidrug regimens at times of optimal tumor oxygenation. The combination of weekly CPA and TPZ was well tolerated and did not translate into any apparent toxicity. Future investigations will examine the impact of higher dosages of TPZ and the use of other classes of bioreductive drugs with better pharmacokinetic properties and greater tumor diffusion.
Supplementary Material
Acknowledgments
Supported by a grant from the Susan G. Komen for the cure Foundation grant BCTR0504032 (YJ), NIH Grant CA120919 (NK, ED, SH), and NIH CA49248 (DJW). We thank Chong-Sheng Chen and David Waxman (DJW) for their assistance, suggestions and advice.
ABBREVIATIONS
- CPA
Cyclophosphamide
- EPR
Electron Paramagnetic Resonance
- Glut-1
Glucose transporter-1
- MTD
Maximally Tolerated Dose
- PLGF
Placental Growth Factor
- TPZ
Tirapazamine
- TSP-1
Thrombospondin-1
- VEGF
Vascular Endothelial Growth Factor
- VEGFR1,2
VEGF receptor 1 or 2
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers website along with the published article.
References
- 1.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 2.Gulledge CJ, Dewhirst MW. Tumor oxygenation: a matter of supply and demand. Anticancer Res. 1996;16:741–749. [PubMed] [Google Scholar]
- 3.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 4.Ma J, Waxman DJ. Modulation of the antitumor activity of metronomic cyclophosphamide by the angiogenesis inhibitor axitinib. Mol Cancer Ther. 2008;7:79–89. doi: 10.1158/1535-7163.MCT-07-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Glade Bender J, Cooney EM, Kandel JJ, Yamashiro DJ. Vascular remodeling and clinical resistance to antiangiogenic cancer therapy. Drug Resist Updat. 2004;7:289–300. doi: 10.1016/j.drup.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Yu JL, Rak JW, Coomber BL, Hicklin DJ, Kerbel RS. Effect of p53 status on tumor response to antiangiogenic therapy. Science. 2002;295:1526–1528. doi: 10.1126/science.1068327. [DOI] [PubMed] [Google Scholar]
- 8.Marx J. Cancer. Encouraging results for second-generation antiangiogenesis drugs. Science. 2005;308:1248–1249. doi: 10.1126/science.308.5726.1248. [DOI] [PubMed] [Google Scholar]
- 9.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–436. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 10.Emmenegger U, Morton GC, Francia G, Shaked Y, Franco M, Weinerman A, Man S, Kerbel RS. Low-dose metronomic daily cyclophosphamide and weekly tirapazamine: a well-tolerated combination regimen with enhanced efficacy that exploits tumor hypoxia. Cancer Res. 2006;66:1664–1674. doi: 10.1158/0008-5472.CAN-05-2598. [DOI] [PubMed] [Google Scholar]
- 11.Zeman EM, Brown JM, Lemmon MJ, Hirst VK, Lee WW. SR-4233: a new bioreductive agent with high selective toxicity for hypoxic mammalian cells. Int J Radiat Oncol Biol Phys. 1986;12:1239–1242. doi: 10.1016/0360-3016(86)90267-1. [DOI] [PubMed] [Google Scholar]
- 12.Fitzsimmons SA, Lewis AD, Riley RJ, Workman P. Reduction of 3-amino-1,2,4-benzotriazine-1,4-di-N-oxide (tirapazamine, WIN 59075, SR 4233) to a DNA-damaging species: a direct role for NADPH:cytochrome P450 oxidoreductase. Carcinogenesis. 1994;15:1503–1510. doi: 10.1093/carcin/15.8.1503. [DOI] [PubMed] [Google Scholar]
- 13.Jones GD, Weinfeld M. Dual action of tirapazamine in the induction of DNA strand breaks. Cancer Res. 1996;56:1584–1590. [PubMed] [Google Scholar]
- 14.Yu LJ, Matias J, Scudiero DA, Hite KM, Monks A, Sausville EA, Waxman DJ. P450 enzyme expression patterns in the NCI human tumor cell line panel. Drug Metab Dispos. 2001;29:304–312. [PubMed] [Google Scholar]
- 15.Nagasawa H, Yamashita M, Mikamo N, Shimamura M, Oka S, Uto Y, Hori H. Design, synthesis and biological activities of antiangiogenic hypoxic cytotoxin, triazine-N-oxide derivatives. Comp Biochem Physiol A Mol Integr Physiol. 2002;132:33–40. doi: 10.1016/s1095-6433(01)00526-8. [DOI] [PubMed] [Google Scholar]
- 16.Durand RE, Olive PL. Physiologic and cytotoxic effects of tirapazamine in tumor-bearing mice. Radiat Oncol Investig. 1997;5:213–219. doi: 10.1002/(SICI)1520-6823(1997)5:5<213::AID-ROI1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Jounaidi Y, Hecht JED, Waxman DJ. Retroviral transfer of human cytochrome P450 genes for oxazaphosphorine-based cancer gene therapy. Cancer Res. 1998;58:4391–4401. [PubMed] [Google Scholar]
- 18.Hou H, Khan N, Grinberg OY, Yu H, Grinberg SA, Lu S, Demidenko E, Steffen RP, Swartz HM. The effects of Efaproxyn (efaproxiral) on subcutaneous RIF-1 tumor oxygenation and enhancement of radiotherapy-mediated inhibition of tumor growth in mice. Radial Res. 2007;168:218–225. doi: 10.1667/RR0962.1. [DOI] [PubMed] [Google Scholar]
- 19.Hou H, Khan N, O’Hara JA, Grinberg OY, Dunn JF, Abajian MA, Wilmot CM, Makki M, Demidenko E, Lu S, Steffen RP, Swartz HM. Effect of RSR13, an allosteric hemoglobin modifier, on oxygenation in murine tumors: an in vivo electron paramagnetic resonance oximetry and bold MRI study. Int J Radial Oncol Biol Phys. 2004;59:834–843. doi: 10.1016/j.ijrobp.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 20.Hon H, Lariviere JP, Demidenko E, Gladstone D, Swartz H, Khan N. Repeated tumor pO(2) measurements by multi-site EPR oximetry as a prognostic marker for enhanced therapeutic efficacy of fractionated radiotherapy. Radiother Oncol. 2009;91:126–131. doi: 10.1016/j.radonc.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan N, Williams BB, Hou H, Li H, Swartz HM. Repetitive tissue pO2 measurements by electron paramagnetic resonance oximetry: current status and future potential for experimental and clinical studies. Antioxid Redox Signal. 2007;9:1169–1182. doi: 10.1089/ars.2007.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swartz HM, Clarkson RB. The measurement of oxygen in vivo using EPR techniques. Phys Med Biol. 1998;43:1957–1975. doi: 10.1088/0031-9155/43/7/017. [DOI] [PubMed] [Google Scholar]
- 23.Liu KJ, Gast P, Moussavi M, Norby SW, Vahidi N, Walczak T, Wu M, Swartz HM. Lithium phthalocyanine: a probe for electron paramagnetic resonance oximetry in viable biological systems. Proc Natl Acad Sci USA. 1993;90:5438–5442. doi: 10.1073/pnas.90.12.5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen CS, Jounaidi Y, Su T, Waxman DJ. Enhancement of intratumoral cyclophosphamide pharmacokinetics and antitumor activity in a P450 2B11-based cancer gene therapy model. Cancer Gene Ther. 2007;14:935–944. doi: 10.1038/sj.cgt.7701092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qu A. Mixed Models. Theory and Applications. In: Demidenko E, editor. Biometrics. Vol. 62. 2006. pp. 304–305. [Google Scholar]
- 26.Demidenko E. The assessment of tumour response to treatment. J R Stat Soc Ser C (Appl Stat) 2006;55:365–377. [Google Scholar]
- 27.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- 28.Hamano Y, Sugimoto H, Soubasakos MA, Kieran M, Olsen BR, Lawler J, Sudhakar A, Kalluri R. Thrombospondin-1 associated with tumor microenvironment contributes to low-dose cyclophosphamide-mediated endothelial cell apoptosis and tumor growth suppression. Cancer Res. 2004;64:1570–1574. doi: 10.1158/0008-5472.can-03-3126. [DOI] [PubMed] [Google Scholar]
- 29.Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci USA. 2003;100:12917–12922. doi: 10.1073/pnas.2135406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654–662. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- 31.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 32.Scripture CD, Sparreboom A, Figg WD. Modulation of cytochrome P450 activity: implications for cancer therapy. Lancet Oncol. 2005;6:780–789. doi: 10.1016/S1470-2045(05)70388-0. [DOI] [PubMed] [Google Scholar]
- 33.Man S, Bocci G, Francia G, Green SK, Jothy S, Hanahan D, Bohlen P, Hicklin DJ, Bergers G, Kerbel RS. Antitumor effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water. Cancer Res. 2002;62:2731–2735. [PubMed] [Google Scholar]
- 34.Burstein HJ, Manola J, Younger J, Parker LM, Bunnell CA, Scheib R, Matulonis UA, Garber JE, Clarke KD, Shulman LN, Winer EP. Docetaxel administered on a weekly basis for metastatic breast cancer. J Clin Oncol. 2000;18:1212–1219. doi: 10.1200/JCO.2000.18.6.1212. [DOI] [PubMed] [Google Scholar]
- 35.Fennelly D, Aghajanian C, Shapiro F, O’Flaherty C, McKenzie M, O’Connor C, Tong W, Norton L, Spriggs D. Phase I and pharmacologic study of paclitaxel administered weekly in patients with relapsed ovarian cancer. J Clin Oncol. 1997;15:187–192. doi: 10.1200/JCO.1997.15.1.187. [DOI] [PubMed] [Google Scholar]
- 36.Joussen AM, Kruse FE, Volcker HE, Kirchhof B. Topical application of methotrexate for inhibition of corneal angiogenesis. Graefes Arch Clin Exp Ophthalmol. 1999;237:920–927. doi: 10.1007/s004170050387. [DOI] [PubMed] [Google Scholar]
- 37.Hirata S, Matsubara T, Saura R, Tateishi H, Hirohata K. Inhibition of in vitro vascular endothelial cell proliferation and in vivo neovascularization by low-dose methotrexate. Arthritis Rheum. 1989;32:1065–1073. doi: 10.1002/anr.1780320903. [DOI] [PubMed] [Google Scholar]
- 38.Colleoni M, Rocca A, Sandri MT, Zorzino L, Masci G, Nole F, Peruzzotti G, Robertson C, Orlando L, Cinieri S, de BF, Viale G, Goldhirsch A. Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol. 2002;13:73–80. doi: 10.1093/annonc/mdf013. [DOI] [PubMed] [Google Scholar]
- 39.Bocci G, Nicolaou KC, Kerbel RS. Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs. Cancer Res. 2002;62:6938–6943. [PubMed] [Google Scholar]
- 40.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cobleigh MA, Langmuir VK, Sledge GW, Miller KD, Haney L, Novotny WF, Reimann JD, Vassel A. A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol. 2003;30:117–124. doi: 10.1053/j.seminoncol.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 42.Mayer RJ. Two steps forward in the treatment of colorectal cancer. N Engl J Med. 2004;350:2406–2408. doi: 10.1056/NEJMe048098. [DOI] [PubMed] [Google Scholar]
- 43.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 44.Ma J, Pulfer S, Li S, Chu J, Reed K, Gallo JM. Pharmacodynamic-mediated reduction of temozolomide tumor concentrations by the angiogenesis inhibitor TNP-470. Cancer Res. 2001;61:5491–5498. [PubMed] [Google Scholar]
- 45.Murata R, Nishimura Y, Hiraoka M. An antiangiogenic agent (TNP-470) inhibited reoxygenation during fractionated radiotherapy of murine mammary carcinoma. Int J Radiat Oncol Biol Phys. 1997;37:1107–1113. doi: 10.1016/s0360-3016(96)00628-1. [DOI] [PubMed] [Google Scholar]
- 46.Fenton BM, Paoni SF, Ding I. Effect of VEGF receptor-2 antibody on vascular function and oxygenation in spontaneous and transplanted tumors. Radiother Oncol. 2004;72:221–230. doi: 10.1016/j.radonc.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Hicks KO, Pruijn FB, Secomb TW, Hay MP, Hsu R, Brown JM, Denny WA, Dewhirst MW, Wilson WR. Use of three-dimensional tissue cultures to model extravascular transport and predict in vivo activity of hypoxia-targeted anticancer drugs. J Natl Cancer Inst. 2006;98:1118–1128. doi: 10.1093/jnci/djj306. [DOI] [PubMed] [Google Scholar]
- 48.Hicks KO, Pruijn FB, Sturman JR, Denny WA, Wilson WR. Multicellular resistance to tirapazamine is due to restricted extravascular transport: a pharmacokinetic/pharmacodynamic study in HT29 multicellular layer cultures. Cancer Res. 2003;63:5970–5977. [PubMed] [Google Scholar]
- 49.Kieran MW, Turner CD, Rubin JB, Chi SN, Zimmerman MA, Chordas C, Klement G, Laforme A, Gordon A, Thomas A, Neuberg D, Browder T, Folkman J. A feasibility trial of antiangiogenic (metronomic) chemotherapy in pediatric patients with recurrent or progressive cancer. J Pediatr Hematol Oncol. 2005;27:573–581. doi: 10.1097/01.mph.0000183863.10792.d4. [DOI] [PubMed] [Google Scholar]
- 50.Carmeliet P. Manipulating angiogenesis in medicine. J Intern Med. 2004;255:538–561. doi: 10.1111/j.1365-2796.2003.01297.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.