Abstract
Several animal models have been used for the study of mechanosensory hair cells and hearing loss. Because of the difficulty of tissue acquisition and large animal size, these traditional models are impractical for high-throughput screening. The zebrafish has emerged as a powerful animal model for screening drugs that cause or prevent hair cell death. The unique characteristics of the zebrafish enable rapid in vivo imaging of hair cells and hair cell death. We have used this model to screen for and identify multiple drugs that protect hair cells from aminoglycoside-induced death. Identification of multiple drugs and drug-like compounds that inhibit multiple hair cell death pathways might enable the development of protective cocktails to achieve complete hair cell protection.
Introduction
Hearing loss is a global problem. Worldwide, there are more than 250 million individuals with moderate-to-severe or greater hearing loss. With an aging global population, it is estimated by the year 2050 there will be approximately 900 million individuals with presbycusis (age-related hearing loss) [1]. Interestingly, hearing loss has not garnered the attention typically given by the pharmaceutical industry to chronic slowly progressing diseases that affect a large percentage of the population. There are no Food and Drug Administration (FDA)-approved drugs for the treatment or prevention of sensorineural hearing loss.
Strategies for treating hearing loss on a molecular and cellular level have typically focused primarily on the hair cells, the end organ of the auditory pathway. Remarkably, hair cells of the inner ear in birds, reptiles, frogs and other aquatic vertebrates have the capacity to regenerate. Hair cells of the mammalian inner ear, however, demonstrate no significant[E1] regenerative capacity. Much current research has thus focused on either stimulating regeneration of mammalian hair cells or preventing existing hair cells from dying. Additional research has focused on promoting survival of the spiral ganglion cells that innervate the hair cells [2]. There is some evidence that increasing spiral ganglion cell survival might improve the performance of cochlear implants, although other studies indicate that the correlation between ganglion cell survival and perceptual ability using a cochlear implant is marginal. These neurons, therefore, are an additional potential target for hearing preservation, but are out of the scope of this review [3–5].
Opportunities for medical intervention
Four clinical scenarios lend themselves to pharmaceutical intervention and drug discovery, whether through the development of new drugs or the identification of new uses for old drugs: (i) sudden sensorineural hearing loss; (ii) prevention of ototoxic injury (drug- or noise-induced); (iii) prevention of slowly progressive hearing loss (e.g. presbycusis); and (iv) restoration of hearing after permanent hearing loss. From a cellular and molecular standpoint, each of these scenarios presents a different problem. Treatment of sudden sensorineural hearing loss entails treatment of hair cells that have presumably undergone sublethal (and potentially reversible) damage. Prevention of ototoxic injury requires early administration of protective drugs before hair cell death to halt intracellular death pathways or inhibit ototoxic drug uptake. Treatment of progressive hearing loss suggests intervention and blockade of slowly developing hair cell death. Finally, restoration of hearing suggests either restoring function to nonfunctioning hair cells, or creating and/or regenerating new hair cells to repopulate the inner ear. This last scenario (restoration of hearing) will probably require either chemical or genetic modulation of existing hair cells or supporting cells, or introduction of stem cells to create new hair cells. The first three scenarios are particularly applicable to the development of new drugs that protect against hair cell death.
Current candidate protective drugs
A cursory literature search reveals more than 50 candidate drugs that protect hair cells, including (but not limited to) caspase inhibitors, Jun-kinase inhibitors, D-methionine, aspirin and other antioxidants. Although some of these agents (particularly D-methionine and aspirin) have reached the point of clinical trials, none are currently FDA approved. In addition – for those that have reached clinical trials – the protection afforded by agents such as N-acetylcysteine and aspirin has been incomplete [6,7] and might be very dose dependent [8].[E2]
Traditional animal models and advantages of each
Traditionally, several mammals, birds and amphibians have been used to study hair cells. Avian models have the advantage of having an anatomically simple cochlea (basilar papilla) that lacks the coiled, bone-encased characteristics of the mammalian cochlea. This enables easier surgical dissection, histologic evaluation and quantification. In addition, birds have the capacity for hair cell regeneration after damage [9,10]. This ability to regenerate has led to many studies that define crucial differences between the avian and mammalian inner ear. Avian models however, lack the genetic flexibility that is possible in other animal models.
Among mammals, mice have the obvious advantages of being able to house larger numbers at less expense[E3] and offering more genetic opportunities. This includes the availability of knockout, transgenic and mutant mice strains [11,12], as well as the potential for analyzing more complicated interactions by assessment of synergistic effects and quantitative trait loci analyses. A significant[E4] disadvantage of mice, however, is their variable response to ototoxic agents such as aminoglycosides and noise. Mice are known for their notorious resistance to damage from even known ototoxic agents such as aminoglycosides, making evaluation of potentially protective drugs difficult in this animal. This variability makes other mammalian models such as guinea pigs, rats and chinchillas preferable choices for some types of analyses of hair cell damage and protection.
Impracticality of drug screening in most animal models
Although traditional vertebrate laboratory animal models are excellent for answering many questions about hair cell death and survival, they are not useful for drug screening. This is largely due to the relative inaccessibility of the inner ear. Most in vitro preparations for studying hair cells involve free-floating whole organ cultures such as cochlear or utricular explants. Furthermore, adult inner ear cochlear tissue is notoriously difficult to keep alive and healthy for more than a few hours in vitro. The time required to sacrifice and dissect the large amounts of inner ear tissue required for drug screening makes the use of mammalian or avian tissue impractical for screening. In addition, the cost and number of animals required for screening is prohibitive.
Hair cell lines
For the reasons noted above, hair cell lines are more practical for drug screening than whole cochlear or utricular cultures. Several immortal cell lines derived from cochlear and vestibular tissue have been developed [13,14]. Kalinec et al. [15] developed the House Ear Institute-organ of Corti 1 (HEI-OC1) cell line from cochlear cultures from the Immortomouse™. In addition to expressing several proteins (such as Myosin VIIa and Atoh1) suggestive of a hair cell phenotype, the HEI-OC1 cell line also demonstrated sensitivity to known ototoxins such as gentamicin and cisplatin. Interestingly, however, the HEI-OC1 cells also expressed Nestin, which is typically expressed in neonatal organ of Corti [16], and OCP2, a protein typically expressed in supporting cells [17], suggesting that there are significant[E5] differences from mature hair cells. These hair cell lines have been used to evaluate a variety of agents, such as ebselen [18] and flunarizine [19], as potential protectants against cisplatin-induced hair cell death. Although promising, we must keep in mind that hair cell lines might more closely resemble immature hair cells or even stem cell precursors. In addition, they might have different susceptibility to ototoxic drugs, particularly because they are, in fact, selected for their capacity to survive. Those reservations aside, hair cell lines do offer a model in which high-throughput screening for drugs that protect against hair cell death can occur. Although hair cell lines have been suggested as a possible screening tool for hair cell protectants, to our knowledge no drug has gone the route of screening/identification[E6] in hair cell lines followed by validation in mammalian systems.
The zebrafish: a powerful, simple animal model
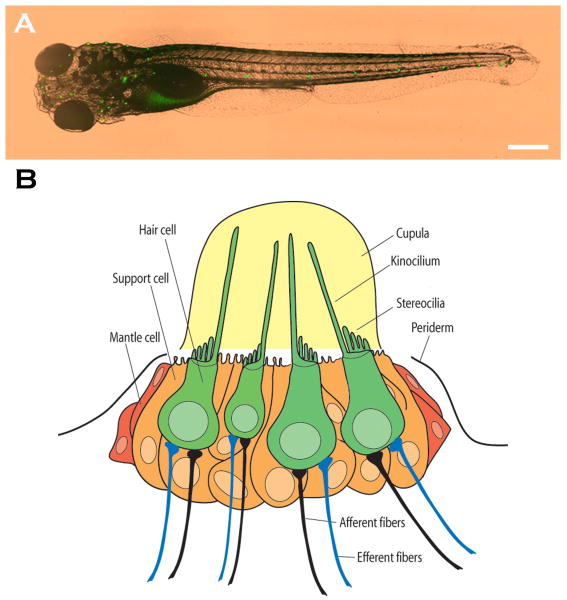
The zebrafish offers several advantages that make it a powerful animal model for studying hair cells in general, as well as for performing high-throughput drug screens and genetic screens for molecular mechanisms that can protect hair cells in particular. The zebrafish, like all aquatic vertebrates, has hair cells on the outside of its body in a sensory system called the lateral line. This system is used for detecting minute differences in water currents to different parts of the body. The hair cells are organized into small groups called neuromasts (Figure 1). Physiologically, their behavior is very similar to that of inner ear hair cells with depolarization occurring in response to deflection of stereocilia towards a single kinocilium. At the electron microscopic level, the intracellular structure of the lateral line hair cell is also very similar to that of inner ear hair cells, particularly those of the vestibular epithelium [20,21].
Figure 1.
[E15] (a) Live preparation of fluorescently labeled zebrafish larva five days post-fertilization. Neuromasts of the lateral line are stained with YO-PRO-1. Scale bar = 0.5 mm. (b) Schematic illustration of a neuromast. Hair cells are depicted in green with long kinocilia and shorter stereocilia projecting from the apical end of the cells and afferent and efferent nerve fibers at the basal end. Support cells (orange cells) intercalate between the hair cells. Reproduced, with permission from the Association for Research in Otolaryngology, from Ref. [31][E16].
Initially, interest in zebrafish focused on its value as a model system for studying vertebrate development, particularly nervous system development. During the past decade, however, this animal has been used as a model for clinically driven investigations, and many discoveries have been made that are applicable to humans. For example, a study of cardiac drugs that slow down the cardiac cycle in humans (prolonged Q-T interval) found that 22 of 23 of these drugs had similar effects in zebrafish [22]. In addition, drugs that affect lipid metabolism, narcotics and anticoagulants all have identical effects in zebrafish [23]. Furthermore, several genes that are required for normal hair cell development and function have been discovered in zebrafish and have been found to have mammalian orthologs. Examples include mutations in cadherin23, which is a candidate for the stereocilia tip link [24]; the trpn1 mutant, which is a candidate for the mechanotransduction channel [25]; and the mariner mutant, which has a myosin7a mutation analogous to the mutation responsible for the Usher IB syndrome [26,27].
In vivo imaging of hair cells and hair cell death
The utility of the zebrafish for studying hair cells comes from four properties: (i) high fecundity – a single mating of adult zebrafish can produce hundreds of offspring; (ii) fluorescent labeling – hair cells of the lateral line selectively pick up several fluorescent vital dyes, such as DASPEI, YO-PRO1 and FM1-43; (iii) optical clarity – at five days post-fertilization, the zebrafish body is clear, enabling in vivo imaging of fluorescently labeled hair cells; and (iv) molecular/genetic flexibility[E7] – the zebrafish is genetically very well characterized, mutagenesis protocols are well established and genetic ‘knock-downs’ using morpholino-based antisense oligonucleotides can be generated easily.
These four properties have enabled careful and detailed studies of hair cell death in the zebrafish lateral line. Williams and Holder [20] first described neomycin-induced hair cell death in the larval zebrafish lateral line in the context of describing natural hair cell turnover. Harris et al. [28] used DASPEI vital dye staining and hair cell counts to demonstrate dose-dependent neomycin-induced lateral line hair cell death and subsequent hair cell regeneration. Since then, other aminoglycosides [21,29], other known ototoxic agents such as cisplatin [30] and new ototoxic agents [31] have been identified and characterized using the zebrafish lateral line. In addition, several studies have begun examining the cell death response to these ototoxic agents in more detail [21,29,32,33]. These studies have demonstrated the utility and power of this animal model for rapid qualitative in vivo assessment of hair cell death and protection, as well as large-scale, quantitative studies of hair cell death.
The zebrafish as a screening tool
Because of its small size, whole-organism-based genetic and drug screens can be performed successfully in the larval zebrafish. Genetic screens entail screening known mutant lines [34] or ENU[E8] mutagenesis, then screening for a particular phenotype or for modulation of an induced phenotype. Drug/chemical screens[E9] entail exposure of the entire zebrafish to libraries of compounds with examination for phenotypic effects or modulation of a phenotype produced by a mutation or another drug. This is in sharp contrast to target-based screens, which screen for drugs that affect a specific molecular pathway of interest. In contrast to cell-culture-based screens, whole-organism-based screens have the additional advantage of evaluating the effects on[E10] drugs on cells in their natural anatomical and biological environment, while also getting early systemic toxicity information. A zebrafish whole-organism screen was used very successfully by North et al. [35] in a search for new modulators of hematopoietic stem cells. By incubating zebrafish embryos with chemicals from three chemical libraries, ten compounds that affected the prostaglandin pathway and altered the number of hematopoietic stem cells were identified. This screen led to the discovery that prostaglandin E2 synthesis enhanced the number of hematopoietic stem cells in both zebrafish and mice – a finding that might prove valuable in the treatment of patients undergoing bone marrow transplantation.
Development of a drug screening protocol for hair cell protectants
The studies described above formed the platform on which a screening protocol for discovering drug-like chemical agents and therapeutic drugs that either protect hair cells from known ototoxic substances or are ototoxic in themselves.[E11] The availability of large numbers of zebrafish and their small size as free-swimming larvae, in combination with the ease of rapid in vivo imaging, makes the zebrafish lateral line an ideal system for screening. In brief (Figure 2), five days post-fertilization zebrafish are labeled with the fluorescent dye YO-PRO1 for 30 minutes, which selectively labels hair cell nuclei. One fish is then placed into each well of a 96-well plate with a clear optical base. Owing to their small size, as many as two or three zebrafish larvae can be placed in each well if necessary. Fish can then be exposed to a series of drugs depending on the exact screening protocol. For protective drug screening in our laboratory, fish are first treated with YO-PRO1 to label the lateral line hair cells, then exposed to libraries of potential protective drugs, followed by treatment with known ototoxic drugs such as aminoglycosides or cisplatin. The 96-well plate is then placed directly on an inverted microscope with a motorized stage. Fluorescence microscopy is used to image hair cells of the lateral line in the fish in each well to evaluate whether hair cells have been protected from exposure to the ototoxic drug; this would be considered a ‘hit’. Typically, a single plate with 80 potential protectants requires 30 minutes for evaluation. All hits from the initial screen are then confirmed with repeat testing followed by thorough quantitative studies.
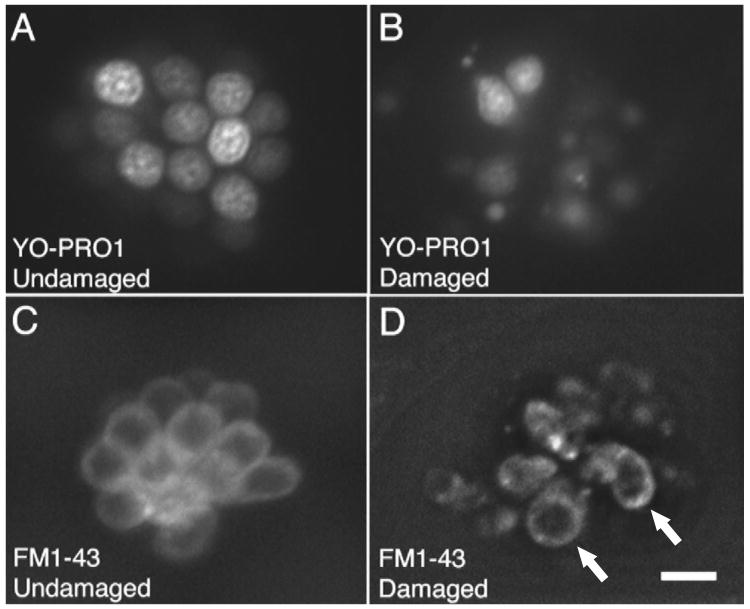
Figure 2.
Examples of normal and damaged fluorescently labeled hair cells of the zebrafish lateral line. YO-PRO1 selectively labels hair cell nuclei in (a) normal and (b) neomycin-damaged neuromasts. Hair cell protection can thus be assessed easily during screening. For quantitative hair cell counts, FM1-43FX is used to count (c) normal and (d) neomycin-damaged hair cells. In the undamaged neuromast (c), there are approximately 12 visible hair cells. In the damaged neuromast (d), there are two surviving hair cells (arrows) after treatment with one hour of neomycin. Scale bar in (d) = 10 μM and applies to all panels. Reproduced, with permission from the Association for Research in Otolaryngology, from Ref. [40].[E17]
Small-molecule screens
Using this drug screening protocol, a small molecule library of >10 000 compounds (Chembridge) was screened for small molecules that inhibited neomycin-induced hair cell death [36]. Fish were pretreated with drugs from the library for one hour, followed immediately by one hour in neomycin. From this library, two small molecules were identified as protective. Interestingly, both small molecules (named PROTO-1 and PROTO-2) were from a class of compounds called benzothiophene carboxamides. Additional testing showed that both drugs demonstrated dose-dependent protection against neomycin and were protective against a wide range of neomycin doses. The protective effects were then confirmed in organotypic mouse utricle cultures, demonstrating that these drugs found to have protective effects in the fish had similar effects in mammalian tissue.
Drug library screens
It is important to note, however, that PROTO-1 and PROTO-2 are not yet drugs. They are small molecules that have unknown bioavailability and unknown toxicity. With the well-known arduous process of drug development and FDA approval, even in the best of situations, it might be several years before PROTO-1 and/or -2 are approved for use in humans.
The rigors of drug development have led some investigative teams to examine drugs already in use for potential beneficial effects in ‘off-label’ situations. Although drugs are developed with a specific target in mind, virtually all drugs have a multitude of off-target effects. A prime example of this has been the recent serendipitous discovery of the effects of the cardiac drug propranolol on the growth of hemangiomas. Two infants with cardiac conditions requiring treatment with the beta blocker propranolol were noted to have decreased growth of coincident cutaneous hemangiomas [37]. Since this initial discovery, numerous other centers have made similar findings, which might ultimately revolutionize how these common lesions are treated. This impressive example of off-target effects demonstrates the importance of screening libraries of known drugs to discover occult effects.
Several libraries of FDA-approved drugs and bioactives, such as the NINDS Custom Collection 2 (Microsource, Inc.) and the BIOMOL FDA-approved Drug Library (Enzo Life Sciences, Inc.), have been developed that are composed of compounds that have already been used in humans. Identification of drugs of interest from these libraries, therefore, has the potential benefit of being able to bypass a large portion of the drug development and approval process. Much is known about their pharmacokinetics and extensive safety testing has been completed. This strategy has been employed in several studies – notably, a screen of the NINDS Custom Collection 2 by Rothstein et al. [38] that identified beta lactam antibiotics as potential neuroprotective agents owing to their ability to increase glutamate transporter expression. Subsequent in vivo studies by the same group demonstrated improved neuronal survival in a mouse model of amyotrophic lateral sclerosis. A second study screening for drugs using the NINDS Custom Collection 2 identified five drugs that blocked mutant huntingtin-induced neuronal cell death in a Huntington's disease in vitro neuronal model [39].
With this in mind, we screened the NINDS Custom Collection 2 for drugs that inhibited neomycin-induced hair cell death in the zebrafish lateral line. Using the same rapid screening protocol described above, we screened all 1040 compounds of the library [40]. From this screen, seven drugs with novel protective activity were identified (Table 1). The optimal drug concentration for protection in the zebrafish lateral line varied between 50 and 100 μM for the seven drugs. All seven drugs protected against a wide range of neomycin doses. Importantly, none of the drugs blocked the normal bactericidal activity of neomycin. Uptake studies were performed to determine whether the protective effects were due to inhibition of neomycin uptake. It was found that four out of seven drugs protected against neomycin-induced hair cell death by blocking uptake of neomycin, whereas the other three seemed to interfere with intracellular death pathways activated by the neomycin. To confirm the effects in a mammalian system, the remaining three were evaluated with organotypic mouse utricle cultures. One of the three drugs, tacrine, was found to have protective effects in both the zebrafish lateral line in vivo and the mouse utricle in vitro. Tacrine is now being tested in in vivo mammalian trials and, if successful, might be a candidate for use in humans. It has previously been used long term in human subjects as a possible therapy for Alzheimer's dementia, albeit with some difficulties with hepatotoxicity [41]. It is likely that the shorter treatment periods required to prevent ototoxic injury would have a reduced risk of hepatotoxicity.
Table 1. Protective drugs identified by zebrafish lateral line screen of NINDS Custom Collection II for protection against neomycin-induced hair cell deatha.
| Protective drug | Known activity |
|---|---|
| Amsacrine | Topoisomerase 2 poison; used as chemotherapeutic agent. Used clinically in other countries; not yet FDA approved. |
| Carvedilol | Beta-2 adrenergic blocker; used for treatment of hypertension and heart failure; FDA approved. |
| Cepharanthine | Plasma membrane stabilizer; used for the treatment of nasal allergy, snake venom hemolysis; possible chemotherapeutic adjunct. Used clinically in other countries; not yet FDA approved. |
| Drofenine | Acetylcholinesterase inhibitor; used as antispasmodic. Used clinically in other countries; not yet FDA approved. |
| Hexamethyleneamiloride | Diuretic, Na/H exchange inhibitor. Derivative of amiloride; FDA approved. Diuretic. |
| Phenoxybenzamine | Alpha-1 adrenergic blocker; used as antihypertensive. FDA approved. |
| 9-amino-1,2,3,4-.tetrahydroacridine (Tacrine) | Anticholinergic; acetylcholinesterase inhibitor, used for treatment of Alzheimer's dementia. FDA approved. |
Modified, with permission from the Association for Research in Otolaryngology, from Ref. [40].
The Chembridge and NINDS Custom Collection screens demonstrate the utility of the zebrafish lateral line for the identification of protective small molecules that might be candidates for drug development, as well as the identification of new protective effects in already established drugs. We have used this technique to identify drugs of interest in the zebrafish that have then had validated effects in mammalian systems, and we are in the process of evaluating their effects in vivo in the inner ears of mammals.
Ototoxicity screening
It is important to note that a large percentage of hearing loss remains idiopathic, and at least some of this hearing loss can probably be attributed to occult ototoxicity. Although the focus of this report is on the use of the zebrafish to discover drugs that are capable of protecting inner ear hair cells from ototoxic events, the zebrafish lateral line also has potential uses in the realm of drug safety. Specifically, this preparation is being used for the identification of potential ototoxic compounds. During drug development, patients are monitored for liver and kidney toxicity – little attention is paid to hearing. Typically, ototoxicity is only identified after anecdotal reports of hearing loss lead to more formal testing. Whereas ototoxic effects of some drugs such as aminoglycosides and cisplatin are well known and have been studied for decades, it is likely that many drugs currently in use or in development have occult ototoxic effects. These drugs might induce lesser degrees of hearing loss/hair cell death[E12] that are easily missed in children, who are less likely to report hearing loss, and the elderly, whose hearing loss is most likely to be attributed to presbycusis.
By modifying the hair cell protection screening protocol, the NINDS Custom Collection 2 was screened for ototoxic effects in the zebrafish lateral line [31]. Larval zebrafish were labeled with YO-PRO1 and then exposed for one hour to compounds from the drug library. Hair cells of the lateral line were then examined with fluorescence microscopy to assess hair cell damage. Any drugs that demonstrated ototoxicity in the initial screen were retested and then underwent full dose-response curves. By doing so, fourteen potentially novel ototoxic drugs were identified (Table 2). Two of these drugs, pentamidine and propantheline, were then tested in the mouse utricle in vitro and demonstrated similar ototoxic effects. Preliminary in vivo testing of pentamidine in rats suggests this drug also causes a mild hearing loss when administered over a six-week period. We thus have a new tool to rapidly screen drugs for potentially adverse otologic outcomes. This tool can be applied to drugs that are currently in therapeutic use and at an early stage of testing for drugs under development. Given the validation of this screening procedure that has already taken place, we are confident that this ‘ototoxicity screen’ can improve the safety of care for patients, particularly those started on new experimental drugs.
Table 2. Candidate ototoxic drugs identified by zebrafish lateral line screen of NINDS Custom Collection II for drugs that cause hair cell deatha.
| Ototoxic drug | Class | Known ototoxicity? |
|---|---|---|
| Chloramphenicol | Antibiotic | Rare case reports |
| Chlortetracycline HCL | Antibiotic | No |
| Pentamidine isethionate | Antiprotozoal | No |
| Spermadine | Ornithine decarboxylase inhibitor | No |
| Tobramycin | Antibiotic | Yes |
| Propantheline bromide | Anticholinergic | No |
| Ethacrynic acid | Loop diuretic | Yes |
| Pomiferin | Antioxidant | No |
| Chlorophyllide | Antineoplastic, chlorophyll derivative | No |
| Estradiol valerate | Estrogen | Rare case reports |
| Neomycin | Antibiotic | Yes |
| Pentetrazole | CNS/respiratory/circulatory stimulant | Yes, animal studies |
| Guaiazulene | Antioxidant, color additive agent | No |
| Rosolic acid | Diagnostic aid | No |
| Cisplatin | Antineoplastic | Yes |
| Vincamine | Vasodilator | No |
| Kanamycin | Antibiotic | Yes |
| Demeclocycline HCL | Antibiotic | No |
| Mefloquine | Antiprotozoal | Yes |
| Candesartan | Angiotensin 1 receptor antagonist | No |
| Simvastatin | HMGCoA reductase inhib., antihyperlipidemic | No |
Modified, with permission from the Association for Research in Otolaryngology, from Ref. [31].
Why screen for more protective drugs?
With multiple protective drugs already identified and in various stages of clinical evaluation, one might wonder whether continued screening and discovery of protective drugs is worthwhile. One need only look at the complicated and numerous cell death pathways and the complex relationship between pathways to understand the need for more protective drugs.
Within the literature on the inner ear, one finds discrepancies and differences in how hair cells die. Whereas many have demonstrated caspase activation in aminoglycoside-induced hair cell death [42,43], others have proposed caspase-independent pathways with activation of cathepsin as the main mechanism of hair cell death [44]. Different ototoxic agents also seem to activate different death pathways. Jun kinase inhibitors have been shown to inhibit aminoglycoside[E13] but not cisplatin-induced hair cell death [45,46]. More recently, Owens et al. [29] used the zebrafish lateral line to define differences in dose-dependent damage between different aminoglycosides, suggesting that different aminoglycosides might cause hair cell death through different pathways.
More importantly, it is becoming apparent that inhibition of one pathway can lead to upregulation of other death pathways. For example, zVAD-fmk (a well-known broad-spectrum caspase inhibitor) has been shown to lead to an increase in necrotic and autophagic death [47– 49]. Conversely, it has been demonstrated that inhibition of autophagy can trigger apoptotic cell death in vitro [50].
It is thus probable that with any ototoxic agent, multiple death pathways are activated, dependent on the ototoxicant itself, the dose and the timecourse of exposure. With this in mind, it is likely that clinically reliable protection from hair cell death will require multiple protective compounds that block caspase-dependent and caspase-independent pathways. To develop these protective ‘cocktails’, we must have rapid and efficient drug screens to identify additional protectants that can then be used in combination to achieve full hair cell protection. We believe that the zebrafish lateral line can be used to rapidly screen large libraries for drugs that protect against hair cell death in the inner ear. In addition, because there is probably overlap between death pathways activated by different ototoxic agents, it is probable that drugs that protect against aminoglycoside-induced hair cell death will also be protective against other damaging agents such as cisplatin and noise.
Will zebrafish solve all the problems?
The answer, of course, is no! Obviously, there are differences between fish and mammals. There is no compartmentalization of fluids in the lateral line – the hair cell apices and stereocilia extend out into the surrounding water. There are no inner and outer hair cells within a neuromast. Furthermore, hair cells of the lateral line regenerate, with new hair cells detected within 24 hours of hair cell injury [51]. As a result, all findings in zebrafish must be confirmed in mammalian tissue. However, we think the zebrafish lateral line provides a powerful preparation to identify genes, drugs and potential drugs that have potential for protecting hearing, and which can then be evaluated more thoroughly in other animal models.
Footnotes
‘Teaser’ sentence: The zebrafish lateral line has emerged as a powerful tool to screen chemical libraries for drugs that protect against hair cell death.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vio MM, Holme RH. Hearing loss and tinnitus: 250 million people and a US$10 billion potential market. Drug Discov Today. 2005;10:1263–1265. doi: 10.1016/S1359-6446(05)03594-4. [DOI] [PubMed] [Google Scholar]
- 2.Gillespie LN, Shepherd RK. Clinical application of neurotrophic factors: the potential for primary auditory neuron protection. Eur J Neurosci. 2005;22:2123–2133. doi: 10.1111/j.1460-9568.2005.04430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nadol JB, Jr, et al. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98:411–416. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- 4.Nadol JB., Jr Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg. 1997;117:220–228. doi: 10.1016/s0194-5998(97)70178-5. [DOI] [PubMed] [Google Scholar]
- 5.Leake PA, et al. Factors influencing neurotrophic effects of electrical stimulation in the deafened developing auditory system. Hear Res. 2008;242:86–99. doi: 10.1016/j.heares.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sha SH, et al. Aspirin to prevent gentamicin-induced hearing loss. N Engl J Med. 2006;354:1856–1857. doi: 10.1056/NEJMc053428. [DOI] [PubMed] [Google Scholar]
- 7.Feldman L, et al. Gentamicin-induced ototoxicity in hemodialysis patients is ameliorated by N-acetylcysteine. Kidney Int. 2007;72:359–363. doi: 10.1038/sj.ki.5002295. [DOI] [PubMed] [Google Scholar]
- 8.Sugahara K, et al. JNK signaling in neomycin-induced vestibular hair cell death. Hear Res. 2006;221:128–135. doi: 10.1016/j.heares.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science. 1988;240:1772–1774. doi: 10.1126/science.3381100. [DOI] [PubMed] [Google Scholar]
- 10.Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science. 1988;240:1774–1776. doi: 10.1126/science.3381101. [DOI] [PubMed] [Google Scholar]
- 11.Quint E, Steel KP. Use of mouse genetics for studying inner ear development. Curr Top Dev Biol. 2003;57:45–83. doi: 10.1016/s0070-2153(03)57002-8. [DOI] [PubMed] [Google Scholar]
- 12.Avraham KB. Mouse models for deafness: lessons for the human inner ear and hearing loss. Ear Hear. 2003;24:332–341. doi: 10.1097/01.AUD.0000079840.96472.DB. [DOI] [PubMed] [Google Scholar]
- 13.Barald KF, et al. Immortalized cell lines from embryonic avian and murine otocysts: tools for molecular studies of the developing inner ear. Int J Dev Neurosci. 1997;15:523–540. doi: 10.1016/s0736-5748(96)00108-6. [DOI] [PubMed] [Google Scholar]
- 14.Kalinec F, et al. Establishment and characterization of conditionally immortalized organ of corti cell lines. Cell Biol Int. 1999;23:175–184. doi: 10.1006/cbir.1998.0339. [DOI] [PubMed] [Google Scholar]
- 15.Kalinec GM, et al. A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol Neurootol. 2003;8:177–189. doi: 10.1159/000071059. [DOI] [PubMed] [Google Scholar]
- 16.Malgrange B, et al. Proliferative generation of mammalian auditory hair cells in culture. Mech Dev. 2002;112:79–88. doi: 10.1016/s0925-4773(01)00642-6. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, et al. cDNA cloning, tissue distribution, and chromosomal localization of Ocp2, a gene encoding a putative transcription-associated factor predominantly expressed in the auditory organs. Genomics. 1995;27:389–398. doi: 10.1006/geno.1995.1068. [DOI] [PubMed] [Google Scholar]
- 18.Kim SJ, et al. Ebselen attenuates cisplatin-induced ROS generation through Nrf2 activation in auditory cells. Hear Res. 2009;251:70–82. doi: 10.1016/j.heares.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 19.So H, et al. Evidence that cisplatin-induced auditory damage is attenuated by downregulation of pro-inflammatory cytokines via Nrf2/HO-1. J Assoc Res Otolaryngol. 2008;9:290–306. doi: 10.1007/s10162-008-0126-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams JA, Holder N. Cell turnover in neuromasts of zebrafish larvae. Hear Res. 2000;143:171–181. doi: 10.1016/s0378-5955(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 21.Owens KN, et al. Ultrastructural analysis of aminoglycoside-induced hair cell death in the zebrafish lateral line reveals an early mitochondrial response. J Comp Neurol. 2007;502:522–543. doi: 10.1002/cne.21345. [DOI] [PubMed] [Google Scholar]
- 22.Milan DJ, et al. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation. 2003;107:1355–1358. doi: 10.1161/01.cir.0000061912.88753.87. [DOI] [PubMed] [Google Scholar]
- 23.Langheinrich U. Zebrafish: a new model on the pharmaceutical catwalk. Bioessays. 2003;25:904–912. doi: 10.1002/bies.10326. [DOI] [PubMed] [Google Scholar]
- 24.Sollner C, et al. Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature. 2004;428:955–959. doi: 10.1038/nature02484. [DOI] [PubMed] [Google Scholar]
- 25.Sidi S, et al. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science. 2003;301:96–99. doi: 10.1126/science.1084370. [DOI] [PubMed] [Google Scholar]
- 26.Ernest S, et al. Mariner is defective in myosin VIIA: a zebrafish model for human hereditary deafness. Hum Mol Genet. 2000;9:2189–2196. doi: 10.1093/hmg/9.14.2189. [DOI] [PubMed] [Google Scholar]
- 27.Seiler C, et al. Myosin VI is required for structural integrity of the apical surface of sensory hair cells in zebrafish. Dev Biol. 2004;272:328–338. doi: 10.1016/j.ydbio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Harris JA, et al. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio) J Assoc Res Otolaryngol. 2003;4:219–234. doi: 10.1007/s10162-002-3022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owens KN, et al. Response of mechanosensory hair cells of the zebrafish lateral line to aminoglycosides reveals distinct cell death pathways. Hear Res. 2009;253:32–41. doi: 10.1016/j.heares.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ou HC, et al. Cisplatin-induced hair cell loss in zebrafish (Danio rerio) lateral line. Hear Res. 2007;233:46–53. doi: 10.1016/j.heares.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiu LL, et al. Using the zebrafish lateral line to screen for ototoxicity. J Assoc Res Otolaryngol. 2008;9:178–190. doi: 10.1007/s10162-008-0118-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murakami SL, et al. Developmental differences in susceptibility to neomycin-induced hair cell death in the lateral line neuromasts of zebrafish (Danio rerio) Hear Res. 2003;186:47–56. doi: 10.1016/s0378-5955(03)00259-4. [DOI] [PubMed] [Google Scholar]
- 33.Santos F, et al. Lateral line hair cell maturation is a determinant of aminoglycoside susceptibility in zebrafish (Danio rerio) Hear Res. 2006;213:25–33. doi: 10.1016/j.heares.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 34.Amsterdam A, et al. A large-scale insertional mutagenesis screen in zebrafish. Genes Dev. 1999;13:2713–2724. doi: 10.1101/gad.13.20.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.North TE, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Owens KN, et al. Identification of genetic and chemical modulators of zebrafish mechanosensory hair cell death. PLoS Genet. 2008;4:e1000020. doi: 10.1371/journal.pgen.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leaute-Labreze C, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 38.Rothstein JD, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 39.Wang W, et al. Compounds blocking mutant huntingtin toxicity identified using a Huntington's disease neuronal cell model. Neurobiol Dis. 2005;20:500–508. doi: 10.1016/j.nbd.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 40.Ou HC, et al. Identification of FDA-approved drugs and bioactives that protect hair cells in the zebrafish (Danio rerio) lateral line and mouse (Mus musculus) utricle. J Assoc Res Otolaryngol. 2009;10:191–203. doi: 10.1007/s10162-009-0158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watkins PB, et al. Hepatotoxic effects of tacrine administration in patients with Alzheimer's disease. J Am Med Assoc. 1994;271:992–998. [PubMed] [Google Scholar]
- 42.Cunningham LL, et al. Caspase activation in hair cells of the mouse utricle exposed to neomycin. J Neurosci. 2002;22:8532–8540. doi: 10.1523/JNEUROSCI.22-19-08532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng AG, et al. Hair cell death in the avian basilar papilla: characterization of the in vitro model and caspase activation. J Assoc Res Otolaryngol. 2003;4:91–105. doi: 10.1007/s10162-002-3016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang H, et al. Caspase-independent pathways of hair cell death induced by kanamycin in vivo. Cell Death Differ. 2006;13:20–30. doi: 10.1038/sj.cdd.4401706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, et al. Caspase inhibitors, but not c-Jun NH2-terminal kinase inhibitor treatment, prevent cisplatin-induced hearing loss. Cancer Res. 2004;64:9217–9224. doi: 10.1158/0008-5472.CAN-04-1581. [DOI] [PubMed] [Google Scholar]
- 46[E14].Ou HC, et al. c-Jun N-terminal kinase inhibition blocks aminoglycoside but not cisplatin-induced hair cell death in the zebrafish lateral line. Midwinter Research Meeting of the Association for Research in Otolaryngology.2006. [Google Scholar]
- 47.Lin Y, et al. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu L, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 49.Vandenabeele P, et al. Caspase inhibitors promote alternative cell death pathways. Sci STKE. 2006;358:pe44. doi: 10.1126/stke.3582006pe44. [DOI] [PubMed] [Google Scholar]
- 50.Boya P, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma EY, et al. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2008;28:2261–2273. doi: 10.1523/JNEUROSCI.4372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]