Abstract
Background
Angiogenesis plays an important role in a wide range of physiological processes, and many diseases are associated with the dysregulation of angiogenesis. Radix Astragali is a Chinese medicinal herb commonly used for treating cardiovascular disorders and has been shown to possess angiogenic effect in previous studies but its active constituent and underlying mechanism remain unclear. The present study investigates the angiogenic effects of calycosin, a major isoflavonoid isolated from Radix Astragali, in vitro and in vivo.
Methodology
Tg(fli1:EGFP) and Tg(fli1:nEGFP) transgenic zebrafish embryos were treated with different concentrations of calycosin (10, 30, 100 µM) from 72 hpf to 96 hpf prior morphological observation and angiogenesis phenotypes assessment. Zebrafish embryos were exposed to calycosin (10, 100 µM) from 72 hpf to 78 hpf before gene-expression analysis. The effects of VEGFR tyrosine kinase inhibitor on calycosin-induced angiogenesis were studied using 72 hpf Tg(fli1:EGFP) and Tg(fli1:nEGFP) zebrafish embryos. The pro-angiogenic effects of calycosin were compared with raloxifene and tamoxifen in 72 hpf Tg(fli1:EGFP) zebrafish embryos. The binding affinities of calycosin to estrogen receptors (ERs) were evaluated by cell-free and cell-based estrogen receptor binding assays. Human umbilical vein endothelial cell cultures (HUVEC) were pretreated with different concentrations of calycosin (3, 10, 30, 100 µM) for 48 h then tested for cell viability and tube formation. The role of MAPK signaling in calycosin-induced angiogenesis was evaluated using western blotting.
Conclusion
Calycosin was shown to induce angiogenesis in human umbilical vein endothelial cell cultures (HUVEC) in vitro and zebrafish embryos in vivo via the up-regulation of vascular endothelial growth factor (VEGF), VEGFR1 and VEGFR2 mRNA expression. It was demonstrated that calycosin acted similar to other selective estrogen receptor modulators (SERMs), such as raloxifene and tamoxifen, by displaying selective potency and affinity to estrogen receptors ERα and ERβ. Our results further indicated that calycosin promotes angiogenesis via activation of MAPK with the involvement of ERK1/2 and ER. Together, this study revealed, for the first time, that calycosin acts as a selective estrogen receptor modulator (SERM) to promote angiogenesis, at least in part through VEGF-VEGFR2 and MAPK signaling pathways.
Introduction
Angiogenesis is the establishment of the mature blood vessel network through expansion and remodeling of the pre-existing vascular primordium. Blood vessel formation through angiogenesis involves the induction of new sprouts, coordinated and directed endothelial cell migration, proliferation, sprout fusion (anastomosis) and lumen formation [1]. It is a process tightly regulated by a variety of pro-angiogenic factors such as the estrogen receptors (ERs). ERs are a group of transcriptional factors that belong to the nuclear receptor superfamily and are activated by estrogen. In addition to its reproductive function, ER also plays an important role in the cardiovascular system [2]. Previous studies have demonstrated that ER expressed in endothelial cells mediates angiogenesis through both classical genomic, and rapid non-genomic, mechanisms [3], [4], [5]. Ligands of ER such as 17β-estradiol (E2), estradiol and raloxifene have been shown to induce endothelial cells proliferation and migration [6], [7]. Meanwhile, some isoflavonoids possessing estrogenic properties that are regarded as selective estrogen receptor modulators (SERMs), also provide cardiovascular benefits, including regulation of endothelial cells proliferation, differentiation, adhesion, migration and kinase activation through interacting with ER [8], [9].
Natural products, such as certain Chinese medicines, contain a variety of angiogenic compounds. It has been demonstrated that Rg1 and Rb1, the two prevalent saponins of Ginseng, have opposing effects in modulating angiogenesis [10]. Another Chinese medicine Radix Astragali, which is rich in isoflavonids, is often used either as a single herb or in combination with other Chinese medicines as formula for treating myocarditis [11], heart failure [12], myocardial infarction [13], pulmonary hypertension [14], [15], [16], [17], chronic hepatitis [18], diabetes [19], [20] and systemic lupus erythematosus [21] among others. Danggui buxue tang (DBT), a Chinese herbal concoction composed of Radix Astragali and Angelica sinensis, is commonly prescribed to treat menopausal irregularity and menstrual disorders [22], [23], [24]. DBT triggered specific phosphorylations of ERα and ERK1/2 in the cultured human breast cancer cell line, MCF-7 [25].
Despite Radix Astragali have been shown to stimulate angiogenesis in some studies, the mechanism underlying its angiogenic activity remains unclear [26]. The major bioactive constituents of Radix Astragali are saponins and flavonoids, including astragaloside (I∼VIII), calycosin, formononetin, ononin and their glucosides [27], [28]. Among these isoflavonoids, calycosin is the candidate with most potential to develop as a small-molecule angiogenic agent, due to its benefits upon endothelial cells [29]. Calycosin protects HUVECs from hypoxia-induced barrier impairment by increasing intracellular energetic sources and promoting regeneration of cAMP levels, as well as improving cytoskeleton remodeling. Our previous study illustrated that Radix Astragali extract (RAE) possesses pro-angiogenic effects upon human umbilical vein endothelial cells (HUVECs), which involve the VEGF-VEGFR2 and PI3K-Akt-eNOS pathways [30]. HPLC chromatography revealed that the compositions of formononetin, calycosin, (6aR, 11aR)-9,10-dimethoxy-3-hydroxypterocarpan and saponins (astragaloside I, II and IV) in the RAE were 8.15%, 0.77%, 0.01% and 0.88% of the whole extract, respectively. In regards to the preliminary screening of the angiogenic effects of these constituents, calycosin was found to be the most potent pro-angiogenic agent among all. This present study examines whether calycosin acts on ER and promotes angiogenesis in HUVEC cultures in vitro and a transgenic zebrafish model in vivo.
Results
Pro-angiogenic effect of calycosin in zebrafish
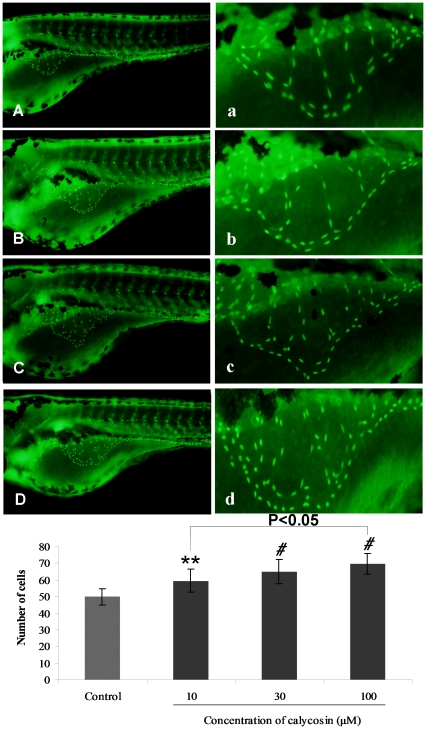
In zebrafish, angiogenic vessel development does not begin until 20 hpf (hours-post fertilization), and changes in subintestinal vein vessels (SIVs) are detected after 72 hpf. Fig. 1A shows that the SIVs of Tg(fli1:EGFP) zebrafish line treated with 0.1% DMSO at 96 hpf developed as a smooth basket-like structure. Following calycosin treatment (10, 30, 100 µM) from 72 hpf to 96 hpf, the diameter of SIVs increased in a dose-dependent manner (Fig. 1B–D). Quantitative analysis confirmed a significant (P<0.05 and P<0.001) dose-dependent effect of calycosin on diameter of SIVs compared with the control group (Fig. 1E).
Figure 1. The effects of calycosin treatment on blood vessel formation in SIVs of Tg(fli1:EGFP) zebrafish embryos.
(A) Control: embryo treated with 0.1% DMSO at 96 hpf, SIVs appear as a smooth basket-like structure. (B–D) Calycosin: embryo treated with 10, 30, 100 µM calycosin at 72 hpf for 24 h, leads to enlarged SIV basket stretching into the posterior yolk extension. (a–d) Enlarged SIV region (×4.5) of A–D respectively. White arrows indicating the enlarged vessels, yellow and red arrows indicate sprouting and intersectioning branches respectively. (E) Calycosin increases SIV diameter in a dose-dependent manner. Data are plotted as mean±SEM, (n = 3), *P<0.05, #P<0.001.
In order to determine whether the change of blood vessel phenotype (Fig. 1B–D) involves merely a transient vasodilation effect, or genomic action on stimulating endothelial cells proliferation, Tg(fli1:nEGFP) zebrafish embryos were used to demonstrate the angiogenic effect of calycosin. Tg(fli1:nEGFP) fish were engineered similarly to Tg(fli1:EGFP) except that Tg(fli1:nEGFP) harbor nuclear-localized GFP expression, permitting real-time in vivo analysis of individual endothelial cells [31]. These results show that calycosin treated (10, 30, 100 µM) SIVs contained significantly (P<0.01 and P<0.001) more endothelial cells (Fig. 2B–D) throughout the SIV region than the control group (Fig. 2A). Quantitative analysis indicates that calycosin induced an approximately 1.5 times increase in endothelial cells population compared with the control (Fig 2E).
Figure 2. The effects of calycosin on endothelial cells population in SIVs of Tg(fli1:nEGFP) zebrafish embryos.
Each green light point represents one endothelial cell (GFP+). (A) Control: embryo treated with 0.1% DMSO at 96 hpf. (B–D) Calycosin: embryo treated with 10, 30, 100 µM calycosin at 72 hpf for 24 h, leads to an increase in endothelial cells. (a–d) Enlarged SIV region (×4.5) of A–D respectively. (E) Calycosin increases the number of endothelial cells in the SIV region in a dose-dependent manner. Data are plotted as mean±SEM, (n = 3), **P<0.01, #P<0.001.
Detection of mRNA expression in calycosin treated zebrafish
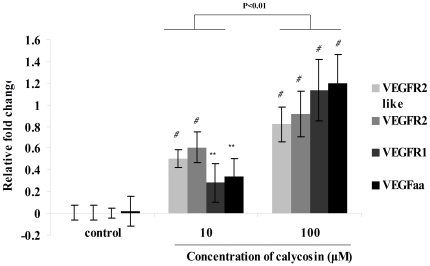
In order to identify molecular targets of the angiogenic effects of calycosin in zebrafish, mRNAs from different groups were isolated and reverse transcribed to cDNA, and relative gene expression determined using real-time PCR. VEGFA is a fundamental mediator of physiological and pathophysiological angiogenesis [32], and acts through tyrosine kinase receptors. VEGFR2 (fetal liver kinase, also known as KDR and Flk-1) has a higher affinity for VEGF and is a major transducer of the VEGF signal in endothelial cells [33], [34].
The bar charts in Fig. 3 represent the gene expression of VEGFA after treatment with 100 µM calycosin for 6 h. There was an increase trend of mRNA expression level compared to the control (1.2-fold at 100 µM), and calycosin caused a significant increase in mRNA expression of VEGFR1 (1.1-fold at 100 µM; P<0.001), Flk1A (0.8-fold at 100 µM; P<0.001) and Flk1B (0.9-fold at 100 µM; P<0.001). Hence, these results suggest that the up-regulation of expression of these genes caused by calycosin could contribute to the pro-angiogenic effects of calycosin observed in zebrafish.
Figure 3. Gene expression of calycosin treated zebrafish.
Data are expressed as mean ±SEM from three individual experiments. **P<0.01, #P<0.001 vs control group; P<0.01 vs calycosin group.
VEGFRs are important in calycosin-induced angiogenic effects
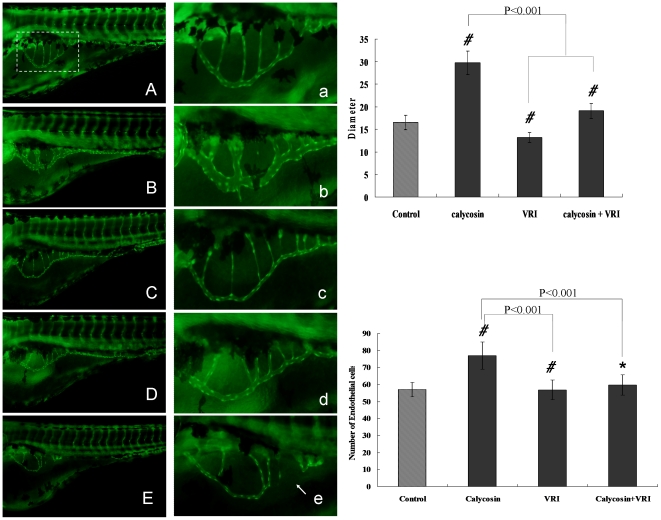
VEGFR tyrosine kinase inhibitor II (VTKI, VRI), a pyridinyl-anthranilamide compound that displays both antiangiogenic and antitumor properties, has been shown to potently inhibit the kinase activities of VEGFR1 and VEGFR2 [35]. We found that VRI, when in high concentration (1 µg/ml), caused significant (P<0.001) defects in angiogenesis in zebrafish embryonic development (Fig. 4E). Indeed, a lower concentration of VRI (100 ng/ml), which itself had no effect (Fig. 4C), caused significant (P<0.001) defects in calycosin-induced angiogenesis in zebrafish embryonic development (Fig. 4D). Quantitative analysis confirmed that a low concentration of VRI (100 ng/ml) was sufficient to reverse the calycosin-induced angiogenic effects to control levels (Fig. 4F & 4G). This indicates that, in exerting its effect, calycosin interacts with VEGF receptors (VEGFRs), further confirming that calycosin-induced angiogenesis, at least in part, involves the VEGF- VEGFR2 signaling pathway.
Figure 4. The effects of VEGFR tyrosine kinase inhibitor on calycosin-induced angiogenesis in zebrafish embryos.
(A) Control: embryo treated with 0.1% DMSO at 96 hpf. (B) Calycosin: embryo treated with calycosin (100 µM) at 72 hpf for 24 h. (C & E) VRI: embryo treated with low concentration (100 ng/ml, C) and high concentration (1 µg/ml, E) of VRI at 72 hpf for 24 h. (D) VRI and calycosin: embryo treated with both VRI (100 ng/ml) and calycosin (100 µM) at 72 hpf for 24 h. (a–e) Enlarged SIV region (×4.5) of A–E respectively. (F) Effects of calycosin and/or VRI on the diameter of SIV compared with the control group. Data are plotted as mean±SEM, (n = 3), #P<0.0001. (G) Effects of calycosin and/or VRI on the number of endothelial cells in SIV region compared with the control group. Data are plotted as mean±SEM, (n = 3), *P<0.05, #P<0.001.
Calycosin acts directly but differentially with ERα and ERβ
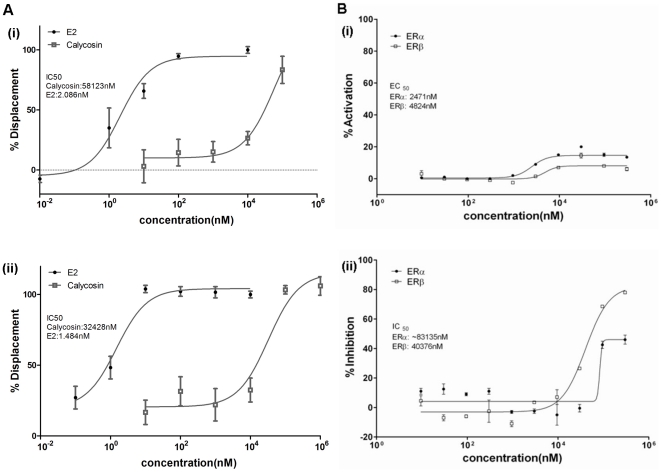
Since ERs are potential targets of calycosin [25], its binding affinities to ERα and ERβ were evaluated by fluorescent polarization competitive binding assay. 17-β-estradiol (E2), a native agonist for both ERα and ERβ, was used as a positive control. In this study, E2 displayed strong binding affinity for ERα and ERβ (ERα: IC50 = 2.086 nM, Fig. 5A–i; ERβ: IC50 = 1.484 nM, Fig. 5A–ii). Calycosin displaced Fluormone™ ES2 and bound to ERα and ERβ in a dose-dependent manner (Fig. 5A–i & Fig. 5A–ii). The binding affinities of calycosin to ERα and ERβ were not as strong as that of E2, with an IC50 value approximately 104-fold higher than that of E2 and its lower maximum displacement. On the other hand, the IC50 value of calycosin at ERα (IC50 = 58.123 µM, Fig. 5A–i) was very similar to that at ERβ (IC50 = 32.428 µM, Fig. 5A–ii).
Figure 5. Cell-free and cell-based estrogenic assays.
(A) Dose-response curves for competitive binding assay. Calycosin and 17-β-estradiol (E2) at the concentrations shown competitively bind with (i) ERα and (ii) ERβ, which caused displacement of Fluormone™ ES2 from ER; (B) Dose-response curves for GeneBLAzer β-lactamase reporter-gene assay. (i) Agonistic activities and (ii) antagonistic activities of calycosin at ERα and ERβ were determined in ERα-UAS-bla GripTite™ and ERβ-UAS-bla GripTite™ cell lines respectively. Results are presented as mean±SEM. (n≥2 independent experiments), P<0.01 between different ER subtypes followed by two-way ANOVA.
To further examine the transcriptional agonistic/antagonistic action of calycosin on ERs, GeneBLAzer β-lactamase reporter-gene experiments were performed. Calycosin showed weak agonistic activities at both ERα and ERβ (maximum activity was 14.6% and 8.6%, respectively, Fig. 5B–i). In contrast, the antagonistic activities of calycosin against E2 at ERα and ERβ were significant (maximum inhibition was 46% and 82%, respectively; P<0.01; Fig. 5B–ii). Thus, our results suggest that calycosin is a partial agonist/antagonist for both ERα and ERβ.
Calycosin also displayed receptor-selective potency and efficacy in the reporter gene assay. In the agonist activity assay, calycosin showed ERα selectivity with a 2-fold reduction in EC50 value and a 2-fold increase in maximal activation compared with ERβ (Fig. 5B–i). However, calycosin was more potent and efficacious at ERβ than at ERα in the antagonist activity assay, showing a 2-fold reduction in IC50 value and a 2-fold increase in maximal inhibition (Fig. 5B–ii).
Comparison of angiogenic effects of calycosin with other classical SERMs in zebrafish embryos.
Raloxifene is a SERM approved for clinical use in osteoporosis, and has been suggested to induce cardioprotection in women at high risk of coronary heart disease [36]. Another example of a SERM is tamoxifen, which is an antagonist of the estrogen receptor and is used in treating breast cancer [37]. E2 represents the major estrogen in humans, which modulates various vascular functions, including inflammation, wound healing, and angiogenesis [38], [39], [40]. As shown in Fig. 6, only calycosin exhibited a significant angiogenic effect in SIVs (Fig. 6e, thick arrow), while no obvious changes were observed in the raloxifene (10 µM), tamoxifen (3 µM) and 17-βEstradiol (10 µM) groups (Fig. 6b–d, arrows) at their highest non-toxic doses in zebrafish embryos.
Figure 6. The effects of calycosin, raloxifene and tamoxifen in SIVs of Tg(fli1:EGFP).
(A) Controls: were treated with 0.1% DMSO at 96 hpf, showing no effect on vessel formation (B–E) were treated with 10 µM raloxifene, 3 µM tamoxifen, 10 µM 17-β-Estradiol and 100 µM calycosin at 72 hpf for 24 h. (a–e) Enlarged SIV region (×4.5) of A–E respectively. Abnormal phenotype of blood vessel formation in SIVs was indicated by white arrow, showing slight increase in vessel diameter. Significant increase in vessel diameter was indicated by thick white arrow.
Calycosin promotes angiogenesis in HUVEC in vitro
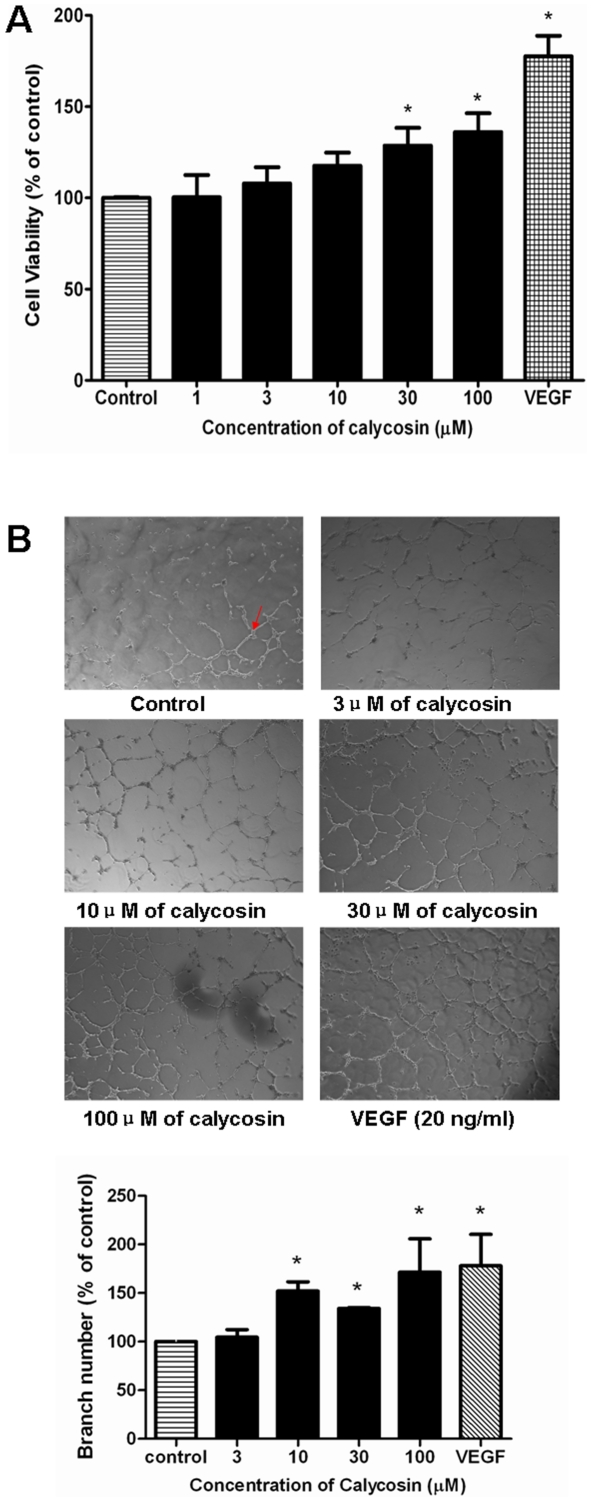
The effect of calycosin on HUVEC proliferation was evaluated using an XTT assay. Following a 24 h starvation, HUVECs were cultured in low serum medium supplemented with calycosin (1 µM–100 µM; 48 h). Cell viability was estimated by determining the amount of formazon product formed in the cell culture medium. As shown in Fig. 7A, calycosin promoted cell proliferation in a dose-dependent manner. The maximum increase of cell viability induced by calycosin was 36% at 100 µM, compared to vehicle control. A significant (P<0.05) increase in cell proliferation was also observed in VEGF-treated cells (77%), which served as the positive control.
Figure 7. The effects of calycosin on HUVECs in vitro.
(A) Effects of calycosin on proliferation of HUVEC by XTT assay. HUVECs were seeded in 96-well plates and incubated with calycosin at different concentrations. Cell proliferation was assessed using XTT assay; (B) Tube formation of calycosin-treated HUVECs on Matrigel. HUVECs cultured on 3-dimensional Matrigel in treatment of calycosin (3 µM, 10 µM, 30 µM and 100 µM). Cells receiving 0.1% DMSO served as vehicle control. Number of branching points in different concentrations of calycosin-treated HUVECs was calculated by computer software (Metamorph). Results are expressed as percentage of control (100%) in mean±SEM (n≥3 independent experiments), *P<0.05 versus control.
The process of angiogenesis is complex, and typically consists of proliferation and alignment to form tubular structures [41]. To test the ability of calycosin to induce HUVEC capillary tube formation, a Matrigel model was used. When HUVECs were cultured on Matrigel – a solid gel of mouse basement membrane proteins – cells aligned easily and formed hollow, tube-like structures. Fig. 7B shows that a very low level of tube formation was observed when HUVECs were plated on Matrigel in low-serum medium, whereas morphological changes were observed after treatment with calycosin. Quantitative analysis indicates that calycosin stimulated HUVECs to form more branching points (Fig. 7B). The number of branching points increased in a dose-dependent manner and reached its maximum (71%) at a calycosin concentration of 100 µM. A significant (P<0.05) increase in branching points was also observed in VEGF-treated cells (71%), which served as the positive control.
Calycosin induces angiogenesis via activation of MAPK signaling pathway
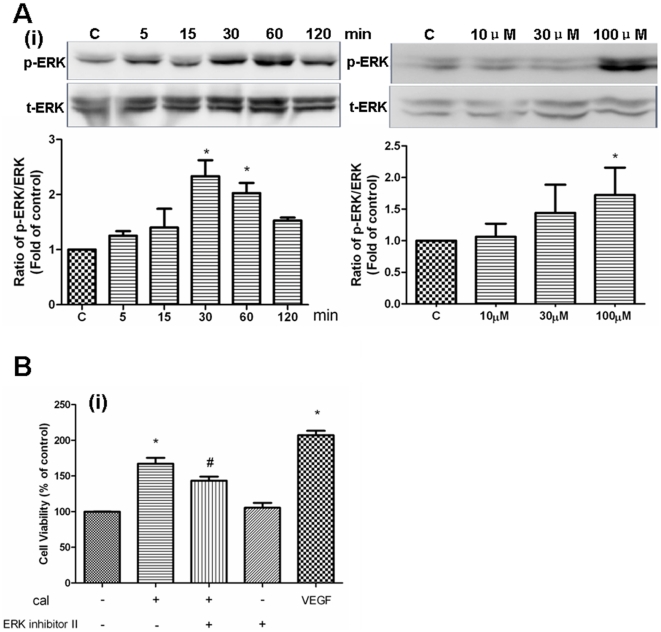
ERK1/2, one of the major targets of the MAPK signaling pathway, has been implicated in the regulation of angiogenesis for different functions including cell proliferation, migration and survival [41], [42]. To evaluate the rapid activation of these kinases, western blotting was used to examine the phosphorylation of ERK1/2 following calycosin treatment.
Firstly, phospho-ERK1/2 and total-ERK1/2 were detected following treatment with calycosin after different time durations. Calycosin stimulated the phosphorylation of ERK1/2 in a time-dependent manner (Fig. 8A–i), which reached a plateau at 30–60 min, and rapidly declined thereafter. However, the total protein levels of ERK1/2 remained unaffected throughout the course of these experiments. Furthermore, phosphorylation of ERK1/2 in HUVECs was enhanced in a dose-dependent manner after incubating with different concentrations of calycosin (Fig. 8A–i). The phosphorylation of ERK1/2 reached its maximum at a calycosin concentration of 100 µM, consistent with the results of the XTT assay. These data demonstrate that calycosin stimulated rapid activation of ERK1/2 in a time- and dose-dependent manner.
Figure 8. Role of MAPK signaling in calycosin-induced angiogenesis.
(A) Effects of calycosin on ERK1/2 activation. HUVEC were incubated with calycosin (100 µM) at indicated time or with calycosin in different concentrations for 30 min. Expressions of phospho-ERK1/2 and total-ERK1/2 were analyzed by western blotting and quantified by densitometry. The values indicate the relative densitometric units. Results are represented as mean±SEM (n = 3 independent experiments), * P<0.05 versus control. (B) Effect of ERK activation inhibitor peptide II on calycosin-induced HUVEC proliferation. HUVECs were pre-treated with 0.5 µM ERK activation inhibitor peptide II (ERK inhibitor II) for 1 h before the addition of calycosin (100 µM). Changes in HUVEC proliferation were determined 48 h later by XTT assay. 20 ng/ml VEGF was used as the positive control in this experiment. “cal” is the abbreviation of calycosin. Results are expressed as percentage of vehicle control (100%) in mean±SEM (n≥3 independent experiments), *P<0.05 versus vehicle control, # P<0.05 versus calycosin.
To further confirm the involvement of ERK1/2 in calycosin-mediated angiogenesis, a specific blocker was applied to examine its effect on calycosin-induced proliferation. HUVEC proliferation was significantly (P<0.05) increased after incubating with calycosin, but this was significantly (P<0.05) inhibited after pre-treatment with ERK activation inhibitor peptide II (Fig. 8B–i). Altogether, these results indicate that ERK1/2-dependent pathways are involved in calycosin-induced HUVEC proliferation.
Calycosin induces HUVEC proliferation via interaction with ER
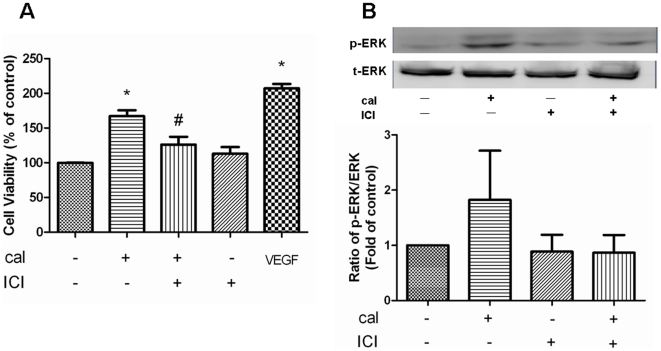
To confirm whether ER is involved in the angiogenic activity of calycosin, the effects of ER inhibitors on calycosin-induced HUVEC proliferation, and ERK1/2 activation, were examined. Fig. 9A demonstrates that calycosin significantly promoted the HUVEC proliferation by 67% (P<0.05), while the ER inhibitor (IVI182, 780) significantly reduced the proliferation by 40% (P<0.05). Western blotting revealed that expression of phospho-ERK1/2 was markedly enhanced in calycosin-treated HUVECs, whereas ICI182, 780 (30 µM) suppressed phosphorylation of ERK1/2 to control levels (Fig. 9B). Total ERK1/2 protein levels were unaffected by these treatments. Altogether, these results show that the effects of calycosin on HUVEC proliferation and ERK1/2 activation could be reversed by ER inhibition.
Figure 9. Role of ER in calycosin-induced angiogenesis.
(A) Effects of ICI182, 780 on calycosin-induced HUVEC proliferation. HUVECs were pre-treated with ICI182, 780 (30 µM) before the addition of calycosin (100 µM). Data are expressed as percentage of vehicle control (100%) in mean±SEM (n = 3 independent experiments), *P<0.05 versus control, #P<0.05 versus calycosin. (B) Effect of ICI182, 780 on calycosin-induced activation of ERK1/2. Calycosin-stimulated phosphorylation of ERK1/2 was completely reversed by the absence of ICI182, 780 (30 µM). Expression of phospho-ERK1/2 and total-ERK1/2 was analyzed by western blotting and quantified by densitometry. The values indicate the relative densitometric units of the p-ERK1/2 bands with the density of the control band set arbitrarily at 1.0. Results are represented as mean±SEM. “cal” and “ICI” are the abbreviations of calycosin and ICI182, 780 respectively.
Discussion
Proliferation of endothelial cells is a key process in angiogenesis [43]. The present study demonstrates that calycosin enhances endothelial cells proliferation in HUVECs in vitro, and in zebrafish embryos in vivo. Both blood vessel diameter and number of endothelial cells increased following calycosin treatment of transgenic zebrafish. Thus, these findings suggest that calycosin possesses pro-angiogenic activity.
Furthermore, these results show that the calycosin-induced phenotypic change in zebrafish involved activation of angiogenesis-related signaling pathways. Changes in mRNA expression levels of several angiogenesis-specific markers were determined. VEGF, also known as vascular permeability factor (VPF), was originally described as an EC-specific mitogen, a potent angiogenic factor [44], as well as an essential growth factor for vascular ECs. Formation of new blood vessels is orchestrated by a plenitude of different proteins, including cell adhesion molecules, ECM components and VEGFRs. Gene targeting experiments have provided insights into the functions of VEGFRs [45], [46]. Although inactivation of each individual VEGFR can cause embryonic lethality at mid-gestation, they have different functions [47], [48]. VEGFR2 is the receptor that initiates the main signaling pathways activated by VEGF. The main function of VEGFR1 appears to be in regulating binding between VEGF and VEGFR2 [49]. In this investigation, the results of real-time PCR illustrate that calycosin extract increased VEGF expression, as well as having a tendency to upregulate expression of VEGFR1 and VEGFR2. Moreover, VKRI, an inhibitor of VEGFR1 and VEGFR2, was shown to potently inhibit the kinase activities of these two proteins [35]. These data confirmed the predominant involvement of these angiogenesis-specific targets in calycosin-induced increases in endothelial cell number and blood vessel diameter at SIVs in zebrafish, and further supported the hypothesis that these clear phenotypic changes were as a result of angiogenesis stimulation.
Menopausal women suffer from many health problems such as hot flushes, sweating and mood swings; they are also more prone to cardiovascular disease, bone density reduction and osteoporosis. These problems are mainly due to deficiencies of ovarian hormones, especially estrogen. Therefore, hormone replacement therapy (HRT) is often applied to relieve such menopausal symptoms, and offer protection against osteoporosis and cardiovascular diseases [50]. However, recent epidemiological studies, and randomized trials, have revealed that women who used HRT had an increased risk of developing breast cancer, strokes and thromboembolisms [50], [51]. These reports contributed to the development of SERM, which is defined as molecules binding with ER and producing a change in the biological activities of the receptor with cell, or tissue, specificity.
Cell-free and cell-based estrogenic assays both revealed that calycosin competitively bound with ERα and ERβ. In addition, calycosin also displayed selective potency and affinity to ERα and ERβ in reporter-gene assays. Clinical and animal studies have suggested multiple benefits of SERM, and several SERMs have already been clinically approved, including raloxifene and tamoxifen. Recent findings have demonstrated the beneficial effects of these two classical SERMs upon the vascular system [52], [53], [54], [55]. Since raloxifene and tamoxifen share the same/similar antagonistic action with calycosin at ERβ, we compared the angiogenic effects of the three compounds in zebrafish embryos. Of the three, only calycosin promoted significant angiogenic development in the SIVs of zebrafish embryos.
A previous study investigated the activities of compounds demonstrated to be active in zebrafish embryo bioassays, in both zebrafish and mammalian cell lines [56]. Interestingly, only half of the 14 compounds were shown to be active in both embryos and either one of the cell lines, revealing that they exerted direct action upon cells. In our results, calycosin not only promoted angiogenesis in zebrafish but also enhanced endothelial cells proliferation and tube formation in HUVECs in vitro, both of which are standard tests for angiogenesis. Although no study has been carried out to identify bioequivalent doses between cell cultures and zebrafish, our results suggest that calycosin, at least in part, exerts direct action upon endothelial cells. Thus, we can further investigate the mechanism of action of calycosin in cell culture.
Many studies have shown that MAPK signaling pathway activation plays a vital role in the proliferation, migration and morphogenesis of endothelial cells induced by pro-angiogenic factors [57], [58]. To further elucidate the mechanism of the angiogenic activity of calycosin, activation of MAPK signaling was detected. It was shown that calycosin stimulated ERK1/2 activation rapidly in HUVECs (Fig. 8A). In addition, an ERK1/2-specific inhibitor effectively reversed calycosin-induced HUVEC proliferation (Fig. 8B). Thus, these results indicate that calycosin promotes angiogenesis via activation of MAPK with the involvement of ERK1/2.
Since calycosin selectively modulates ER transcriptional activation, as well as promoting angiogenesis, to further elucidate the relationship between these two activities, the effects of ER inhibitor ICI182, 780 on calycosin-induced HUVEC proliferation and the expression of phospho-ERK1/2 were examined. In vitro and in vivo studies have demonstrated that estrogen and ER agonists promote angiogenesis in endothelial cells via ERs [3], [59]. It has also been shown that inhibition of ER reduces angiogenesis induced by an ER agonist [5]. Here, we showed that ICI182, 780 significantly (P<0.05) decreased calycosin-induced HUVEC proliferation (Fig. 9A). Moreover, recent studies indicate that 17-β-estradiol stimulates ERK1/2 phosphorylation through ERα activation in endothelial cells [60]. In this sense, our data revealed that calycosin-stimulated ERK1/2 activation was also abrogated by ER inhibition (Fig. 9B). Altogether, our data suggest that calycosin stimulates activation of ER and MAPK signaling pathways, which may contribute to the pro-angiogenic activity of calycosin.
In conclusion, this present study provides evidence that calycosin from Radix Astragali acts as a novel SERM, since calycosin was shown to competitively bind with ERα and ERβ, as well as selectively modulate ER transcriptional activities. We also show that calycosin treatment promotes several features of angiogenesis in HUVECs in vitro. Our studies elucidate the mechanism of the angiogenic activity of calycosin on HUVEC cells, where it promotes angiogenesis through activation of ER and the MAPK signaling pathway to play multiple roles in regulating cell proliferation and morphogenesis. Finally, our findings provide inspiration for further development of Radix Astragali and calycosin as therapeutic agents for the treatment of problems associated with estrogen deficiency, such as cardiovascular diseases in post-menopausal women.
Materials and Methods
Ethics Statement
All animal experiments were conducted according to the ethical guidelines of ICMS, University of Macau and the protocol was approved by ICMS, University of Macau.
Chemicals and reagents
Kaighn's modification of Ham's F12 medium (F-12K), fetal bovine serum (FBS), phosphate-buffered saline (PBS), charcoal-stripped fetal bovine serum (CS-FBS), penicillin-streptomycin (PS), 0.25% (w/v) trypsin/1 mM EDTA and nitric oxide indicators DAF-FM diacetate were all purchased from Invitrogen (Carlsbad, CA, USA). Endothelial cell growth supplement (ECGS), heparin, gelatin, ER antagonist ICI182, 780, 17β-estradiol, Raloxifene hydrochloride, Tamoxifen, SNP and wortannin were supplied by Sigma (St Louis, MO). Growth factor reduced (GFR) Matrigel™ basement membrane matrix was obtained from BD Biosciences (Bedford, MA). ERK activation inhibitor peptide II was obtained from Biocalchem (Darmstadt, Germany). Vascular endothelial growth factors (VEGF) were obtained from R&D Systems (Minneapolis, MN). Anti-p-ERK1/2 antibody, anti-ERK1/2 antibody and goat anti-rabbit IgG HRP-conjugated antibody were all purchased from Cell Signaling Technology (Berverly, MA). Dimethyl sulfoxide (DMSO) was acquired from SIGMA and the calycosin(≥99%) was extracted at our laboratory. Calycosin was dissolved in DMSO to form a 100 mM solution. VEGFR tyrosine kinase inhibitor II (VTKI) was purchased from Calbiochem Company/EMD Chemicals Inc (Cat. No. 676481) and was dissolved in DMSO to form a 1 mg/ml solution.
Maintenance of zebrafish and its embryos
EGFP is expressed in all endothelial cells and each nucleus of Tg(fli-1:EGFP) and Tg(fli-1:nEGFP) zebrafish embryos. All types of zebrafish were maintained as described in the Zebrafish Handbook [61].
Embryo collection and drug treatment
Zebrafish embryos were generated by natural pair-wise mating (3–12 months old) and were raised at 28.5°C in embyro water. Healthy, hatched zebrafish were picked out at 3 dpf and distributed into a 12-well microplate with 10 to 15 fish in each well. Different concentrations (10, 30, 100 µM) of calycosin, raloxifene or tamoxifen solutions were then added to wells and incubated at 28°C for 24 h. Embryos receiving DMSO (0.1%) served as vehicle controls and were equivalent to no treatment. Each experiment was repeated at least three times, with 30 embryos per group. VTKI was dissolved in DMSO as stock. Zebrafish embryos, Tg(fli-1:EGFP) and Tg(fli-1:nEGFP), were treated with inhibitor dissolved in embryo water from 3 dpf at the concentration indicated, controlled by DMSO treated embryos. Embryos were maintained using standard methods.
Morphological observation of zebrafish
At 96 hpf, zebrafish were removed from microplates and observed for viability and gross morphological changes under a fluorescence microscope (Olympus IX81 Motorized Inverted Microscope, Japan) equipped with a digital camera (DP controller, Soft Imaging System, Olympus). Images were analyzed with Axiovision 4.2 and Adobe Photoshop 7.0.
Assessment of vascular changes
Three random points in SIVs of Tg(fli1:EGFP) zebrafish embryos were chosen for vessel diameter measurement using AxiovisionLE 4.1. Numbers of endothelial cells in SIVs of Tg(fli1:nEGFP) zebrafish embryos were assessed by direct counting of the total number of green light points. Each green light point represents one endothelial cell (GFP+).
Total RNA extraction, reverse transcription, and real-time PCR
Zebrafish embryos at 72 hpf were treated with calycosin for 6 h. Total RNA was extracted from 30 zebrafish embryos of each treatment group using the RNeasy Mini Kit (Qiagen, USA) in accordance with the manufacturer's instructions. RNA was reverse transcribed to single-strand cDNA using SuperScript™ III First-Strand Synthesis System for RT–PCR (Invitrogen™, USA), followed by real-time PCR using the TaqMan® Universal PCR Master Mix and 250 nM custom TaqMan primers for zebrafish Flk1A, Flk1B, VEGFR1, VEGFA2 (Applied Biosystems, USA) in the ABI 7500 Real-Time PCR System (Applied Biosystems). The expression of Flk1A, VEGFA2 mRNA was normalized to the amount of bactin1, using the relative quantification method described by the manufacturer.
The zebrafish bactin1 primers were 5′-CAAGATTCCATACCCAGGAAGGA-3′ (F) and 5′-CAAGATTCCATACCCAGGAAGGA-3′(R) (Applied Biosystems, USA).
The zebrafish Flk1A (kdrl) primers were 5′- GACCATAAAACAAGTGAGGCAGAAG-3′ (F) and 5′- CTCCTGGTTTGACAGAGCGATA-3′(R) (Applied Biosystems, USA).
The zebrafish Flk1B (kdr) primers were 5′- CAAGTAACTCGTTTTCTCAACCTAAGC-3′ (F) and 5′-GGTCTGCTACACAACGCATTATAAC-3′(R) (Applied Biosystems, USA).
The zebrafish FLT1 primers were 5′-AACTCACAGACCAGTGAACAAGATC-3′ (F) and 5′-GCCCTGTAACGTGTGCACTAAA-3′(R) (Applied Biosystems, USA).
The zebrafish VEGFA2 primers were 5′-GATGTGATTCCCTTCATGGATGTGT-3′ (F) and 5′-GGATACTCCTGGATGATGTCTACCA-3′ (R) (Applied Biosystems, USA).
HUVEC culture
Human umbilical vein endothelial cells (ATCC, Manassas) were cultured in F-12K medium with 2 mM L-glutamine, 1.5 g/l sodium bicarbonate, 100 µg/ml heparin, 30 µg/ml endothelial cell growth supplement and 10% FBS at 37°C in a humidified atmosphere of 5% CO2. Tissue culture flasks, 96-well plates and 6-well plates were pre-coated with 0.1% gelatin. Cells were exposed to culture medium with 10% CS-FBS instead of normal FBS for at least 1 day before experiments. Cultures were then starved with low-serum medium (contain 0.5% CS-FBS) for either 24 h in cell proliferation assays, or overnight in other assays. All assays were conducted using low cell passage cells (2–5 passages).
ER fluorescence polarization competitive binding assay
The binding affinity of calycosin to ER-α and β was evaluated by the commercially available competitor assay (Invitrogen, Carlasbad, CA) according to the manufacturer's instructions. In brief, ER was added to fluorescently tagged ER ligand (Fluormone™ ES2) to form ER/Fluormone™ ES2 complexes with a high fluorescent polarization value. Displacement of fluorescently tagged ligands by unlabeled ligands decreased fluorescent polarization, resulting in a low value. In this system, changes in intensity of polarization reflect displacement of fluorescently tagged ligands. In 96-well plates, serial dilution of calycosin (1 nM to 3×105 nM) or the ER agonist 17-β-estradiol (104 nM to 10−2 nM) were added to ER/Fluormone™ ES2 complexes to compete with ES2 for binding to ER. Plates were incubated at room temperature for 2 h and fluorescent polarization values measured using a Multilabel Counter (Perkin Elmer, Singapore). Results were expressed as percentages of maximum displacement induced by 17-β-estradiol (10 µM).
Cell-based ER transcriptional response by GeneBLAzer β-lactamase reporter-gene assay
Assays were preformed by Invitrogen (USA) as described in literature [62]. Briefly, GeneBLAzer β-lactamase reporter-gene assays were performed to measure the agonistic or antagonistic activities of calycosin at ER. For ER agonist activity assay, 4 µl of a 10×serial dilution of 17-β-estratiol served as the control agonist (starting concentration 10 µM, 3-fold dilute manner), or calycosin (starting concentration 300 µM, 3-fold dilute manner), was added to the appropriate wells of a 384-well plate. 32 µl of cell suspension and 4 µl of Assay Media were added to each well to bring the final volume to 40 µl. Plates were incubated for 16–24 h, then 8 µl of 1 µM substrate loading solution was added to each well, and plates incubated for another 2 h at room temperature. For the antagonist activity assay, cells were grown and prepared as above. 4 µl of a 10×serial dilution of 4-hydroxytamoxifen (starting concentration 100 nM) for ERα, RU-496 (starting concentration 10 µM) for ERβ or calycosin (starting concentration 300 µM, 3-fold dilute manner) was added to cells. Cells were pre-incubated with calycosin and the antagonist control for 30–60 min, then 4 µl of 17-β-estradiol was added to wells at the pre-determined EC80 concentration. Plates were then incubated for another 16–24 h. 8 µl of 1 µM substrate loading solution was added to each well, and plates incubated for 2 h at room temperature. All results were measured using a fluorescence plate reader. Results of agonist activity assays were expressed as percentage activation of the defined maximum activation induced by 17-β-estradiol (10 µM). For the antagonist activity assay, inhibition responses were expressed as percentage inhibition in the presence of EC80 concentration of 17-β-estradiol according to the previous agonist activity assay.
HUVEC viability by XTT assay
HUVECs were trypsinised and seeded at 104 cells/well in 96-well gelatin coated plates. After 24 h, complete medium was removed and renewed with hormone-free low serum (0.5% CS-FBS) medium, and samples incubated for 24 h in order to starve HUVECs to achieve a quiescent state. After these pre-incubations, different concentrations (1 µM-100 µM) of calycosin medium were replaced. Cells receiving DMSO (0.1%) served as vehicle controls, and were equivalent to no treatment. For inhibition assays, HUVECs were pretreated with inhibitors (10 µM ICI182, 780 and 1 µM ERK activation inhibitor peptide II) for 60 min before addition of calycosin (100 µM). Cells receiving DMSO (0.1%) served as vehicle control and were equivalent to no treatment. On the other hand, cells cultured in VEGF (20 ng/ml) served as positive controls. After 48 h, cell proliferation was assessed by XTT for 4 h. The spectrophotometrical absorbance of each well was measured by a Multilabel counter (Perkin Elmer, Singapore). The wavelength used to measure absorbance of the formazan product was 490 nm and the reference wavelength was 690 nm. Cell viability data were expressed as percentage of cell viability calculated.
Tube formation assay on HUVEC
The effect of calycosin on HUVEC differentiation was examined by in vitro tube formation on Matrigel [63]. Confluent HUVECs were harvested and diluted (9×104 cells) in 500 µl low serum medium containing 3–100 µM calycosin, which were then seeded on 1∶1 Matrigel (v/v) coated 24-well plates in triplicate at 37°C for 7 h. Cells receiving DMSO (0.1%) served as vehicle controls, and were equivalent to no treatment. Besides, cells cultured in 20 ng/ml VEGF served as positive controls (data not shown). The network-like structures were examined under an inverted microscope (at 50× magnification). The tube-like structures were defined as endothelial cord formations that were connected at both ends. The number of branching points in three random fields per well was quantified by Metamorph Imaging Series software.
Western blotting analysis
Cells were treated with 100 µM calycosin for different time durations (5–120 min) in the time course study. 20 ng/ml VEGF was used as a positive control while medium with 0.1% DMSO served as a negative control. To observe dose-dependent effects of calycosin, 10 µM, 30 µM and 100 µM calycosin were used to treat HUVECs for 30 min in culture medium. For inhibition assays, HUVECs were pretreated with 10 µM ICI182, 780 for 60 min prior to the addition of 100 µM calycosin. Cells were then washed with PBS and lysed for 30 min on ice with lysis buffer (0.5 M NaCl, 50 mM Tris, 1 mM EDTA, 0.05% SDS, 0.5% Triton X-100, 1 mM PMSF, pH 7.4). Cell lysates were centrifuged at 11000×g for 20 min at 4°C. Protein concentrations in the supernatants were measured using the bicinchoninic acid assay (Pierce, Rockford, IL). Supernatants were electrophoresed on 12% SDS-PAGE, and transferred to polyvinylidene diuoride (PVDF) membranes, which were then blocked with 5% non-fat milk. Immunoblot analysis was undertaken by incubation with anti-p-ERK1/2 antibody and anti-ERK1/2 antibody at 4°C overnight. After washing, membranes were incubated for 1 h at room temperature with horseradish peroxidase-conjugated goat anti-rabbit IgG. Proteins were detected using an advanced enhanced ECL system (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Semi-quantifications were performed with densitometric analysis by Quantity One software.
Statistical analysis
Data was analyzed with unpaired two-tailed Student's t-tests or one-way ANOVA followed by Tukey's multiple comparison test, using GraphPad Prism 5.0 software (San Diego, CA). Curve fitting was carried out using GraphPad Prism 5.0 (nonlinear fit, variable slope sigmoidal dose-response model). Data were expressed as mean±SEM from individual experiments. Differences were considered as significant at P<0.05.
Acknowledgments
We are very grateful to Dr Patrick Ying-Kit Yue, Department of Biology, The Hong Kong Baptist University, for his advice and assistance on in vitro binding assay.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study is supported by grant from the Science and Technology Development Fund of Macau SAR (Ref. No. 045/2007/A3) and Research Committee, University of Macau (Ref. No. RG085 and UL017/09-Y1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Franco CA, Liebner S, Gerhardt H. Vascular morphogenesis: a Wnt for every vessel? Curr Opin Genet Dev. 2009;19:476–483. doi: 10.1016/j.gde.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Barkhem T, Nilsson S, Gustafsson JA. Molecular mechanisms, physiological consequences and pharmacological implications of estrogen receptor action. Am J Pharmacogenomics. 2004;4:19–28. doi: 10.2165/00129785-200404010-00003. [DOI] [PubMed] [Google Scholar]
- 3.Losordo DW, Isner JM. Estrogen and angiogenesis: A review. Arterioscler Thromb Vasc Biol. 2001;21:6–12. doi: 10.1161/01.atv.21.1.6. [DOI] [PubMed] [Google Scholar]
- 4.Kim KH, Moriarty K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids. 2008;73:864–869. doi: 10.1016/j.steroids.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim-Schulze S, McGowan KA, Hubchak SC, Cid MC, Martin MB, et al. Expression of an estrogen receptor by human coronary artery and umbilical vein endothelial cells. Circulation. 1996;94:1402–1407. doi: 10.1161/01.cir.94.6.1402. [DOI] [PubMed] [Google Scholar]
- 6.Morales DE, McGowan KA, Grant DS, Maheshwari S, Bhartiya D, et al. Estrogen promotes angiogenic activity in human umbilical vein endothelial cells in vitro and in a murine model. Circulation. 1995;91:755–763. doi: 10.1161/01.cir.91.3.755. [DOI] [PubMed] [Google Scholar]
- 7.Doshida M, Ohmichi M, Tsutsumi S, Kawagoe J, Takahashi T, et al. Raloxifene increases proliferation and up-regulates telomerase activity in human umbilical vein endothelial cells. J Biol Chem. 2006;281:24270–24278. doi: 10.1074/jbc.M513251200. [DOI] [PubMed] [Google Scholar]
- 8.Valachovicova T, Slivova V, Sliva D. Cellular and physiological effects of soy flavonoids. Mini Rev Med Chem. 2004;4:881–887. doi: 10.2174/1389557043403387. [DOI] [PubMed] [Google Scholar]
- 9.Kostelac D, Rechkemmer G, Briviba K. Phytoestrogens modulate binding response of estrogen receptors alpha and beta to the estrogen response element. J Agric Food Chem. 2003;51:7632–7635. doi: 10.1021/jf034427b. [DOI] [PubMed] [Google Scholar]
- 10.Seng WL, Eng K, Lee J, McGrath P. Use of a monoclonal antibody specific for activated endothelial cells to quantitate angiogenesis in vivo in zebrafish after drug treatment. Angiogenesis. 2004;7:243–253. doi: 10.1007/s10456-004-4181-7. [DOI] [PubMed] [Google Scholar]
- 11.Chen XJ, Bian ZP, Lu S, Xu JD, Gu CR, et al. Cardiac protective effect of Astragalus on viral myocarditis mice: comparison with Perindopril. Am J Chin Med. 2006;34:493–502. doi: 10.1142/S0192415X06004028. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Z, Wang W, Wang F, Zhao K, Han Y, et al. Effects of Astragaloside IV on heart failure in rats. Chin Med. 2009;4:6. doi: 10.1186/1749-8546-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu XL, Ji H, Gu SY, Shao Q, Huang QJ, et al. Cardioprotective effects of Astragali Radix against isoproterenol-induced myocardial injury in rats and its possible mechanism. Phytother Res. 2008;22:389–394. doi: 10.1002/ptr.2332. [DOI] [PubMed] [Google Scholar]
- 14.He J, Jing Z, Gu Q. [Collagen expression of intra-acinar pulmonary arteries and right ventricle and intervention of Radix Astragali in rats with hypoxic pulmonary hypertension]. Zhonghua Yi Xue Za Zhi. 1999;79:654–656. [PubMed] [Google Scholar]
- 15.Xi S, Ruan Y, Liu Y. [Morphometric investigation on hypoxic structural remodeling of intraacinar pulmonary arteries]. Zhonghua Jie He He Hu Xi Za Zhi. 1998;21:303–305. [PubMed] [Google Scholar]
- 16.Chen X, Ruan Y, Xi S, Si W, Zhang L. [Therapeutic effect of radix Astragali on hypoxia pulmonary hypertension in rats]. Zhongguo Zhong Yao Za Zhi. 1997;22:432–434, inside back cover. [PubMed] [Google Scholar]
- 17.Liu JC, An CS, Wang JF, Li FY, Li JH. [Influence of Radix Astragali on nitric oxide and endothelin-1 in pulmonary tissue in hypoxemic pulmonary hypertension in rats]. Zhonghua Er Ke Za Zhi. 2006;44:46–48. [PubMed] [Google Scholar]
- 18.Liu K. [Preliminary report on various symptoms of chronic hepatitis treated with radix Astragali and its regulative effect on levels of serum hormone]. Zhong Xi Yi Jie He Za Zhi. 1990;10:330–333, 323. [PubMed] [Google Scholar]
- 19.Chan CM, Chan YW, Lau CH, Lau TW, Lau KM, et al. Influence of an anti-diabetic foot ulcer formula and its component herbs on tissue and systemic glucose homeostasis. J Ethnopharmacol. 2007;109:10–20. doi: 10.1016/j.jep.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Xu A, Wang H, Hoo RL, Sweeney G, Vanhoutte PM, et al. Selective elevation of adiponectin production by the natural compounds derived from a medicinal herb alleviates insulin resistance and glucose intolerance in obese mice. Endocrinology. 2009;150:625–633. doi: 10.1210/en.2008-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai XY, Xu YL, Lin XJ. [Effects of radix Astragali injection on apoptosis of lymphocytes and immune function in patients with systemic lupus erythematosus]. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2006;26:443–445. [PubMed] [Google Scholar]
- 22.Choi RC, Gao QT, Cheung AW, Zhu JT, Lau FT, et al. A Chinese Herbal Decoction, Danggui Buxue Tang, Stimulates Proliferation, Differentiation and Gene Expression of Cultured Osteosarcoma Cells: Genomic Approach to Reveal Specific Gene Activation. Evid Based Complement Alternat Med. 2009. [DOI] [PMC free article] [PubMed]
- 23.Song ZH, Ji ZN, Lo CK, Dong TT, Zhao KJ, et al. Chemical and biological assessment of a traditional chinese herbal decoction prepared from Radix Astragali and Radix Angelicae Sinensis: orthogonal array design to optimize the extraction of chemical constituents. Planta Med. 2004;70:1222–1227. doi: 10.1055/s-2004-835855. [DOI] [PubMed] [Google Scholar]
- 24.Dong TT, Zhao KJ, Gao QT, Ji ZN, Zhu TT, et al. Chemical and biological assessment of a chinese herbal decoction containing Radix Astragali and Radix Angelicae Sinensis: Determination of drug ratio in having optimized properties. J Agric Food Chem. 2006;54:2767–2774. doi: 10.1021/jf053163l. [DOI] [PubMed] [Google Scholar]
- 25.Gao QT, Choi RC, Cheung AW, Zhu JT, Li J, et al. Danggui buxue tang–a Chinese herbal decoction activates the phosphorylations of extracellular signal-regulated kinase and estrogen receptor alpha in cultured MCF-7 cells. FEBS Lett. 2007;581:233–240. doi: 10.1016/j.febslet.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Fan TP, Yeh JC, Leung KW, Yue PY, Wong RN. Angiogenesis: from plants to blood vessels. Trends Pharmacol Sci. 2006;27:297–309. doi: 10.1016/j.tips.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Shi ZY, Bao Z, Jiang Y, Tu PF. [Quantitative analysis of calycosin glycoside and formononetin in Radix astragali from different sources]. Zhongguo Zhong Yao Za Zhi. 2007;32:779–783. [PubMed] [Google Scholar]
- 28.Song JZ, Yiu HH, Qiao CF, Han QB, Xu HX. Chemical comparison and classification of Radix Astragali by determination of isoflavonoids and astragalosides. J Pharm Biomed Anal. 2008;47:399–406. doi: 10.1016/j.jpba.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 29.Fan Y, Wu DZ, Gong YQ, Zhou JY, Hu ZB. Effects of calycosin on the impairment of barrier function induced by hypoxia in human umbilical vein endothelial cells. Eur J Pharmacol. 2003;481:33–40. doi: 10.1016/j.ejphar.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Hu G, Lin HC, Hong SJ, Deng YH, et al. Radix Astragali extract promotes angiogenesis involving vascular endothelial growth factor receptor-related phosphatidylinositol 3-kinase/Akt-dependent pathway in human endothelial cells. Phytother Res. 2009;23:1205–1213. doi: 10.1002/ptr.2479. [DOI] [PubMed] [Google Scholar]
- 31.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- 32.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- 33.Klagsbrun M, D'Amore PA. Vascular endothelial growth factor and its receptors. Cytokine Growth Factor Rev. 1996;7:259–270. doi: 10.1016/s1359-6101(96)00027-5. [DOI] [PubMed] [Google Scholar]
- 34.Zachary I. Signaling mechanisms mediating vascular protective actions of vascular endothelial growth factor. Am J Physiol Cell Physiol. 2001;280:C1375–1386. doi: 10.1152/ajpcell.2001.280.6.C1375. [DOI] [PubMed] [Google Scholar]
- 35.Furet P, Bold G, Hofmann F, Manley P, Meyer T, et al. Identification of a new chemical class of potent angiogenesis inhibitors based on conformational considerations and database searching. Bioorg Med Chem Lett. 2003;13:2967–2971. doi: 10.1016/s0960-894x(03)00626-7. [DOI] [PubMed] [Google Scholar]
- 36.Bracamonte MP, Rud KS, Miller VM. Mechanism of raloxifene-induced relaxation in femoral veins depends on ovarian hormonal status. J Cardiovasc Pharmacol. 2002;39:704–713. doi: 10.1097/00005344-200205000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Jordan VC. Tamoxifen (ICI46,474) as a targeted therapy to treat and prevent breast cancer. Br J Pharmacol. 2006;147(Suppl 1):S269–276. doi: 10.1038/sj.bjp.0706399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cid MC, Schnaper HW, Kleinman HK. Estrogens and the vascular endothelium. Ann N Y Acad Sci. 2002;966:143–157. doi: 10.1111/j.1749-6632.2002.tb04211.x. [DOI] [PubMed] [Google Scholar]
- 39.Haynes MP, Li L, Sinha D, Russell KS, Hisamoto K, et al. Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J Biol Chem. 2003;278:2118–2123. doi: 10.1074/jbc.M210828200. [DOI] [PubMed] [Google Scholar]
- 40.Johns A, Freay AD, Fraser W, Korach KS, Rubanyi GM. Disruption of estrogen receptor gene prevents 17 beta estradiol-induced angiogenesis in transgenic mice. Endocrinology. 1996;137:4511–4513. doi: 10.1210/endo.137.10.8828515. [DOI] [PubMed] [Google Scholar]
- 41.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 42.Pages G, Milanini J, Richard DE, Berra E, Gothie E, et al. Signaling angiogenesis via p42/p44 MAP kinase cascade. Ann N Y Acad Sci. 2000;902:187–200. doi: 10.1111/j.1749-6632.2000.tb06313.x. [DOI] [PubMed] [Google Scholar]
- 43.Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18:5356–5362. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- 44.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 45.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 46.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 47.Fong TA, Shawver LK, Sun L, Tang C, App H, et al. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999;59:99–106. [PubMed] [Google Scholar]
- 48.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 49.Park JE, Chen HH, Winer J, Houck KA, Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem. 1994;269:25646–25654. [PubMed] [Google Scholar]
- 50.Wren BG. The benefits of oestrogen following menopause: why hormone replacement therapy should be offered to postmenopausal women. Med J Aust. 2009;190:321–325. doi: 10.5694/j.1326-5377.2009.tb02423.x. [DOI] [PubMed] [Google Scholar]
- 51.Conner P, Lundstrom E, von Schoultz B. Breast cancer and hormonal therapy. Clin Obstet Gynecol. 2008;51:592–606. doi: 10.1097/GRF.0b013e318180b8ed. [DOI] [PubMed] [Google Scholar]
- 52.Leung FP, Yung LM, Leung HS, Au CL, Yao X, et al. Therapeutic concentrations of raloxifene augment nitric oxide-dependent coronary artery dilatation in vitro. Br J Pharmacol. 2007;152:223–229. doi: 10.1038/sj.bjp.0707387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leung HS, Yung LM, Leung FP, Yao X, Chen ZY, et al. Tamoxifen dilates porcine coronary arteries: roles for nitric oxide and ouabain-sensitive mechanisms. Br J Pharmacol. 2006;149:703–711. doi: 10.1038/sj.bjp.0706921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pinna C, Bolego C, Sanvito P, Pelosi V, Baetta R, et al. Raloxifene elicits combined rapid vasorelaxation and long-term anti-inflammatory actions in rat aorta. J Pharmacol Exp Ther. 2006;319:1444–1451. doi: 10.1124/jpet.106.106062. [DOI] [PubMed] [Google Scholar]
- 55.Wong CM, Yung LM, Leung FP, Tsang SY, Au CL, et al. Raloxifene protects endothelial cell function against oxidative stress. Br J Pharmacol. 2008;155:326–334. doi: 10.1038/bjp.2008.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murphey RD, Stern HM, Straub CT, Zon LI. A chemical genetic screen for cell cycle inhibitors in zebrafish embryos. Chem Biol Drug Des. 2006;68:213–219. doi: 10.1111/j.1747-0285.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- 57.Kuida K, Boucher DM. Functions of MAP kinases: insights from gene-targeting studies. J Biochem. 2004;135:653–656. doi: 10.1093/jb/mvh078. [DOI] [PubMed] [Google Scholar]
- 58.Uchiba M, Okajima K, Oike Y, Ito Y, Fukudome K, et al. Activated protein C induces endothelial cell proliferation by mitogen-activated protein kinase activation in vitro and angiogenesis in vivo. Circ Res. 2004;95:34–41. doi: 10.1161/01.RES.0000133680.87668.FA. [DOI] [PubMed] [Google Scholar]
- 59.Suzuma I, Mandai M, Takagi H, Suzuma K, Otani A, et al. 17 Beta-estradiol increases VEGF receptor-2 and promotes DNA synthesis in retinal microvascular endothelial cells. Invest Ophthalmol Vis Sci. 1999;40:2122–2129. [PubMed] [Google Scholar]
- 60.Geraldes P, Sirois MG, Tanguay JF. Specific contribution of estrogen receptors on mitogen-activated protein kinase pathways and vascular cell activation. Circ Res. 2003;93:399–405. doi: 10.1161/01.RES.0000088640.18462.42. [DOI] [PubMed] [Google Scholar]
- 61.Westerfield M. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 3rd Edition. Eugene, OR, University of Oregon Press; 1995. The Zebrafish Book.385 [Google Scholar]
- 62.Wilkinson JM, Hayes S, Thompson D, Whitney P, Bi K. Compound profiling using a panel of steroid hormone receptor cell-based assays. J Biomol Screen. 2008;13:755–765. doi: 10.1177/1087057108322155. [DOI] [PubMed] [Google Scholar]
- 63.Merchan JR, Chan B, Kale S, Schnipper LE, Sukhatme VP. In vitro and in vivo induction of antiangiogenic activity by plasminogen activators and captopril. J Natl Cancer Inst. 2003;95:388–399. doi: 10.1093/jnci/95.5.388. [DOI] [PubMed] [Google Scholar]