Abstract
Objectives
An in-vivo rabbit model was used to study the effect of three hours of experimental induced phonation on messenger RNA expression of the normal vocal fold.
Study Design
Prospective; animal model.
Subjects and Methods
Ten rabbits received experimental phonation for three hours, followed by one hour of recovery. A separate group of five rabbits served as no-phonation controls. We measured messenger RNA expression of matrix metalloproteinase -1, -9, and interleukin-1β using real-time reverse transcribed polymerase chain reaction. Gene expression ratios from phonation and control animals were compared using the Mann Whitney U test.
Results
Phonation (77 +/− 3 dB; 429 +/−141 Hz) resulted in increased matrix metalloproteinase -1 gene expression from rabbits receiving experimental phonation compared to controls, and a non-significant increase in matrix metalloproteinase -9 and interleukin-1β gene expression.
Conclusion
Matrix metalloproteinases play a role in maintaining tissue homeostasis. Investigation of cellular responses to experimental phonation may provide insight into how matrix metalloproteinases and other extracellular matrices contribute to maintenance of the vocal fold and development of pathology.
INTRODUCTION
The vocal fold lamina propria is an area of connective tissue with significant clinical and biological importance. Alterations in the extracellular matrix lead to dysphonia in humans, which presents a frustrating therapeutic challenge. Biologically, the vocal folds are uniquely different from tissues found elsewhere in the body because of the continuous mechanical forces acting on the tissue during vibration. Nowhere else does vibration occur naturally and regularly at frequencies of 100-1000 Hz.1 It has been proposed that vocal fold tissues may develop specialized functions to withstand such mechanical stress.1 Our laboratory has developed a rabbit model to study phonation related molecular alterations in-vivo. Our objective is to investigate the role of these potentially injurious mechanical forces on expression and turnover of the vocal fold extracellular matrix. The rabbit phonation model allows for the investigation of vibration induced gene regulation, extracellular matrix deposition, and tissue remodeling in-vivo. The purpose of our current experiment was to investigate matrix metalloproteinase (MMP) -1, -9, and interleukin-1β (IL-1β) related gene expression from vocal fold tissues exposed to three hours of in-vivo experimental induced phonation, followed by one hour of recovery.
Matrix metalloproteinases (MMPs) belong to a family of enzymes that play an important role during wound healing. The MMP family has been divided into subgroups based on activity against various matrix components. MMP-1 is considered the prototype for all the interstitial collagenases and was the first vertebrate collagenase purified as a protein.2 Specifically, MMP-1 is an interstitial collagenase, which catalyzes the initial step in the breakdown of fibrillar collagen types I and III. MMP-9 is a collagenase and gelatinase, which degrades basement membrane collagens as well as gelatins.3 Connective tissues, such as vocal fold lamina propria are maintained by the degradation and synthesis of extracellular matrix components. MMPs play an important part in maintaining tissue homeostasis, as does IL-1β a pro-inflammatory cytokine, which serves as an initiator of the inflammatory process partly through MMP induction.
Recent studies have reported altered messenger RNA (mRNA) expression of extracellular matrix related genes from fibroblast cultures responding to various magnitudes of mechanical strain.1,4,5 Such cellular responses support the idea of dynamic remodeling and suggest the need for in-vivo studies to investigate phonation induced gene regulation, extracellular matrix deposition, and tissue remodeling. The in-vivo setting is crucial for study of normal physiologic processes as it contains various cells and extracellular matrices in their native 3-dimensional environment. The in-vivo environment is especially important for the study of MMP-1 because it is synthesized and secreted in a latent form and can be activated by other proteases. Confirmation of phonation induced changes in gene expression and tissue protein levels in-vivo may provide evidence for modern-day opinion that the vocal folds undergo a process of dynamic tissue remodeling as mechanical forces act on the tissue during vibration. Our laboratory has been using an in-vivo rabbit model to study phonation related molecular alterations. The rabbit is an ideal model because it shares similarities with humans in terms of vocal fold extracellular matrix components, and is a quiet animal which allows increased control over non experimental vocalization. This level of control is difficult to achieve in vocalizing animals such as dogs, pigs, and rats, which require nerve injury to prohibit voice use outside of the laboratory setting. In the following study, we tested the null hypothesis that the population distribution functions for MMP-1, -9, and IL-1β gene expression were identical against the alternative hypothesis that they differ by location from rabbits receiving experimental induced phonation and control rabbits.
METHODS
Fifteen New Zealand White breeder rabbits weighing 3-5 kg were involved in this study. Induction of anesthesia was achieved using Ketamine 35mg/kg, Xylazine 5mg/kg, and Acepromazine 0.75mg/kg administered intramuscularly. Heart rate, temperature, and oxygen saturation levels, were monitored throughout the experiment to monitor the animal's state of anesthesia and general well being. Subsequent intramuscular injections of Ketamine (17.5 mg/kg) and Acepromazine (0.375 mg/kg) were provided as needed to maintain a surgical anesthetic plane.
Animals were placed supine on an operating platform. The neck was shaved and prepped for surgery. The larynx and trachea were exposed using a midline neck incision extending from the hyoid bone to the sternal notch. An endotracheal tube was inserted via a low tracheotomy approximately 3.5 cm below the cricoid cartilage for spontaneous respiration and a cuffed endotracheal tube (RUSCh, Germany) was positioned to rest approximately 2 cm below the glottis to deliver airflow through the glottis. The cuff of this tube was inflated to seal off the trachea. Custom bipolar stainless steel hooked wire electrodes were inserted medio-laterally into the cricothyroid muscle. A flowmeter (Barnant, USA) and humidifier (Concha Therm III, CA, USA) were used to deliver compressed humidified air heated to 37° C through the glottis.
A Grass S-88 stimulator (SA Instrumentation, Encinitas, CA) and constant current isolation unit (Grass Telefactor; model PSIU6; West Warwick, RI.) were used to provide electrical stimulation to the cricothyroid muscle at 50 Hz, with 4 mA current, for 1 ms pulse duration. The total train duration was 5 seconds (2 seconds on; 3 seconds off). These stimulation settings were established from our previous work involving the in-vivo phonation model.*
Phonation was produced using continuous airflow delivered at a rate of 143 cm3/sec. This variable remained constant throughout the experiment. A pressure transducer located approximately 2 cm below the glottal aperture was used to record subglottal air pressure. Acoustic signals were recorded and amplified using a single channel audio mixer (Shure M267, CA, USA) coupled to a frequency counter (6002, Global Specialties, CT, USA) and acquired using a data acquisition system (DATAQ Model 720, DATAQ Instruments, Ohio, USA).
Ten animals received experimental induced phonation (cricothyroid stimulation and airflow) for three hours, followed by one hour of recovery (no stimulation; no airflow). A separate group of five rabbits served as no-phonation controls. Control rabbits received cricothyroid stimulation for three hours in the absence of airflow, followed by one hour of recovery (no stimulation). As a result, surgical procedures were similar for both groups and phonation was the only variable manipulated. The one hour recovery period was used to standardize tissue collection across all animals involved in the study.
This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Vanderbilt University.
Real-Time Reverse Transcribed Polymerase Chain Reaction (RT-PCR)
We measured the effects of three hours of experimental induced phonation followed by one hour of recovery on mRNA expression of MMP-1, MMP-9, and IL-1β in the vocal fold using real time RT-PCR. A 1mm tissue specimen of lamina propria was obtained superficially from the middle 1/3 portion of the vocal fold bilaterally from experimental and control animals, immediately following the one hour recovery period. Specimens were immediately submerged in RNAlater stabilization reagent (QIAGEN Inc. USA) incubated at 4°C overnight, and stored at −80°C until extraction. Animals were euthanized without recovery from anesthesia after collection of specimens.
Tissue specimens were placed in120mg Zirconia/Silica beads (1-mm-diameter) and homogenized at 4800 rpm for 90 seconds using a Mini-Beadbeater homogenizer (BioSpec Products, Inc. USA). We extracted mRNA from tissue specimens using the RNeasy® Fibrous Tissue Mini Kit (QIAGEN Inc. USA) and treated the specimens with ribonuclease-free deoxyribonuclease I (QIAGEN Inc. USA) to minimize contamination from genomic DNA. Samples of mRNA were stored at −80°C. Reverse transcription was performed with the iScript™ cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, California) using the manufacturers recommended reaction protocol.
We used the iQ™SyBR Green Supermix Kit to perform real-time PCR in a 50 μL volume reaction mixture composed of 500 nM primer1, 500 nM primer 2, 25 μL iQ SyBR Green Supermix and 1.2 μL template cDNA ribonuclease-free water. Rabbit-specific primers were used for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), MMP-1, MMP-9 and IL-1β. The detailed forward and reverse primer sequences and the size of products are summarized in Table 1. Polymerase Chain Reaction (PCR) was performed under the following conditions: 1 cycle at 95°C for 15 min, followed by 50 cycles at 95°C for 30 seconds, 58°C for 60 seconds, 72°C for 60 seconds, and 1 cycle at 50°C to 95°C in 0.5°C increments to make a melting curve.6 Fifteen specimens were analyzed randomly. PCR measurement was repeated two times per specimen. Analyses were performed in a blinded fashion, such that the examiner was blinded to animal identification and group assignment. We used the iCycler iQ Real-time PCR Detection System (Bio-Rad) to detect the PCR products.
TABLE 1.
PRIMER SEQUENCES
| GAPDH 177bp | Forward: 5_-TCG GCA TTG TGG AGG GGC TC-3_ Reverse: 5_-TCC CGT TCA GCT CGG GGA TG-3_ |
| MMP-1 322bp | Forward: 5_-TCA GTT CGT CCT CAC TCC AG-3_ Reverse: 5_-TTG GTC CAC CTG TCA TCT TC-3_ |
| MMP-9 218bp | Forward: 5_-TGC CAG GAG TAC CTG TTC CGC TAT G-3_ Reverse: 5_-TGC CAC TTG AGG TCA CCC TCG AA-3_ |
| IL-1β 354bp | Forward: 5_-TCC AGC TGC GCA TCT CCT GC -3_ Reverse: 5_-CTT CTC CTT GCA CAA AAC TC -3_ |
GAPDH - glyceraldehyde-3-phosphate dehydrogenase; MMP– matrix metalloproteinase; IL– 1β interleukin-1β.
Relative quantitative gene expression was determined by the ratio of target gene concentration to the internal control GAPDH.7 For PCR negative control samples, primers were not added during PCR. PCR products were separated by electrophoresis in 2.5 % agarose gels containing 0.5μg/mL ethidium bromide to verify PCR products according to fragment size.
Statistical Analysis
Gene expression ratios from phonation and control groups were compared using the Mann Whitney U rank-sum test for two independent samples.8 We tested the null hypothesis that the population distribution functions for gene expression ratios were identical against the alternative hypothesis that they differ by location. The threshold for significance was set to p<.05. Analyses were performed using two-tailed p-values. The analysis of the data was performed using SPSS 15.0 for Windows (SPSS, Inc.).
Role of the Funding Source
The study sponsors (NIH, VUMC) had no role in study design, data collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
RESULTS
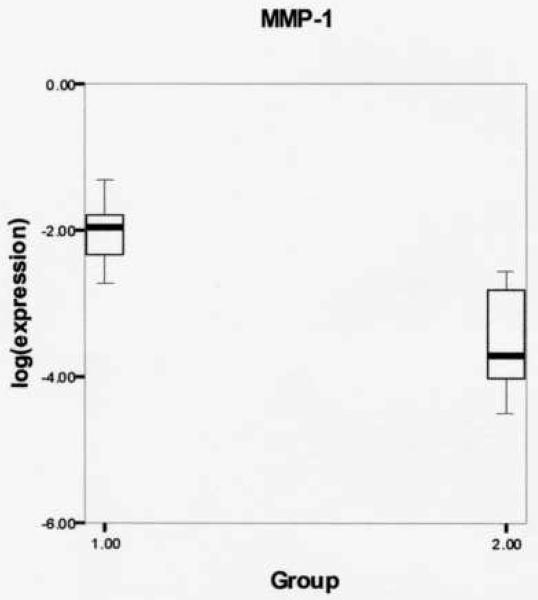
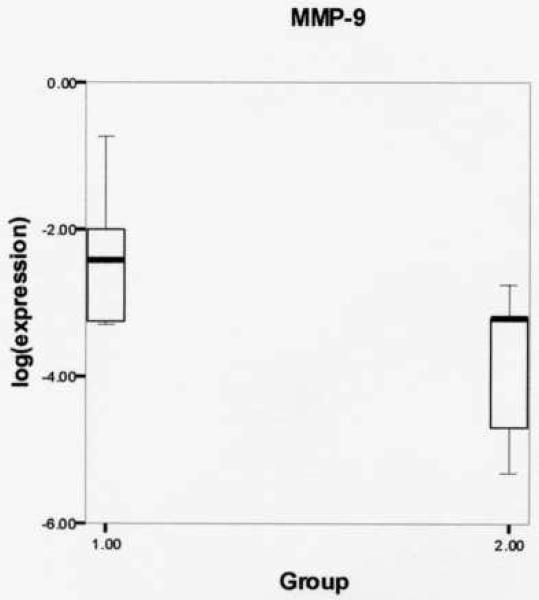
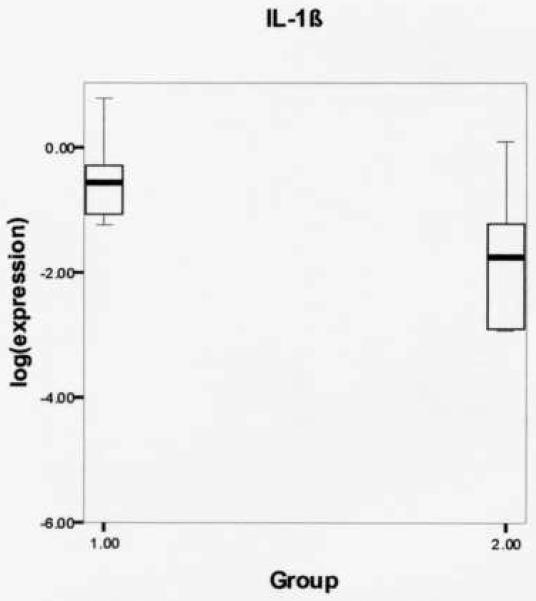
Log-transformed expression ratios were compared between the phonation and control group (Table 2) using the Mann Whitney U two-sample rank-sum test for the variables MMP-1, MMP-9, and IL-1β. Figure 1 shows significantly increased MMP-1 gene expression from rabbits receiving experimental phonation compared to control rabbits (mean (SD): −2.0336 (.45723) vs. −3.5089 (.81638), respectively, P = .004). Figure 2 reveals a non-significant increase in MMP-9 gene expression from experimental vs. controls (mean (SD): −2.3625 (.91368) vs. −3.8245 (1.1040), respectively, P = .075). Figure 3 shows a non-significant increase in IL-1β gene expression from rabbits receiving experimental phonation compared to control rabbits (mean (SD): −.5267 (.61870) vs. −1.7439 (1.2622), respectively, P = .075).
TABLE 2.
LOG TRANSFORMED MATRIX METALLOPROTEINASE-1 (MMP-1), MATRIX METALLOPROTEINASE-9 (MMP-9) AND INTERLEUKIN-1β (IL-1β) GENE EXPRESSION RATIOS FOR PHONATION (1) AND CONTROL GROUPS (2). SPECIMEN NOT AVAILABLE FOR ANALYSIS (---).
| MMP-1 | MMP-9 | IL-1β | |||
|---|---|---|---|---|---|
| Expression | Group | Expression | Group | Expression | Group |
| −1.88596 | 1 | −3.16558 | 1 | −0.96480 | 1 |
| −2.72125 | 1 | −0.72398 | 1 | 0.77364 | 1 |
| −2.64898 | 1 | −3.24033 | 1 | −1.06715 | 1 |
| −1.30948 | 1 | −1.13338 | 1 | 0.05268 | 1 |
| −1.77757 | 1 | −2.25924 | 1 | −0.28899 | 1 |
| −2.32395 | 1 | −2.05154 | 1 | −0.54035 | 1 |
| −1.69179 | 1 | −1.98076 | 1 | −0.30254 | 1 |
| −1.98678 | 1 | −3.27984 | 1 | −1.07769 | 1 |
| −1.95669 | 1 | −3.24185 | 1 | −0.60507 | 1 |
| --- | 1 | −2.54821 | 1 | −1.24626 | 1 |
| −2.80559 | 2 | −4.67489 | 2 | −1.77156 | 2 |
| −4.01278 | 2 | −3.19393 | 2 | −1.23121 | 2 |
| −2.54821 | 2 | −2.74982 | 2 | 0.09934 | 2 |
| −4.48986 | 2 | −3.19518 | 2 | −2.89757 | 2 |
| −3.68825 | 2 | −5.30892 | 2 | −2.91865 | 2 |
Figure 1.
Log-transformed matrix metalloproteinase-1 (MMP-1) gene expression ratios for phonation (1) and control (2) groups. Results revealed increased MMP-1 gene expression from rabbits receiving experimental induced phonation compared to controls (mean (SD): −2.0336 (.45723) vs. −3.5089 (.81638), respectively, P = .004).
Figure 2.
Log-transformed matrix metalloproteinase-9 (MMP-9) gene expression ratios for phonation (1) and control (2) groups. Results revealed a non-significant increase in MMP-9 gene expression from rabbits receiving experimental induced phonation compared to controls (mean (SD): −2.3625 (.91368) vs. −3.8245 (1.1040), respectively, P = .075).
Figure 3.
Log-transformed interleukin-1 β (IL-1β) gene expression ratios for phonation (1) and control (2) groups. Results revealed a non-significant increase in IL-1β gene expression from rabbits receiving experimental induced phonation compared to controls (mean (SD): −.5267 (.61870) vs. −1.7439 (1.2622), respectively, P = .075).
DISCUSSION
Connective tissue is maintained as a balance between synthesis and degradation of matrix components. Both processes are tightly regulated to allow for normal function of tissue. Degradation of connective tissue occurs during inflammatory processes, such as after injury from mechanical stress, and is controlled by MMPs and tissue inhibitors of MMPs (TIMP). MMP-1 is a multifunctional molecule involved in the turnover of collagen in the extracellular matrix as well as the cleavage of non-matrix substances and cell surface molecules.2 MMP-1 is one of only four members of the MMP family capable of degrading fibrillar collagens in their triple helical domain.2 Thermally unstable collagens degraded by MMP-1 unwind to form gelatin, which can be further degraded by other MMPs. MMP-1 can also act on and regulate the function of other biologically active molecules such as fibroblast growth factor by cleaving the proteoglycan perlecan, demonstrating its significant role during physiologic tissue remodeling and revealing the importance of studying these processes in-vivo.9
MMP-1 is undetectable in normal resting tissues. However, expression is increased during physiologic and pathologic tissue remodeling in vivo.10 Regulation of MMP-1 is at the level of gene expression, extracellularly where activation of latent forms occurs and is dependent on the amount of specific inhibitors present in the tissue. De novo synthesis of MMPs can be induced by a variety of cytokines, such as hepatocyte growth factor, epidermal growth factor, transforming growth factor-α, and interleukin-1, an effector molecule synthesized by connective tissue and inflammatory cells. Interestingly, MMP-1 transcription is suppressed by transforming growth factor-β and vitamin A derivatives, all-trans retinoic acid, and the synthetic retinoids.2
Results of the current experiment revealed a significant increase in MMP-1 gene expression from normal vocal fold lamina propria in rabbits receiving three hours of experimental induced phonation followed by one hour of recovery. Findings of increased MMP-1 gene expression from in-vivo phonation are in agreement with similar findings of increased MMP-1 gene expression from in-vitro fibroblast cultures exposed to 100 Hz vibratory strain for 6 hours using a novel bioreactor.1 In the current study using an in-vivo rabbit phonation model, experimental induced phonation (77 +/− 3 dB; 429 +/−141 Hz) resulted in an increase of MMP-1 gene expression in the normal vocal fold lamina propria. These data are the first to demonstrate phonation associated MMP-1 gene alterations in-vivo using a physiologic animal preparation and provide evidence that prolonged phonation within a physiologic range of human voice production (77 +/− 3 dB; 429 +/−141 Hz), results in an upregulation of MMP-1 gene expression in normal vocal fold lamina propria. These data are consistent with studies revealing elevated expression of MMP during system disturbance, such as during wound healing and in response to injury.2
MMP-9, a gelatinase plays a key role in extracellular matrix remodeling and is involved in a number of biologic processes, such as bone growth, skin wound healing, sinusitis, asthma and colitis. MMP-9 displays a broad spectrum of activity against various extracellular matrix components including denatured collagens (gelatins), type IV collagen, type V collagen, and elastin. Expression of MMP-9 is maximally upregulated 24-72 hours after wounding, followed by a return to near baseline levels after reepithelialization.11,12 MMP-9 upregulation is linked to post-injury inflammation and epithelial migration.12
The development of vocal pathology may be attributed to a disruption in the normal balance of matrix synthesis, deposition, and degradation. Interestingly, Karahan et al13 have revealed increased tissue levels of MMP-9 in stromal vascular walls and spindle cells from human vocal fold polyps removed after microlaryngeal surgery. Additionally, Courey et al14 have reported less staining of collagen type IV in the basement membrane zone of lesions clinically diagnosed as polyps compared to lesions diagnosed as nodules, which are characterized by a thickened band of collagen type IV around the basement membrane.15 Results of the current experiment revealed a non-significant increase in MMP-9 gene expression from rabbits receiving three hours of experimental induced phonation followed by one hour of recovery, compared to control. In other tissues, increased MMP-9 expression has been associated with low levels of collagen during the remodeling phase of wound repair and may be linked to decreased scar formation.16 Findings have led some to believe that increased MMP-9 expression may play an important role during scarless tissue healing given that rapid degradation of collagen may be necessary to prevent scar formation.17 Accordingly, MMP-9 gene transcription may play a significant physiologic role in vocal fold extracellular matrix remodeling, regulation of the vocal fold inflammatory response, and the prevention of pathology. Given findings of increased MMP-9 in vocal fold polyps, it would be interesting to investigate the role of MMP-9 in minimizing abnormal matrix accumulation in the vocal fold. Phonation associated alterations in MMP-9 expression may be a factor in minimizing the excess accumulation of collagen during the development of vocal fold pathology. Measurement of gene expression and tissue protein levels from a large cohort will be necessary in future experiments to test this hypothesis.
IL-1β is a cytokine that is pro-inflammatory and is secreted by macrophages, monocytes and epithelial cells during injury. IL-1β levels are increased from vocal fold surface secretions during acute vocal fold injury, with maximal expression one day post injury.18 There has also been a case report showing strong shifts of IL-1β levels from surface secretions obtained from the vocal fold in a human subject following intense vocal loading.19 Results from the current experiment revealed a non-significant increase in IL-1β gene expression from normal vocal fold lamina propria in rabbits exposed to three hours of experimental induced phonation followed by one hour of recovery. It is possible that IL-1β gene expression may have stabilized during the one hour recovery period, thus eliminating differences in mRNA levels. This would be advantageous from a tissue injury standpoint as stabilization of IL-1β may help attenuate the inflammatory response and minimize excessive matrix accumulation. One could speculate that a reduced inflammatory response may prevent some vocal overusers from developing voice problems. Considering that reduced inflammation is associated with scarless fetal wound repair20 it may be that tight control of the inflammatory cascade in normal vocal fold lamina propria is an essential homeostatic mechanism for minimizing vocal fold injury.
One possible drawback of our study is that we did not compute power a priori, which opens the possibility of committing a type II error in those hypotheses we failed to reject. Given the borderline P values obtained for MMP-9 and IL-1β, this possibility cannot be dismissed, although the fact that significance was obtained for MMP-1 suggests that power was adequate for at least this response. We did not compute power post hoc because of its known limitations.21,22
Although increased gene expression was found for MMP-1, it should be noted that pre and post translation events may differ. Therefore, measurement of tissue levels is important to determine whether increased gene expression results in increased MMP-1 levels in tissue, and whether or not this increase translates to a reduction of tissue collagen. This is a logical extension of this work and additional studies using the in-vivo phonation model will be necessary to determine the expression and deposition of MMP, tissue inhibitors of MMP, as well as structural glycoproteins and fibrous proteins.
CONCLUSIONS
We used an in-vivo rabbit phonation model to investigate the effects of experimental induced phonation on mRNA expression of the normal vocal fold. Results revealed increased gene expression of MMP-1 and a non-significant increase in MMP-9 and IL-1β following three hours of experimental induced phonation, followed by a one hour recovery period. Experimental induced phonation was within a physiologic range of human voice production (77 +/− 3 dB; 429 +/−141 Hz). Knowledge of MMP regulation and expression in normal and phonated vocal fold tissues may provide clues into how MMPs and other extracellular matrices contribute to the maintenance of the vocal fold lamina propria and development of pathology. Additionally, improved understanding of phonation induced gene regulation may help us to identify unique molecular targets to treat and prevent dysphonia.
ACKNOWLEDGMENT
Research supported by NIH grant R03 DC 008400 from the National Institute of Deafness and Other Communication Disorders (NIDCD), and Vanderbilt University Medical Center (VUMC) Department of Otolaryngology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Paper presented at the Annual Meeting of the American Academy of Otolaryngology-Head and Neck Surgery, Washington, D.C., Sept. 16-19, 2007.
Submitted manuscript
References
- 1.Titze IR, Hitchcock RW, Broadhead K, et al. Design and validation of a bioreactor for engineering vocal fold tissues under combined tensile and vibrational stresses. J Biomech. 2004;37(10):1521–9. doi: 10.1016/j.jbiomech.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Pardo A, Selman M. MMP-1: the elder of the family. Int J Biochem Cell Biol. 2005;37(2):283–8. doi: 10.1016/j.biocel.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 3.Mauch C. Regulation of connective tissue turnover by cell-matrix interactions. Arch Dermatol Res. 1998;290(Suppl):S30–S36. doi: 10.1007/pl00007451. [DOI] [PubMed] [Google Scholar]
- 4.Yamazaki S, Banes AJ, Weinhold PS, et al. Vibratory loading decreases extracellular matrix and matrix metalloproteinase gene expression in rabbit annulus cells. Spine J. 2002;2(6):415–20. doi: 10.1016/s1529-9430(02)00427-8. [DOI] [PubMed] [Google Scholar]
- 5.Branski RC, Perera P, Verdolini K, et al. Dynamic Biomechanical Strain Inhibits IL-1beta-induced Inflammation in Vocal Fold Fibroblasts. J Voice. 2006 August 11; doi: 10.1016/j.jvoice.2006.06.005. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper JA, Jr, Bailey LO, Carter JN, et al. Evaluation of the anterior cruciate ligament, medial collateral ligament, achilles tendon and patellar tendon as cell sources for tissue-engineered ligament. Biomaterials. 2006;27(13):2747–54. doi: 10.1016/j.biomaterials.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 7.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 8.Kirk R. Experimental Design. 2 ed. Brooks/Cole Publishing Group; Belmont, CA: 1982. [Google Scholar]
- 9.Whitelock JM, Murdoch AD, Iozzo RV, et al. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J Biol Chem. 1996;271(17):10079–86. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- 10.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3(3):207–14. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 11.Madlener M. Differential expression of matrix metalloproteinases and their physiological inhibitors in acute murine skin wounds. Arch Dermatol Res. 1998;290(Suppl):S24–S29. doi: 10.1007/pl00007450. [DOI] [PubMed] [Google Scholar]
- 12.Soo C, Shaw WW, Zhang X, et al. Differential expression of matrix metalloproteinases and their tissue-derived inhibitors in cutaneous wound repair. Plast Reconstr Surg. 2000;105(2):638–47. doi: 10.1097/00006534-200002000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Karahan N, Baspinar S, Yariktas M, et al. Matrix Metalloproteinases (MMP-2 and MMP-9) and Cyclooxygenase-2 (COX-2) Expressions In Vocal Cord Polyps. J Voice. 2007 July 9; doi: 10.1016/j.jvoice.2007.05.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Courey MS, Shohet JA, Scott MA, et al. Immunohistochemical characterization of benign laryngeal lesions. Ann Otol Rhinol Laryngol. 1996;105(7):525–31. doi: 10.1177/000348949610500706. [DOI] [PubMed] [Google Scholar]
- 15.Gray SD, Hammond E, Hanson DF. Benign pathologic responses of the larynx. Ann Otol Rhinol Laryngol. 1995;104(1):13–8. doi: 10.1177/000348949510400103. [DOI] [PubMed] [Google Scholar]
- 16.Manuel JA, Gawronska-Kozak B. Matrix metalloproteinase 9 (MMP-9) is upregulated during scarless wound healing in athymic nude mice. Matrix Biol. 2006;25(8):505–14. doi: 10.1016/j.matbio.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Vinarsky V, Atkinson DL, Stevenson TJ, et al. Normal newt limb regeneration requires matrix metalloproteinase function. Dev Biol. 2005;279(1):86–98. doi: 10.1016/j.ydbio.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Branski RC, Rosen CA, Verdolini K, et al. Biochemical markers associated with acute vocal fold wound healing: a rabbit model. J Voice. 2005;19(2):283–9. doi: 10.1016/j.jvoice.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Verdolini K, Rosen CA, Branski RC, et al. Shifts in biochemical markers associated with wound healing in laryngeal secretions following phonotrauma: a preliminary study. Ann Otol Rhinol Laryngol. 2003;112(12):1021–5. doi: 10.1177/000348940311201205. [DOI] [PubMed] [Google Scholar]
- 20.Dang C, Ting K, Soo C, et al. Fetal wound healing current perspectives. Clin Plast Surg. 2003;30(1):13–23. doi: 10.1016/s0094-1298(02)00067-6. [DOI] [PubMed] [Google Scholar]
- 21.Hoenig JM, Heisey DM. The abuse of power: the pervasive fallacy of power calculations for data analysis. Am Stat. 2001;(55):19–24. [Google Scholar]
- 22.Goodman SN, Berlin JA. The use of predicted confidence intervals when planning experiments and the misuse of power when interpreting results. Ann Intern Med. 1994;121(3):200–6. doi: 10.7326/0003-4819-121-3-199408010-00008. [DOI] [PubMed] [Google Scholar]