Abstract
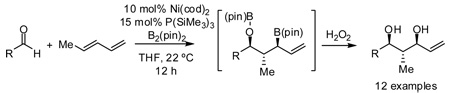
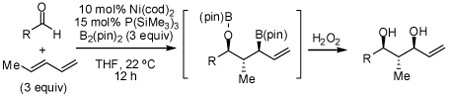
The nickel-catalyzed reaction of carbonyls and dienes was accomplished in a regio-and stereo-selective fashion employing a stoichiometric amount of bis(pinacolato)diboron. In the presence of P(SiMe3)3, this reductive coupling furnishes allyl boronic esters which are regioisomeric to those obtained with PCy3 as the ligand. The coupling product may be subject to oxidation, which furnishes the derived 1,3-diol, or allylation with an additional aldehyde, which furnishes the derived 1,6-diol in a stereoselective fashion.
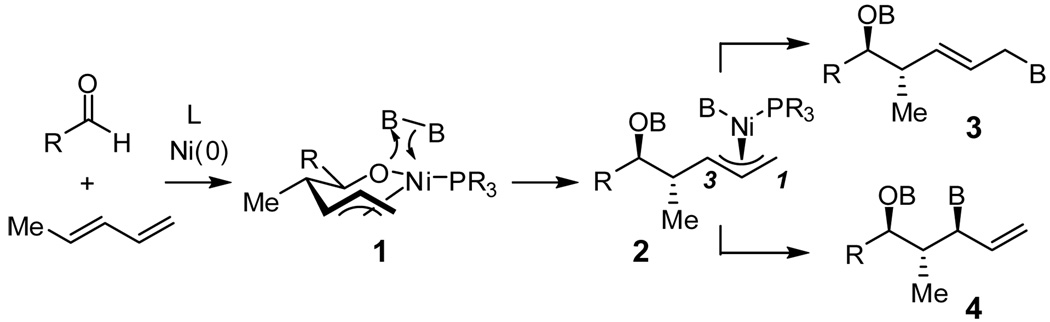
Rapid construction of complex molecules with high regio- and stereoselectivity is a central objective in organic synthesis. In this regard, reactions that result in the formation of multiple new bonds and that establish multiple stereocenters are particularly valuable.1 To address this topic, we recently initiated studies on the borylative coupling of unsaturated substrates.2 In one manifestation of this strategy, we examined the Mori-Tamaru Ni-catalyzed coupling of aldehydes and dienes,3,4 but employed bis(pinacolato)diboron (B2(pin)2) as the reducing agent. In the presence of PCy3 as an ancillary ligand for nickel, reaction product 3 (Scheme 1) is furnished as a single constitutional and stereoisomer. In accord with mechanistic studies by Ogoshi,5 it is likely that this reaction occurs by a pathway involving oxidative cyclometalation to give 1; subsequent transmetalation would furnish 2 and reductive elimination to connect C1 with the boryl ligand would release 3 from the catalyst. We considered that other ligands might alter the regioselectivity of the reductive elimination by connection of C3 and the boryl group, and thereby furnish allylboronate 4 as the reaction product. The structural features of compound 4 – three contiguous stereocenters, an α-chiral allylboronate,6 and a functional group pattern that maps onto polyketides – made this a compelling inquiry. In this report, we describe a remarkable turnover in regioselectivity of the borylative diene/aldehyde coupling when PCy3 is replaced with P(SiMe3)3.
Scheme 1.
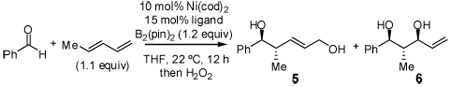
Initial exploratory studies focused on the nickel catalyzed reaction between one equivalent each of 1,3-pentadiene, benzaldehyde, and B2(pin)2. While PCy3 and P(t-Bu)3 both promoted formation of terminal boronate 3 (analysis after oxidative workup with hydrogen peroxide), other alkyl and aryl phosphines, triaminophosphines, and simple phosphites were either ineffective or resulted in poor selectivity (Table 1). However, when commercially available P(SiMe3)3 was employed, compound 4 was observed with excellent selectivity.
Table 1.
Effect of Ligand On the Ni-Catalyzed Diborylative Coupling.
 | ||||
|---|---|---|---|---|
| entry | ligand | 5:6 | % yielda | d.r.b |
| 1 | none | >20:1 | 39 | 1:1 |
| 2 | PCy3 | >20:1 | 69 | >20:1 |
| 3 | P(t-Bu)3 | >20:1 | 71 | 6:1 |
| 4 | PPh3 | >20:1 | 63 | 5:1 |
| 5 | P(NMe2)3 | >20:1 | 16 | >20:1 |
| 6 | P(OEt)3 | >20:1 | 40 | 4:1 |
| 7 | PEt3 | 1:2 | 34 | 2:1 |
| 8 | PMe3 | 1:3 | 55 | 4:1 |
| 9 | P(SiMe3) | 1:12 | 45 | >20:1 |
Isolated yield of major product.
d.r. of major product.
In addition to compound 4, by-products comprising two aldehydes and one diene, or two dienes and one aldehyde, were also observed. Reasoning that reaction of 4 with unreacted aldehyde might compete with the catalytic process at later stages of reaction and deliver some of these by-products, the concentrations of B2(pin)2 and pentadiene were increased. This strategy furnished optimal conditions for this three-component process. As depicted in Table 2, upon oxidation the borylative coupling reaction converts benzaldehyde and 1,3-pentadiene to the derived 1,3-diol with >20:1 diastereoselectivity.7 Examination of other substrates revealed that, in general, the reaction is effective for aromatic and heteroaromatic aldehydes and generally delivers the 1,3-diol with excellent regio- and stereocontrol. To determine whether this transformation might apply to more common synthetic building blocks, the aliphatic aldehydes in entries 8–12 were also studied. These experiments suggest that the reaction can be effective with both linear and branched aliphatic aldehydes; additionally, a simple α-chiral aldehyde was found to react with Felkin selectivity thereby opening the possibility for asymmetric synthesis (entry 12).
Table 2.
Ni(cod)2/P(SiMe3)3-Catalyzed Diborylative Coupling.
 | ||||
|---|---|---|---|---|
| entry | aldehyde | product | d.r.a | % yieldb |
| 1 | 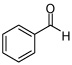 |
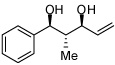 |
>20:1 | 67 |
| 2 |  |
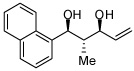 |
>20:1 | 73 |
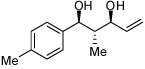
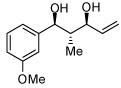
| 3 | 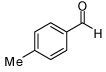 |
 |
>20:1 | 64 |
| 4 | 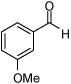 |
 |
>20:1 | 57 |
| 5 | 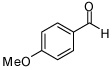 |
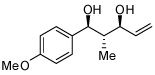 |
>20:1 | 57 |
| 6 | 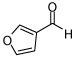 |
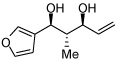 |
>20:1 | 55 |
| 7 | 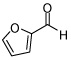 |
 |
>20:1 | 54 |
| 8 | 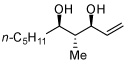 |
>20:1 | 58c | |
| 9 | 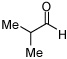 |
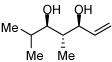 |
>20:1 | 50c |
| 10 | 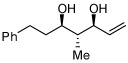 |
>20:1 | 45c | |
| 11 | 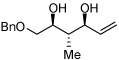 |
>20:1 | 37c | |
| 12 | 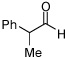 |
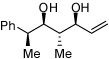 |
6:1d | 49c |
Determined by 1H NMR analysis of unpurified reaction mixture.
Isolated yield of purified material. Value is an average of two experiments.
Run with 1.1 equiv of diene and 1.2 equiv of B2(pin)2.
Ratio refers to Felkin:anti Felkin selectivity. Reaction for 24 h.
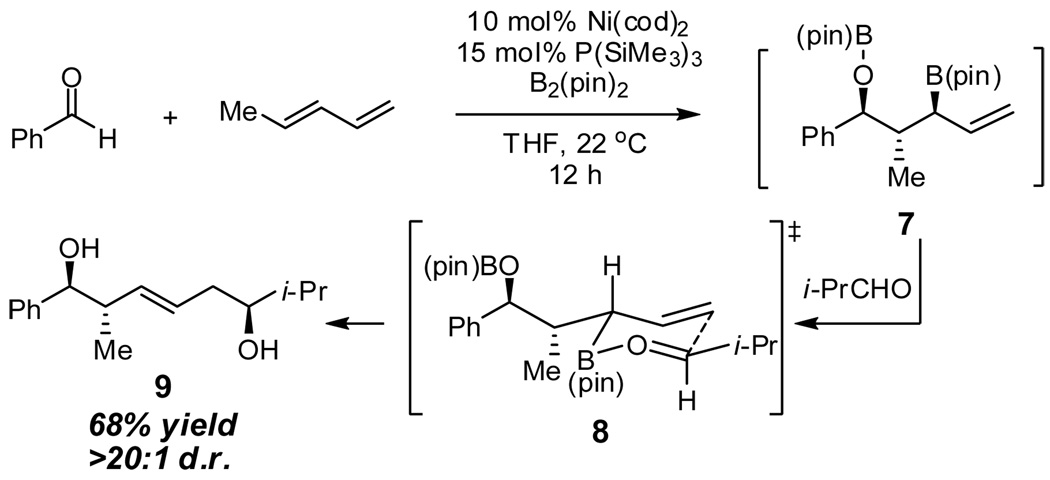
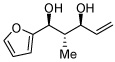
As alluded to in the introduction, general structure 4 possesses an α-chiral allylic boronate, a motif that often engages in highly selective carbonyl allylation reactions. To probe the capacity for structures such as 4 to participate in stereoselective allylations, benzaldehyde and 1,3-pentadiene were subjected to borylative coupling and, after 12 hours, isobutyraldehyde was added to the reaction mixture. This single-pot reaction sequence delivered 1,6-diol 9 (Scheme 2) in good yield, as a single regioisomer, and with excellent levels of 1,5-stereoinduction (>20:1 d.r.) and olefin stereocontrol. Considering the olefin configuration in the reaction product, it appears plausible that boronate 7 reacts with isobutyraldehyde by way of transition structure 8 with the α-substituent occupying a pseudoequatorial position.8
Scheme 2.
P(SiMe3)3 is a relatively unknown ligand in transition metal catalysis9 and the reversal in regioselectivity when it is used in place of PCy3 or P(t-Bu)3 deserves comment. Data in Table 1 reveal that smaller ligands may favor formation of 4 (entries 7 and 8 versus entries 2 and 3), which might suggest that P(SiMe3)3 simply serves as a precursor to PH3 (reaction with adventitious moisture).10 However, in situ 31P NMR analysis of reactions in the presence of P(SiMe3)3 shows that most of the ligand remains unmodified (31P δ = −251.4 ppm) over the course of the catalytic reaction.11 The fact that the cone angle of P(SiMe3)3 is similar to that of P(t-Bu)3 (178° versus 182°)12 suggests that the difference in regioselectivity observed with these ligands may arise from electronic rather than steric differences. While the 13C NMR chemical shift13 and IR analysis (A1 CO stretching frequency) of (Me3Si)3PNi(CO)3 suggest that P(SiMe3)3 and trialkylphosphines are electronically similar ligands, analyses by both Bartik14 and Helm15 indicate that P(SiMe3)3 can act as an electron acceptor. Thus a tentative hypothesis is that the large cone angle of P(SiMe3)3, combined with an ability to act as an electron acceptor, may facilitate reductive elimination of 4 from 2, prior to allyl isomerization required for formation of 3.16
Supplementary Material
Acknowledgment
This work was supported by the NIGMS (GM-59417) and the NSF (BC Mass Spec Center; grant # DBI-0619576). We thank AllyChem, Co., Ltd. for B2(pin)2. H.Y.C. is grateful for a Rodin Fellowship.
Footnotes
Supporting Information Available: Characterization and procedures. This information is available free of charge through the internet at http://pubs.acs.org.
References
- 1.For a recent review of multicomponent reactions, see: Touré BB, Hall DG. Chem. Rev. 2009;109:4439.. doi: 10.1021/cr800296p.
- 2.Cho HY, Morken JP. J. Am. Chem. Soc. 2008;130:16140. doi: 10.1021/ja806113v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Sato Y, Takimoto M, Hayashi K, Katsuhara T, Takagi K, Mori M. J. Am. Chem. Soc. 1994;116:9771. [Google Scholar]; (b) Sato Y, Takimoto M, Mori M. Tetrahedron Lett. 1996;37:887. [Google Scholar]; (c) Takimoto M, Hiraga Y, Sato Y, Mori M. Tetrahedron Lett. 1998;39:4543. [Google Scholar]; (d) Sato Y, Takanashi T, Hoshiba M, Mori M. Tetrahedron Lett. 1998;39:5579. [Google Scholar]; (e) Sato Y, Saito N, Mori M. Tetrahedron. 1998;54:1153. [Google Scholar]; (f) Sato Y, Takimoto M, Mori M. J. Am. Chem. Soc. 2000;122:1624. [Google Scholar]; (g) Sato Y, Saito N, Mori M. J. Am. Chem. Soc. 2000;122:2371. [Google Scholar]; (h) Sato Y, Sawaki R, Mori M. Organometallics. 2001;27:5510. [Google Scholar]; (i) Sato Y, Saito N, Mori M. J. Org. Chem. 2002;67:9310. doi: 10.1021/jo020438c. [DOI] [PubMed] [Google Scholar]; (j) Sato Y, Sawaki R, Saito N, Mori M. J. Org. Chem. 2002;73:656. doi: 10.1021/jo0106086. [DOI] [PubMed] [Google Scholar]
- 4.(a) Kimura M, Ezoe A, Shibata K, Tamaru Y. J. Am. Chem. Soc. 1998;120:4033. [Google Scholar]; (b) Kimura M, Fujimatsu H, Ezoe A, Shibata K, Shimizu M, Matsumoto S, Tamaru Y. Angew. Chem., Int. Ed. 1999;38:397. doi: 10.1002/(SICI)1521-3773(19990201)38:3<397::AID-ANIE397>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]; (c) Shibata K, Kimura M, Shimizu M, Tamaru Y. Org. Lett. 2001;3:2181. doi: 10.1021/ol0100879. [DOI] [PubMed] [Google Scholar]; (d) Kimura M, Ezoe A, Mori M, Iwata K, Tamaru Y. J. Am. Chem. Soc. 2006;128:8559. doi: 10.1021/ja0608904. [DOI] [PubMed] [Google Scholar]
- 5. Ogoshi S, Tonomori K, Oka M, Kurosawa H. J. Am. Chem. Soc. 2006;128:7077. doi: 10.1021/ja060580l. Studies of carbonyl-alkyne couplings: Hratchian HP, Chowdhury SK, Gutiérrez-Garcia VM, Amarasinghe KKD, Heeg MJ, Schlegel HB, Montgomery J. Organometallics. 2004;23:4636. McCarren PR, Liu P, Cheong PH-Y, Jamison TF, Houk KN. J. Am. Chem. Soc. 2009;131:6654. doi: 10.1021/ja900701g..
- 6.Select syntheses of α-chiral allyl borons: Hoffmann RW, Dresely S. Angew. Chem., Int. Ed. 1986;25:189. Ditrich K, Bube T, Stürmer R, Hoffmann RW. Angew. Chem., Int. Ed. 1986;25:1028. Hoffmann RW, Neil G, Schlapbach A. Pure Appl. Chem. 1990;62:1993. Pietruszka J, Schöne N. Angew. Chem., Int. Ed. 2003;42:5638. doi: 10.1002/anie.200352210. Pietruszka J, Schöne N. Eur. J. Org. Chem. 2004:5011. Pelz NF, Woodward AR, Burks HE, Sieber JD, Morken JP. J. Am. Chem. Soc. 2004;126:16328. doi: 10.1021/ja044167u. Beckmann E, Hoppe D. Synthesis. 2005:217. Ito H, Kawakami C, Sawamura M. J. Am. Chem. Soc. 2005;127:16034. doi: 10.1021/ja056099x. Fang GY, Aggarwal VK. Angew. Chem., Int. Ed. 2007;46:359. doi: 10.1002/anie.200603659. Carosi L, Hall DG. Angew. Chem., Int. Ed. 2007;46:5913. doi: 10.1002/anie.200700975..
- 7.For recent diol construction using organoboron reagents, see: González AZ, Román JG, Alicea E, Canales E, Soderquist JA. J. Am. Chem. Soc. 2009;131:1269. doi: 10.1021/ja808360z. Chen M, Handa M, Roush WR. J. Am. Chem. Soc. 2009;131:14602. doi: 10.1021/ja904599h..
- 8.Large boron ligands (i.e. tetraphenylethylene glycol) favor the axial orientation whereas small ligands (propanediol) favor the equatorial orientation. See: Hoffmann RW, Weidmann U. J. Organomet. Chem. 1980;195:137. Flamme EM, Roush WR. J. Am. Chem. Soc. 2002;124:13644. doi: 10.1021/ja028055j. With Lewis acids, the equatorial orientation is preferred. See: Carosi L, Lachance H, Hall DG. Tetrahedron Lett. 2005;46:8981..
- 9.P(SiMe3)3 has been claimed in Pd-catalyzed arylamination. See: Richter AM, Lischewski VDE. Patent 19,963,009. Chem. Abstr. 2001;135:152619. 1991..
- 10.Conversion of P(SiMe3)3 complexes to PH3 complexes with Brønsted acids: Haupt H-J, Krampe O, Flörke U. Z. Anorg. Allg. Chem. 1996;622:807. Vogel U, Timoshkin AY, Schwan K-C, Bodensteiner M, Scheer M. J. Organomet. Chem. 2006;691:4556..
- 11.An analogous experiment with PCy3 shows that the majority of PCy3 is uncoordinated during catalytic reactions as well.
- 12.Bruckmann J, Krüger C. Acta. Crystallogr., Sect. C: Cryst. Struct. Commun. 1995;C51:1152. [Google Scholar]
- 13.Bodner GM, May MP, McKinney LE. Inorg. Chem. 1980;19:1951. [Google Scholar]
- 14.Bartik T, Himmler T, Schulte H-G, Seevogel K. J. Organomet. Chem. 1984;272:29. [Google Scholar]
- 15.McCampbell TA, Kinkel BA, Miller SM, Helm ML. J. Chem. Crystallogr. 2006;36:271. [Google Scholar]
- 16.Consistent with this hypothesis, the borylative coupling reaction with tris(2,4-di-tert-butylphenyl) phosphite as the ligand (cone angle = 215°, Crous R, Datt M, Foster D, Bennie L, Steenkamp C, Huyser J, Kirsten L, Steyl G, Roodt A. Dalton Trans. 2005:1108. doi: 10.1039/b416917d..) also furnishes 4 selectively, albeit in an inferior yield relative to P(SiMe3)3.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.