Summary
The use of tissue plasminogen activator (tPA) as a thrombolytic treatment in ischemic stroke is limited largely due to concerns for hemorrhagic complications. The underlying mechanisms are still unknown but evidence is beginning to emerge that tPA interacts with key regulators of the neurovascular unit (NVU), and that these interactions may contribute to the undesirable side effects associated with the use of tPA in ischemic stroke. Understanding these connections and tPA’s normal function within the NVU may offer new insights into future therapeutic approaches.
Keywords: Blood brain barrier, LRP, neurovascular unit, PDGF, stroke, tPA
Overview
tPA, a highly specific serine protease in the fibrinolytic cascade, is currently the only thrombolytic agent approved by the US Food and Drug Administration for treatment of ischemic stroke [1]. However, emerging evidence demonstrates that the beneficial thrombolytic effects of tPA within the vascular lumen are counteracted by its activities within the central nervous system (CNS) [2] and clinically, thrombolytic tPA is known to increase the incidence of intracerebral hemorrhage, a severe complication of stroke that is associated with high mortality [1]. Therefore, current guidelines recommend thrombolytic tPA to be used only within the first 3 hours after the onset of symptoms, and this restriction markedly reduces the number of patients who receive this treatment [1]. Therefore, understanding the mechanisms that underlie the relationship between tPA and its effect on cerebrovascular function should facilitate the development of new and safer therapeutic strategies for treating ischemic stroke.
One of the first studies demonstrating that tPA can negatively affect outcome in focal cerebral ischemia (FCI) showed that tPA−/− mice had significantly smaller cerebral infarcts than wild-type mice, and that intravenous administration of tPA to tPA−/− mice increased infarct volume to levels comparable to wild-type controls [2]. This was later confirmed in a different model of FCI using adenoviral vectors expressing tPA [3]. Studies with the primary inhibitor of tPA in the CNS, neuroserpin, also showed that blocking tPA activity can provide neuronal protection and reduce infarct volume [4].
The mechanism whereby tPA deficiency or its inhibition within the CNS could reduce infarct size after stroke is not completely understood; however, an important observation was made when studies showed a correlation between the use of tPA and increased BBB permeability [5]. Shortly thereafter, Yepes et al provided convincing evidence that tPA is both necessary and sufficient to induce early opening of the BBB after stroke [6]. This study demonstrated that tPA−/− mice were protected from early loss of BBB integrity after stroke (necessary) and that direct injection of recombinant tPA into the cerebrospinal fluid (CSF) of non-ischemic mice induced a rapid loss of the BBB integrity (sufficient). This study also provided proof that the opening of the BBB is dependent on the proteolytic activity of tPA, but not on plasminogen, indicative of the existence of another tPA substrate (see below).
tPA’s mechanism of action within the CNS
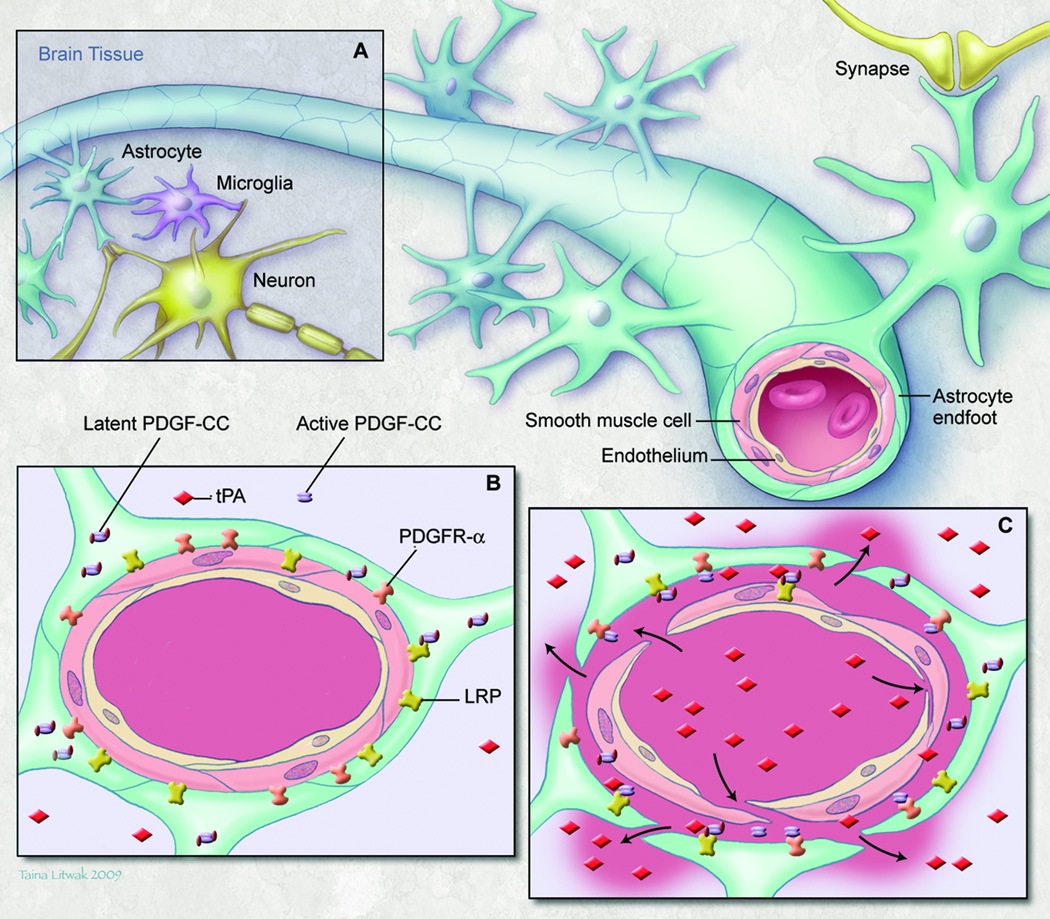
The cerebrovascular bed is unique in that normal CNS function requires a highly regulated extracellular environment to keep the concentrations of most molecules within a narrow range [7]. Thus, the BBB exists to tightly control the trafficking of substances between the blood and the CNS, and to facilitate the rapid reestablishment of extracellular homeostasis following neuronal activity [8]. The physical barrier of the BBB is evident from the inter-endothelial cell tight junctions and an integral basal lamina, formed by both the endothelial cells and astrocyte endfeet. However, the BBB is more than just a barrier and its regulation is complex and dependent on the NVU, which is composed of vascular endothelial cells, mural cells (vascular smooth muscle cells and pericytes), astrocytes, microglia, neurons and extracellular matrix, all working together in a coordinated way to regulate the extracellular environment of the brain parenchyma. (Fig.1 A) [7,8]
Fig.1.
Neurovascular unit (NVU): (a) Components of the NVU (b) Precapillary arteriole with intact NVU (c) Response of the NVU to focal cerebral ischemia; endogenous tPA bound to LRP activates latent PDGF-CC, and active PDGF-CC binds to PDGFRα. Thrombolytic tPA in the blood can cross a compromised BBB and activate additional PDGF-CC which exacerbates loss of BBB integrity.
The association of tPA with the NVU and its correlation with BBB permeability is now well established. Very early after the onset of FCI, tPA activity is increased in ischemic regions where permeability of the BBB is also elevated [4,6]. In addition, tPA’s action on the BBB requires interaction with the CNS side of the NVU as high doses of tPA administered intravenously do not elicit opening of the BBB, whereas low doses of tPA on the CNS side of the NVU do [9].
Significant efforts have been made to delineate the mechanisms of tPA actions in the CNS, which resulted in the discovery of several potential downstream mediators including NMDA receptors [10], matrix metaloprotease 9 (MMP-9) [11], activated protein C (APC) [12], and platelet-derived growth factor CC (PDGF-CC) [9]. All of these downstream mediators except APC have been linked directly to the low density lipoprotein receptor-related protein (LRP) and blocking LRP either with antagonists or specific antibodies reduces BBB permeability and infarct expansion after FCI [6,13]. LRP can regulate the expression of MMP-9 in cerebral endothelial cells in culture in a tPA-dependent manner [11], and APC has been shown to down-regulate tPA-mediated MMP-9 expression in human brain endothelial cells [12]. However, MMP-9–null mice were not protected from early BBB dysfunction after FCI (6h) [6], and while deletion of MMP-9 has been shown to reduce BBB permeability and stroke volume 24 hours after stroke [14], the depletion of circulating leukocytes completely blocks the rise in MMP-9 activity in the first 24 hours after FCI [15]. This suggests that the early increase in MMP-9 activity after FCI is due to infiltrating leukocytes, and consistent with this interpretation, MMP-9 is not expressed in either neurons or astrocytes in the first 24 hours after FCI [16]. These data suggest that MMP-9–mediated events occur on the luminal side of the NVU and might not be critical for the early response of the NVU to FCI.
On the CNS side of the NVU, tPA appears to regulate the BBB via a different process. Our recently published data show that platelet-derived growth factor CC (PDGF-CC) is acting directly downstream of tPA in the CNS [9]. PDGF-CC is expressed as a latent factor that is cleaved by tPA to generate active PDGF-CC capable of triggering PDGF receptor α (PDGFRα) signaling [17]. Injection of active PDGF-CC into the CSF increased the BBB permeability within 1 hour, suggesting that PDGF-CC signaling is immediate and does not require gene expression or synthesis. Furthermore, neutralizing antibodies against PDGF-CC inhibited tPA-induced opening of the BBB as did blockade of PDGFRα [9].
Both PDGF-CC and its receptor in the NVU are located in perivascular cells surrounding cerebrovascular arterioles. Transmission electron microscopy also confirms the apparent formation of edema surrounding cerebrovascular arterioles but not capillaries, within 1 hour after tPA or PDGF-CC injection. There is no gross destruction of vascular structures within this time frame even though the extent of Evans Blue extravasation into the brain parenchyma is significant, suggesting that the activation of PDGF-CC/PDGFRα may represent a regulated physiological process that controls the BBB.
The activation of PDGF-CC by tPA appears to be dependent on LRP; however, the activity of active PDGF-CC is not, suggesting that LRP and tPA interact upstream of active PDGF-CC/PDGFRα interaction [9]. As illustrated in Fig.1B, LRP is present on the astrocyte endfeet along with PDGFRα. In response to events such as FCI, tPA is secreted and bound to LRP facilitating the cleavage of PDGF-CC. Active PDGF-CC in turn binds to and activates PDGFRα leading to the opening of the BBB (Fig. 1C).
Late delivery of tPA (>3h after onset) has been shown to exacerbate intracranial hemorrhagic transformation, a severe complication of ischemic stroke. However, blockade of the PDGF-CC/PDGFR signaling pathway with imatinib, a known PDGFR antagonist, significantly reduces the extent of hemorrhage and improves outcome [9]. These observations suggest a mechanism whereby tPA might promote the development of hemorrhagic transformation.
tPA and vascular tone in cerebral vasculature
Local cerebral blood flow increases rapidly in response to neural activity, a phenomenon termed functional hyperemia or neurovascular coupling. Neurovascular coupling is critical for the maintenance of substrate and energy supply and for the clearance of metabolic byproducts [8]. Although the process of neurovascular coupling is still not completely understood, studies have demonstrated a role for several vasoactive mediators acting through the perivascular astrocytes [18]. For example, nitric oxide released in response to NMDA receptor activation plays a critical role in many models of neurovascular coupling. Other mediators, including extracellular potassium, cyclooxygenase metabolites, and adenosine have also been shown to be involved [8], and very recent evidence has implicated endogenous tPA as a direct modulator of neurovascular coupling [19].
The coupling of local neural activity to local blood flow is controlled by both the resistance vessels on the brain surface (pial arterioles) and the smaller penetrating arterioles. Interestingly, tPA has been shown to be primarily associated with precapillary arterioles in the CNS [6,9,20]. Furthermore, tPA has been reported to reduce vessel reactivity to increased luminal pressure and vasoactive mediators, suggesting that tPA may be involved in regulating vascular tone [21,22], and systemic delivery of tPA at low concentrations can directly reduce cerebral vascular resistance and systemic blood pressure [22]. Taken together, these studies suggest that tPA may modulate cerebrovascular tone during functional hyperemia.
The prospect that tPA has a role in normal neurovascular coupling has been tested by Park et al who used a model of whisker stimulation that increases the activity of specific neurons in the somatosensory cortex [19]. Compared with wild-type mice, cerebral blood flow in the corresponding barrel cortex of tPA−/− mice showed a sustained attenuation after whisker stimulation, suggesting that tPA is required for neurovascular coupling. In addition, administration of tPA to tPA−/− mice restored neurovascular coupling; the NMDA receptor and activation of nitric oxide synthase were implicated as mediators of this response.
The relationship between tPA-induced neurovascular coupling and opening of the BBB is not known. However, molecules that control both processes have their commonalities, and pathways that regulate both are very likely to be interconnected. For example, nitric oxide, reported to be a downstream mediator of tPA action, induces both opening of the BBB and neurovascular coupling [19,23]. Thus it is interesting to speculate that the effects of tPA on the BBB and hemorrhagic transformation in stroke may be causally related to tPA’s role in regulating cerebral vascular tone and cerebral blood flow.
Conclusions
The regulation of BBB/NVU integrity after FCI plays a critical role in the progression of lesion development and hemorrhagic transformation, and may hold the key to better treatments for this devastating disorder. Paradoxically, tPA, the only drug approved to treat stroke, may prove to be uniquely associated with the risk of hemorrhagic transformation. However, discovering downstream mediators of tPA like PDGF-CC/PDGFR could be instrumental in solving this critical dilemma.
Acknowledgements
This work was supported by National Institutes of Health grants HL-55374, HL-54710, and HL-89407 (to D.A. Lawrence); and grants from Karolinska Institutet, Novo Nordisk Foundation, Swedish Research Council, Swedish Cancer Foundation, and the LeDucq Foundation (to U. Eriksson).
Footnotes
Disclosure and Conflict of Interests: The authors state they have no conflict of interest.
References
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Wang YF, Tsirka SE, Strickland S, Stieg PE, Soriano SG, Lipton SA. Tissue plasminogen activator (tPA) increases neuronal damage after focal cerebral ischemia in wild-type and tPA-deficient mice. Nat Med. 1998;4:228–231. doi: 10.1038/nm0298-228. [DOI] [PubMed] [Google Scholar]
- 3.Nagai N, De Mol M, Lijnen HR, Carmeliet P, Collen D. Role of plasminogen system components in focal cerebral ischemic infarction: a gene targeting and gene transfer study in mice. Circulation. 1999;99:2440–2444. doi: 10.1161/01.cir.99.18.2440. [DOI] [PubMed] [Google Scholar]
- 4.Yepes M, Sandkvist M, Wong MK, Coleman TA, Smith E, Cohan SL, Lawrence DA. Neuroserpin reduces cerebral infarct volume and protects neurons from ischemia-induced apoptosis. Blood. 2000;96:569–576. [PubMed] [Google Scholar]
- 5.Zhang Z, Zhang L, Yepes M, Jiang Q, Li Q, Arniego P, Coleman TA, Lawrence DA, Chopp M. Adjuvant treatment with neuroserpin increases the therapeutic window for tissue-type plasminogen activator administration in a rat model of embolic stroke. Circulation. 2002;106:740–745. doi: 10.1161/01.cir.0000023942.10849.41. [DOI] [PubMed] [Google Scholar]
- 6.Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J. Clin. Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 8.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 9.Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, Gao Y, Pietras K, Mann K, Yepes M, et al. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat. Med. 2008;14:731–737. doi: 10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, Vivien D, Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat. Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat. Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- 12.Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z, Liu D, Maggirwar SB, Deane R, Fernandez JA, et al. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat. Med. 2006;12:1278–1285. doi: 10.1038/nm1498. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Polavarapu R, She H, Mao Z, Yepes M. Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein mediate cerebral ischemia-induced nuclear factor-kappaB pathway activation. Am. J. Pathol. 2007;171:1281–1290. doi: 10.2353/ajpath.2007.070472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J. Cereb. Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Copin JC, Merlani P, Sugawara T, Chan PH, Gasche Y. Delayed matrix metalloproteinase inhibition reduces intracerebral hemorrhage after embolic stroke in rats. Exp. Neurol. 2008;213:196–201. doi: 10.1016/j.expneurol.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat. Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 17.Fredriksson L, Li H, Fieber C, Li X, Eriksson U. Tissue plasminogen activator is a potent activator of PDGF-CC. EMBO J. 2004;23:3793–3802. doi: 10.1038/sj.emboj.7600397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 19.Park L, Gallo EF, Anrather J, Wang G, Norris EH, Paul J, Strickland S, Iadecola C. Key role of tissue plasminogen activator in neurovascular coupling. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1073–1078. doi: 10.1073/pnas.0708823105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin EG, del Zoppo GJ. Localization of tissue plasminogen activator in the endothelium of a limited number of vessels. Am. J. Pathol. 1994;144:855–861. [PMC free article] [PubMed] [Google Scholar]
- 21.Cipolla MJ, Lessov N, Clark WM, Haley EC., Jr Postischemic attenuation of cerebral artery reactivity is increased in the presence of tissue plasminogen activator. Stroke. 2000;31:940–945. doi: 10.1161/01.str.31.4.940. [DOI] [PubMed] [Google Scholar]
- 22.Nassar T, Akkawi S, Shina A, Haj-Yehia A, Bdeir K, Tarshis M, Heyman SN, Higazi AA. In vitro and in vivo effects of tPA and PAI-1 on blood vessel tone. Blood. 2004;103:897–902. doi: 10.1182/blood-2003-05-1685. [DOI] [PubMed] [Google Scholar]
- 23.Parathath SR, Parathath S, Tsirka SE. Nitric oxide mediates neurodegeneration and breakdown of the blood-brain barrier in tPA-dependent excitotoxic injury in mice. J. Cell Sci. 2006;119:339–349. doi: 10.1242/jcs.02734. [DOI] [PubMed] [Google Scholar]